Abstract
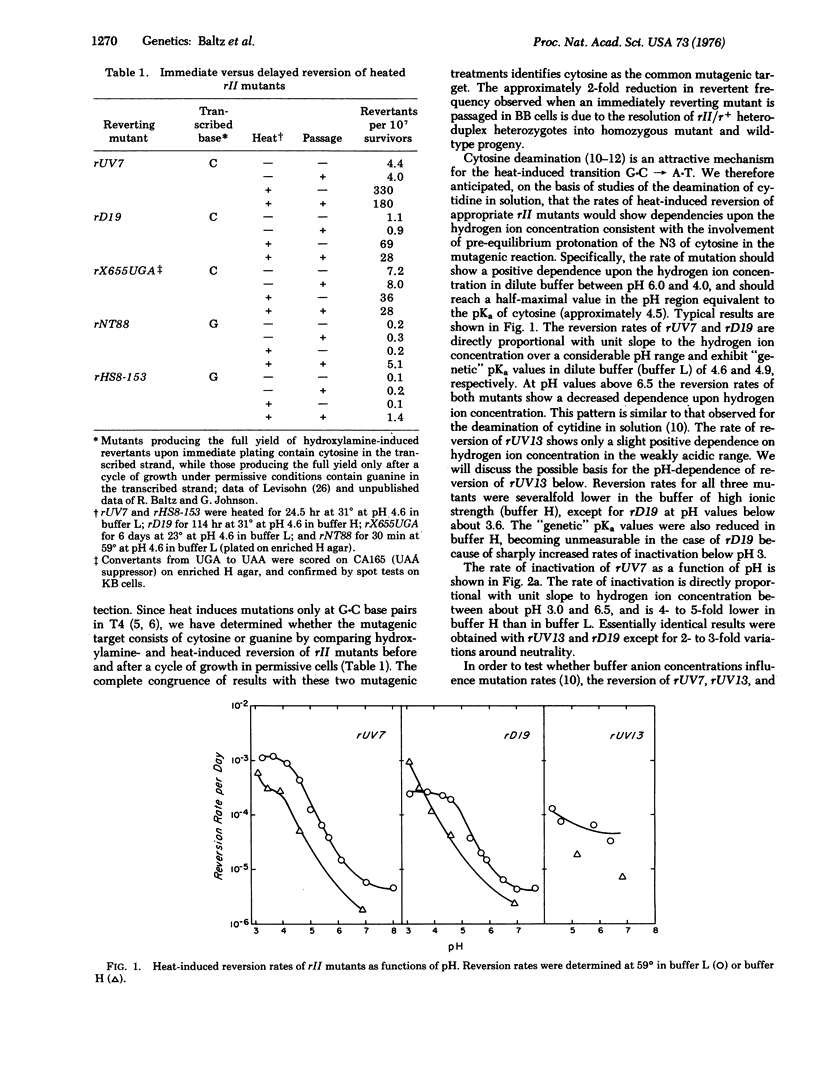
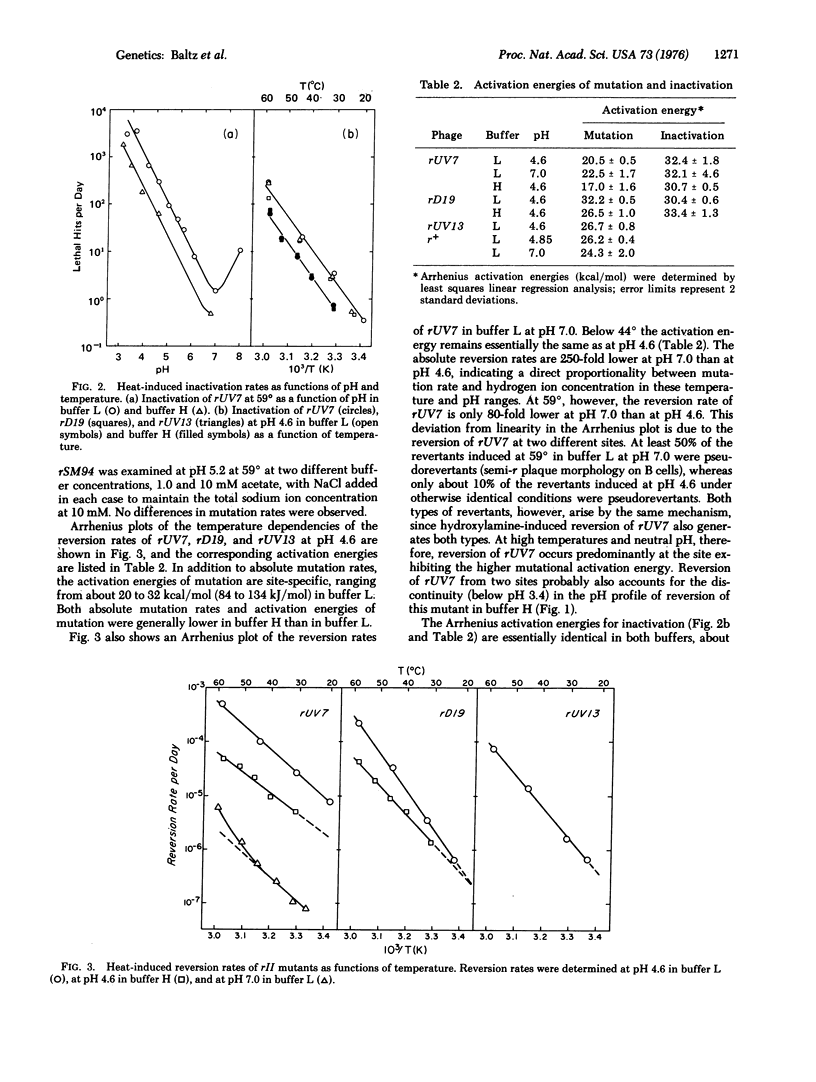
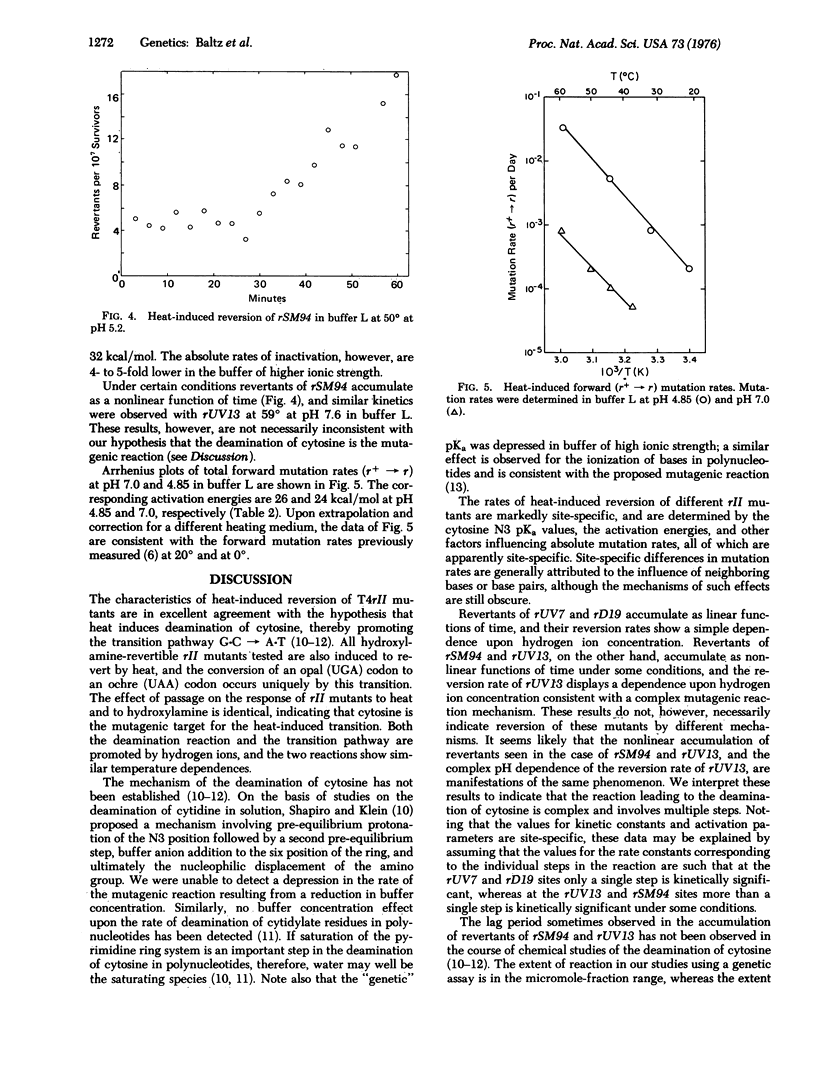
G-C leads to A-T transitions are induced by heat, and arise from the deamination of cytosine (5-hydroxymethylcytosine in the case of bacteriophage T4) generating uracil. The reaction is proton-catalyzed, and is also characteristic of acid mutagenesis. Mutation rates and activation energies of mutation are site-specific, and are presumably influenced by neighboring bases. Rates of heat-induced mutation in bacteriophage T4 under conditions of temperature, pH, and ionic strength similar to those prevailing in higher eukaryotic cells suggest that heat mutagenesis may present a serious challenge to organisms with large genomes, and may comprise an important determinant of the rates of spontaneous mutation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H., Drake J. W. Bacteriophage T4 transformation: an assay for mutations induced in vitro. Virology. 1972 Aug;49(2):462–474. doi: 10.1016/0042-6822(72)90498-9. [DOI] [PubMed] [Google Scholar]
- Bautz E., Freese E. ON THE MUTAGENIC EFFECT OF ALKYLATING AGENTS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1585–1594. doi: 10.1073/pnas.46.12.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Darland G. K. Limits of microbial existence: temperature and pH. Science. 1970 Sep 25;169(3952):1316–1318. doi: 10.1126/science.169.3952.1316. [DOI] [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Characteristics of mutations appearing spontaneously in extracellular particles of bacteriophage T4. Genetics. 1967 Mar;55(3):387–398. doi: 10.1093/genetics/55.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Spontaneous mutations accumulating in bacteriophage T4 in the complete absence of DNA replication. Proc Natl Acad Sci U S A. 1966 Apr;55(4):738–743. doi: 10.1073/pnas.55.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRENBERG L., VON EHRENSTEIN G., HEDGRAN A. Gonad temperature and spontaneous mutation-rate in man. Nature. 1957 Dec 21;180(4599):1433–1434. doi: 10.1038/1801433a0. [DOI] [PubMed] [Google Scholar]
- FREESE E. B. Transitions and transversions induced by depurinating agents. Proc Natl Acad Sci U S A. 1961 Apr 15;47:540–545. doi: 10.1073/pnas.47.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E. On the molecular explanation of spontaneous and induced mutations. Brookhaven Symp Biol. 1959 Nov;NO:63–75. [PubMed] [Google Scholar]
- GREER S., ZAMENHOF S. Studies on depurination of DNA by heat. J Mol Biol. 1962 Mar;4:123–141. doi: 10.1016/s0022-2836(62)80046-1. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Tsau J. Solvolyses of cytosine and cytidine. J Pharm Sci. 1972 Jul;61(7):1052–1061. doi: 10.1002/jps.2600610703. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Levisohn R. Genotypic reversion by hydroxylamine: the orientation of guanine-hydroxymethylcytosine at mutated sites in phage T4 rII. Genetics. 1967 Feb;55(2):345–348. doi: 10.1093/genetics/55.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Karlström O. Heat-induced depyrimidination of deoxyribonucleic acid in neutral solution. Biochemistry. 1973 Dec 4;12(25):5151–5154. doi: 10.1021/bi00749a020. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Generation time and genomic evolution in primates. Science. 1973 Mar 16;179(4078):1144–1147. doi: 10.1126/science.179.4078.1144. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Klein R. S. The deamination of cytidine and cytosine by acidic buffer solutions. Mutagenic implications. Biochemistry. 1966 Jul;5(7):2358–2362. doi: 10.1021/bi00871a026. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y., Thibodeau L. Nuclease for DNA apurinic sites may be involved in the maintenance of DNA in normal cells. Nat New Biol. 1973 Jul 18;244(133):67–69. doi: 10.1038/newbio244067a0. [DOI] [PubMed] [Google Scholar]
- ZAMENHOF S., GREER S. Heat as an agent producing high frequency of mutations and unstable genes in Escherichia coli. Nature. 1958 Aug 30;182(4635):611–613. doi: 10.1038/182611a0. [DOI] [PubMed] [Google Scholar]
- Zamenhof S. EFFECTS OF HEATING DRY BACTERIA AND SPORES ON THEIR PHENOTYPE AND GENOTYPE. Proc Natl Acad Sci U S A. 1960 Jan;46(1):101–105. doi: 10.1073/pnas.46.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]



