Abstract
In the present study, we investigated the dynamic expression of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway related factors in the process of in vitro hippocampal neural stem/progenitor cell differentiation from embryonic Sprague-Dawley rats or embryonic Kunming species mice, using fluorescent quantitative reverse transcription-PCR and western blot analyses. Results demonstrated that the dynamic expression of fibroblast growth factor 8 was similar to fibroblast growth factor receptor 1 expression but not to other fibroblast growth factor receptors. Enzyme-linked immunosorbent assay demonstrated that fibroblast growth factor 8 and Sonic Hedgehog signaling pathway protein factors were secreted by neural cells into the intercellular niche. Our experimental findings indicate that fibroblast growth factor 8 and Sonic Hedgehog expression may be related to the differentiation of neural stem/progenitor cells.
Keywords: neural stem cells, neural progenitor cells, fibroblast growth factor 8, Sonic Hedgehog, signal pathway, secretion, dynamic, differentiation, neurons, neural regeneration
Research Highlights
-
(1)
During the process of neural stem/progenitor cell differentiation, fibroblast growth factor or Sonic Hedgehog proteins or factors may be secreted from other neural cells, and together they compose a niche environment for neurosphere co-culture.
-
(2)
The experimental results were analyzed by reverse transcription-PCR, western blot and enzyme-linked immunosorbent assay, to provide a detailed analysis of mRNA and protein expression.
-
(3)
There were differences between the cell mRNA, protein content and protein secretion levels.
Abbreviations
ELISA, enzyme-linked immunosorbent assay
INTRODUCTION
Fibroblast growth factor is a multi-functional peptide growth factor that regulates cellular proliferation and the growth of organisms[1,2]. Fibroblast growth factor 8 and fibroblast growth factor receptors are expressed in many tissues and species[3]. Sonic Hedgehog signaling pathway is involved in cell fate, patterning in embryonic development, homeostasis, and adult tissue renewal[4].
Neural stem/progenitor cells may differentiate into neurons, astrocytes and oligodendrocytes[5,6]. This differentiation process is considered to be regulated by intrinsic elements and extrinsic signals[5,6,7,8,9]. Among them, signaling pathway molecules such as BMP4, Oct4, Sox2, Nanog, and Nurr1, and the ERK, Wnt, and Notch signaling pathways have been closely studied[5,6,8,9,10]. However, the molecular mechanisms that regulate and mediate the differentiation process are still not well understood[6,7]. Here, we mainly studied the changes in expression and secretion of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway as they relate to neural stem/progenitor cell differentiation in vitro.
RESULTS
Changes in expression and secretion of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during neural stem/progenitor cell differentiation in vitro
Quantitative reverse transcription-PCR, western blot and enzyme-linked immunosorbent assay (ELISA) analyses demonstrated that the mean fibroblast growth factor 8 mRNA expression and protein levels from cultured neural stem/progenitor cells were significantly lower on day 10 of the differentiation culture compared with neural stem/progenitor cells or day 20 (P < 0.05; Figures 1, 2).
Figure 1.
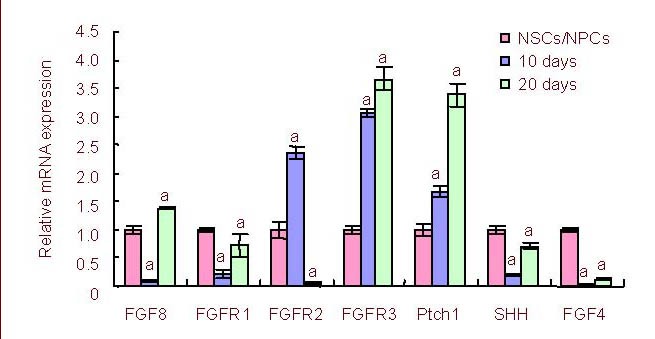
Dynamic expression of FGF8, FGFRs and Sonic Hedgehog signaling pathway molecule mRNA during neural stem/progenitor cells differentiation in vitro was measured on day, 10 and 20 by reverse transcription-PCR.
aP < 0.05, vs. expression of the previous differentiation stage. The results were expressed as absorbance ratio of mRNA expression on day 10 or 20 to that of the neural stem/progenitor cell stage, which was assigned a value of 1 (mean ± SD, n = 4).
FGF: Fibroblast growth factor; FGFR: fibroblast growth factor receptor; NSCs/NPCs: neural stem/progenitor cells.
Figure 2.
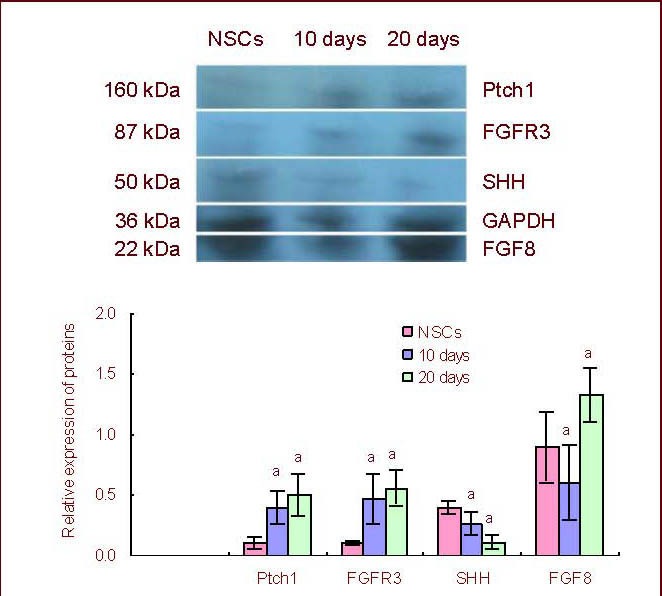
Dynamic expression of FGF8, FGFR3 and Sonic Hedgehog signaling pathway molecule protein levels during neural stem/progenitor cell differentiation in vitro.
aP < 0.05, vs. expression level of the previous differentiation stage (Duncan multiple comparison test). The western blot data are expressed as the absorbance ratio of target protein to GAPDH on day 10 or 20 to that of the neural stem/progenitor cell stage (mean ± SD, n = 4).
FGF: Fibroblast growth factor; FGFR: fibroblast growth factor receptors; NSCs: neural stem cells.
Interestingly, timing of expression was different between fibroblast growth factor receptor 1, fibroblast growth factor receptor 2 and fibroblast growth factor receptors 3. Fibroblast growth factor receptor 1 expression decreased on day 10 but fibroblast growth factor receptor 2 reached a peak of expression on day 10, suggesting an inverse correlation between the two receptors. Fibroblast growth factor receptors 3 mRNA expression and protein levels increased over time between the neural stem/progenitor cell stage to day 20 (Figures 1, 2).
Ptch1, a Sonic Hedgehog signaling pathway surface receptor, also demonstrated a significantly higher expression on day 20 compared with the neural stem/progenitor cell stage cells and those on day 10 (P < 0.01; Figure 1).
Fibroblast growth factor 8 and Sonic Hedgehog factors were continuously secreted into the culture medium during the differentiation process. The peak secretion of Sonic Hedgehog occurred on day 4, while the peak of fibroblast growth factor 8 secretion was on day 20 of neural stem/progenitor cell differentiation (Figure 3).
Figure 3.
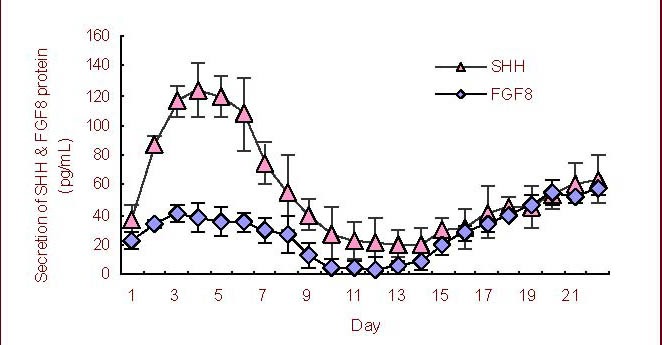
Dynamic secretion of fibroblast growth factor 8 (FGF8) and Sonic Hedgehog (SHH) proteins during neural stem/progenitor cell differentiation in vitro by enzyme-linked immunosorbent assay.
Tissue culture supernatant was used for enzyme-linked immunosorbent assay of FGF8 or SHH proteins everyday between day 1 and 22 (n = 4). Data are expressed as mean ± SD.
Immunofluorescence analysis of the dynamic expression of fibroblast growth factor 8 during neural stem/progenitor cell differentiation in vitro
The relative intensity of nestin+ fluorescence staining on neural stem/progenitor cells during differentiation in vitro indicated no significant difference on any of the days tested (days 3, 10 and 20, P > 0.05; Figure 4).
Figure 4.
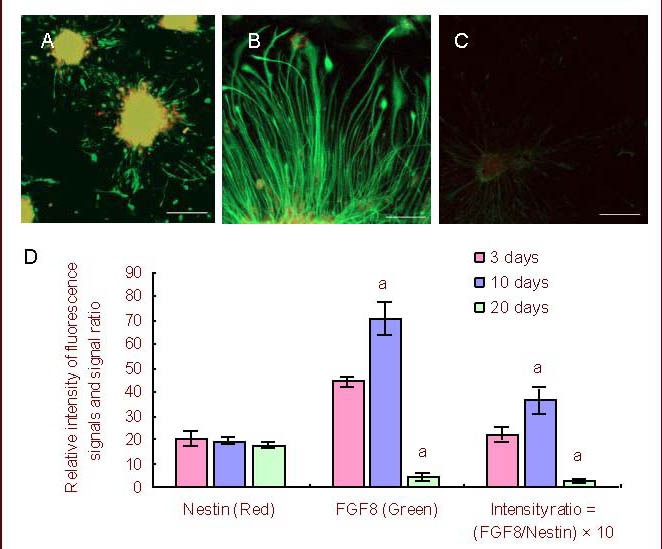
Immunofluorescence analysis of FGF8 expressions and distribution during neural stem/progenitor cell differentiation in vitro.
(A–C) Immunofluorescence staining on days 3, 10 and 20 during NSC/NPC differentiation. Green (FITC): FGF8+; red (TRITC): Nestin+; multicolor: merged. Scale bars: A, 100 μm; B, 50 μm; C, 200 μm.
(D) Relative intensity of FGF8+ (green) and Nestin+ (red) fluorescence signals, and the signal ratios of FGF8 to Nestin (green/red) on days 3, 10 or 20 during NSC/NPC differentiation (mean ± SD, n = 4). Nestin+ fluorescence signal intensity was used as a control.
The ratio of FGF8+ fluorescence intensity to nestin+ fluorescence intensity reflected the relative expression level of FGF8 at each stage. aP < 0.05, vs. that of the prior differentiation stage (Duncan multiple comparison test).
FGF8: Fibroblast growth factor 8; NSC/NPC: neural stem/progenitor cell.
However, the relative intensity of fibroblast growth factor 8+ fluorescence staining was significantly higher on day 10 than on day 3 or 20 (P < 0.05). Thus, the expression of fibroblast growth factor 8 protein was significantly increased on day 10 compared with on days 3 and 20 while nestin levels were relatively stable in the un-differentiated neural stem/progenitor cells.
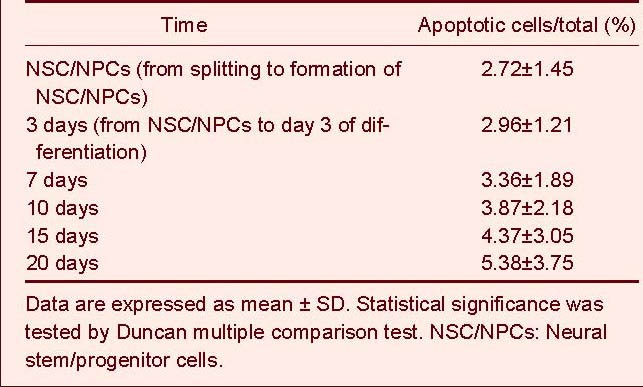
Under the differentiation conditions used in this study, all neurospheres were actively proliferating or undergoing differentiation according to the morphology of the cells observed at different neural stem/progenitor cell differentiation stages in vitro, as well as the results of neural cell apoptosis tests (Figure 5, Table 1). The cultured cells continued to expand and very low levels of accumulated apoptosis were observed up to day 20 of neural stem/progenitor cell differentiation. The percentage of accumulated apoptotic cells was 5.38 ± 3.75% by 20 day of differentiation.
Figure 5.
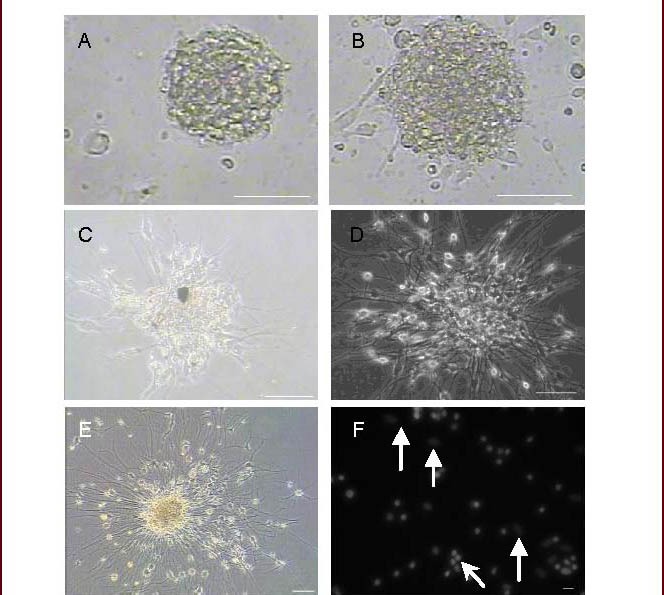
The time course of neural stem/progenitor cell differentiation in vitro (Leica microscope or confocal microscope; scale bar, A–E: 100 μm; F: 20 μm).
(A) Neural stem/progenitor cell. (B–E) Cultured neural stem/progenitor cells were observed by microscopy on days 3, 7, 10, and 20. (F) Hochest33258 staining was performed to observe apoptotic nuclei on day 20 after differentiation. Arrowheads indicate glial cell nuclei; thin arrows indicate non-glial cell nuclei.
Table 1.
Neural stem cell apoptosis at stage of differentiation
DISCUSSION
Quantitative reverse transcription-PCR, western blot and ELISA analysis performed in this study demonstrated that fibroblast growth factor 8 and Sonic Hedgehog signaling pathways may be involved in neural stem/progenitor cell differentiation in vitro[10,11]. We observed the changes in expression and secretion of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during the differentiation of neural stem/progenitor cells. Immunofluorescence analysis of fibroblast growth factor 8 expression on nerve cells during neural stem/progenitor cell differentiation confirmed the dynamic expression of fibroblast growth factor 8. All our experiments suggested that fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules were regulated during neural stem/progenitor cell differentiation and thus may be important for central nervous system neurosphere proliferation in vitro and in vivo[10,11].
Previous studies have shown similar dynamics of fibroblast growth factor 8 expression during mammalian development and growth in vivo[11,12,13].
Although reports have demonstrated the importance of Sonic Hedgehog, fibroblast growth factor 8 in stem cell differentiation to dopaminergic neurons[10,11]. Studies describing the serial dynamic expression and secretion of Sonic Hedgehog and fibroblast growth factor 8 signaling pathway molecules during stem cell differentiation in vitro or in vivo have not been reported. In the present study, we attempted to detect and analyze the dynamic expression and secretion of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during neural stem/progenitor cell differentiation in vitro, with the aim of elucidating the functions of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules in neural stem/progenitor cell differentiation and mammalian nervous system development and generation.
Our study indicated that the expression of fibroblast growth factor 8 and fibroblast growth factor receptors, and Sonic Hedgehog and Ptch1 did not occur concurrently. The dynamic expression of fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 differed from that of fibroblast growth factor 8. In some cases, there were inverse relationships between expression levels. For example, the lowest expression of fibroblast growth factor 8 mRNA and the peak of fibroblast growth factor receptor 2 mRNA expression occurred on day 10 whereas fibroblast growth factor receptor 3 mRNA expression increased over time. However, the expression of fibroblast growth factor receptor 1 mRNA was similar in temporal expression to that of fibroblast growth factor 8. However, whether fibroblast growth factor 8 has a higher affinity for fibroblast growth factor receptor 1 than other fibroblast growth factor receptors was not determined in this study. Whether this is important for neural stem/progenitor cell differentiation is difficult to determine as the different fibroblast growth factor receptors or fibroblast growth factors share similar structures and overlapping functions[14,15]. For instance, fibroblast growth factor 8 can bind to most fibroblast growth factor receptors including fibroblast growth factor receptor 1. Similarly, fibroblast growth factor receptor 1 can bind to other fibroblast growth factors besides fibroblast growth factor 8[14,15]. Therefore, the promiscuous binding between fibroblast growth factor family receptors and ligands suggests that multiple combinations may be important during neural stem/progenitor cell differentiation. In addition, as dimerization can occur between two fibroblast growth factor molecules or fibroblast growth factor receptorss[16,17], the original expression and function of the molecules may be altered[16,17].
MATERIALS AND METHODS
Design
A randomized, controlled study on cell molecular biology.
Time and setting
Experiments were performed at Guangxi University, Hubei University of Medicine, Affiliated Taihe Hospital, China, from July 2007 to September 2011.
Materials
Pregnant female Sprague-Dawley rats or Kunming mice were provided by the Laboratory Animal Center of Hubei University of Medicine, China (license No. SCXK (E) 2005-0008). Pregnant mice were housed individually in cages in a temperature controlled room (20–24°C) on a 12-hour light/dark cycle, and allowed free access to food and water, at 60 ± 10% relative humidity, 30–50 db background noise. All mice used in the study (males and females) were fed in a specific pathogen-free laboratory animal feeding environment. The experimental procedure was in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued and governed by the Ministry of Science and Technology of China[18].
Methods
Isolation and culture of hippocampal neural stem/progenitor cells from Sprague-Dawley rats or Kunming mice
The protocol for isolation of neural stem/progenitor cells was described previously[6,9,19]. In brief, pregnant female mice were anesthetized with ether and executed, and the uteri removed immediately. Ten to twelve 14-day embryonic Kunming mice or six to seven 14-day embryonic Sprague-Dawley rats were used to obtain one flask of primary cells. The fetal murine hippocampus was isolated in a biological safety cabinet. Cells were incubated at 5 × 105/mL in a 25-cm2 tissue culture flask in basic culture medium, consisting of DMEM/F12 (1:1, v/v), 2% B27 supplement, 1% N2 supplement, 0.5 mM L-glutamine and 0.5 mM non-essential amino acid, respectively, 50 IU/mL penicillin and 50 μg/mL streptomycin growth culture medium, and supplemented with 20 ng/mL recombinant human basic fibroblast growth factor (Promega, Madison, WI, USA)[5,6,9,19].
Differentiation of hippocampal neural stem/progenitor cells from Sprague-Dawley rats or Kunming mice
The neural stem/progenitor cells were further incubated for 20 days in poly-L-lysine coated (0.1%, 10 mg/mL) flasks according to our novel defined neural stem/progenitor cell differentiation protocol using the special culture medium described above but which also contained 8% serum replacement (SR, Gibco, Grand Island, NY, USA) and lacked the basic fibroblast growth factor[5,6,9,19]. Half of the volume of the novel culture medium was replaced with fresh medium every 7 days.
Dynamic expression of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecule mRNA real-time quantitative PCR detection
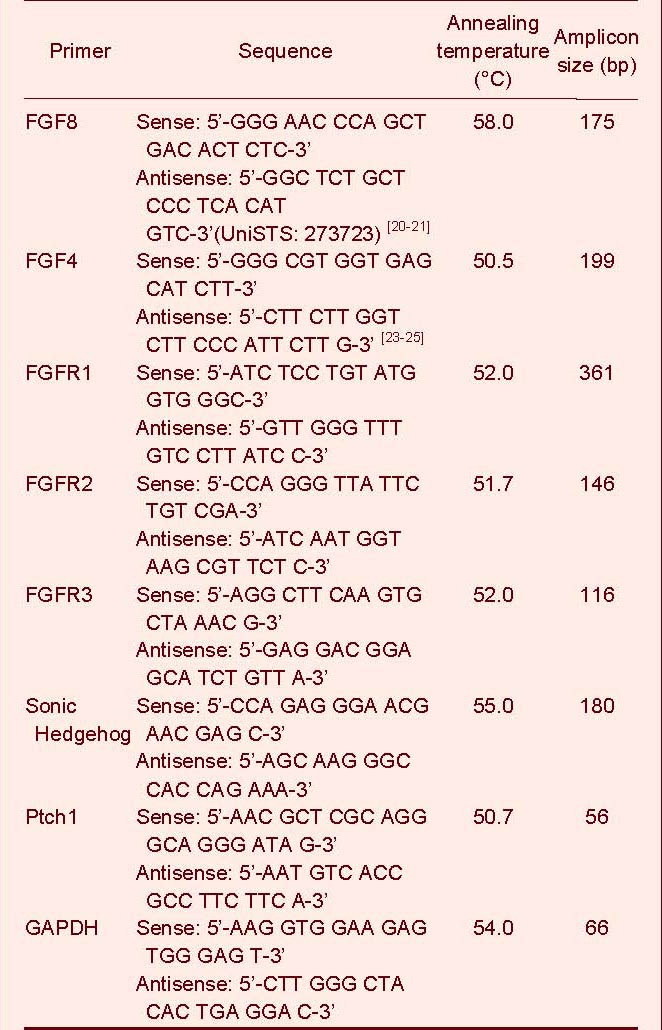
According to our experimental design, more than 200 neurospheres (neural stem/progenitor cells 1–2 × 104 cells) were used for the neural stem/progenitor cell time point or un-differentiated neurospheres mRNA extraction[5,6,9,19]. For the differentiated cells, which were adherent in the flasks, about 0.5–1 × 105 cells (one 25-cm2 flask) were used for mRNA extraction at each timepoint[6,9]. Total cellular RNA was extracted according to the manufacturer's instructions for RNA Isolation and Purification Procedure using the Promega SV Total RNA Isolation System (Promega, Madison, WI, USA). Total RNA was converted to cDNA using a murine Moloney leukemia virus (M-MuLV; Fermentas, Vilnius, Lithuania) for reverse transcription. Real-time quantitative PCR primers were either designed by ourselves or based on Genebank primers from NCBI or literature reports[20,21,22,23,24,25] (Table 2).
Table 2.
Primer sequences
Dynamic expression of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecule protein levels by western blot assay
In brief, more than 200 neurospheres (1–2 × 104 cells) were used for total protein extraction at the neural stem/progenitor cell stage, and about 0.5–1 × 105 neural cells were collected from each 25-cm2 flask on day 10 or 20 after differentiation for total protein extraction[5,6,9,19].
Total proteins were separated by 12% SDS-PAGE and transferred onto polyvinylidene difluoride blotting membranes (Millipore, Billerica, MA, USA) by semi-dried electrophoretic transfer. The membranes were then blocked with 5% nonfat dry milk (Wandashan, Harbin, China) in TBST (100 mM NaCl, 50 mM Tris pH 7.5, 0.15% Tween-20), then incubated with rabbit anti-fibroblast growth factor 8 polyclonal antibody (1:200; Bioss, Beijing, China), rabbit anti-fibroblast growth factor receptors 3 polyclonal antibody (1:200; Bioss), rabbit anti-Patch1 polyclonal antibody (1:800; Sigma, St. Louis, MO, USA), rabbit anti-Sonic Hedgehog polyclonal antibody (1:500; Proteintech, Chicago, IL, USA) and rabbit anti-GAPDH polyclonal antibody (1:200; Millipore) for 12 hours at 4°C, then washed three times with TBST for 10 minutes each. Rabbit anti-GAPDH polyclonal antibody (1:200; Millipore) served as an internal control. The membranes were then incubated for 12 hours at 4°C with horseradish peroxidase-conjugated AffiniPure goat anti-rabbit IgG antibody (1:1 000; Pierce, Thermon Fisher Scientific Inc., Rockford, IL, USA), detected with enhanced chemiluminescence reagent (Millipore) after three washes with TBST for 10 minutes. All the membranes were exposed to X-ray film and the expression of target genes was quantified by detecting specific bands recorded on X-ray film by Band Scan 5.0 system (Glyko, Hayward, CA, USA).
Dynamic secretion of fibroblast growth factor 8 and Sonic Hedgehog by ELISA
ELISA was used to detect secreted proteins in the culture medium, using the following kit: mouse fibroblast growth factor 8 ELISA Kit (Cusabio, Wuhan, China); mouse Sonic hedgehog ELISA Kit (MyBioSource, San Diego, California, USA). Culture medium (500 μL) was taken from each testing well for the ELISA, and replaced with 500 μL of fresh medium. The ELISA was performed according to the manufacturer's instructions. A microplate reader (Σ960, Metertech Inc., Taiwan, China) was used to measure absorbance at 450 nm, with the correction wavelength set at 540 nm or 570 nm.
Immunofluorescence analysis of fibroblast growth factor 8 and nestin expression on neural stem/progenitor cell during differentiation
The analyses of cell immunofluorescence were performed on days 3, 10 and 20 of neural stem/progenitor cell differentiation. Neural cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature. Then, the samples were permeabilized with 0.5% Triton X-100 and blocked with 4% goat serum. After that, samples were incubated with rabbit anti-fibroblast growth factor 8 polyclonal antibody (1:50; Bioss) and mouse anti-nestin monoclonal antibody (1:100; BD Biosciences, San Diego, CA, USA) for 12 hours at 4°C. Cells were then washed three times for 10 minutes, and samples were incubated with secondary antibodies conjugated with fluorescein isothiocyanate (1:100; FITC-conjugated goat anti-rabbit IgG; Thermon Fisher Scientific Inc., USA) and tetraethyl rhodamine isothiocyanate (1:100; TRITC-conjugated goat anti-mouse IgG; Thermon Fisher Scientific Inc.) for 1 hour at room temperature in the dark. Samples were then sealed with fresh gum. Images were acquired by confocal microscopy (Carl Zeiss GmbH, Jena, Germany) and ImageJ 1.42q system (Java 1.6.0_10, National Institutes of Health, USA) were used to analyze and compare the relative intensity of fluorescence signal of each image[26].
Measurement of cell apoptosis at different stages of differentiation
We used the Annexin V-FITC/PI apoptosis assay kit (Bipec, Cambridge, Massachusetts, USA) to detect cell apoptosis by annexin-V and PI staining at different differentiation stages, and evaluated staining by flow cytometry (Beckman-Coulter, Miami, FL, USA). FITC and PI emissions were measured. Apoptotic analysis was represented by the ratio of cumulative apoptotic cells to total cells in the detection flask or well. In addition, Hochest 33258 (Beyotime, Haimen, China) staining was used to determine apoptotic nuclei. Nuclei were observed by confocal microscopy (Carl Zeiss GmbH). Blue light was filtered for better images.
Statistical analysis
Statistical analysis was performed with SPSS 13.0 (SPSS, Chicago, IL, USA). Measurement data are presented as mean ± SD. Differences between related groups were determined with the Duncan multiple comparison test for western blot, immunofluorescence and protein factor secretion experiments. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 81070614; the Key Project of the Natural Science Foundation of Hubei Province of China, No. 2008CDA044; and the Natural Science Foundation of Hubei University of Medicine, No. 2011QDZR-2.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Guangxi University, Hubei University of Medicine and affiliated Taihe Hospital, China.
(Edited by Ruan XZ, Zhao H/Yang Y/Song LP)
REFERENCES
- [1].Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313(2):139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- [2].Vesterlund L, Töhönen V, Hovatta O, et al. Co-localization of neural cell adhesion molecule and fibroblast growth factor receptor 2 in early embryo development. Int J Dev Biol. 2011;55(3):313–319. doi: 10.1387/ijdb.103240lv. [DOI] [PubMed] [Google Scholar]
- [3].Kataoka A, Shimogori T. Fgf8 controls regional identity in the developing thalamus. Development. 2008;135(17):2873–2881. doi: 10.1242/dev.021618. [DOI] [PubMed] [Google Scholar]
- [4].Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411(6835):349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- [5].Yu Y, Gu S, Huang H, et al. Combination of bFGF, heparin and laminin induce the generation of dopaminergic neurons from rat neural stem cells both in vitro and in vivo. J Neurol Sci. 2007;255(1-2):81–86. doi: 10.1016/j.jns.2007.01.076. [DOI] [PubMed] [Google Scholar]
- [6].Wen T, Bao K, Li H. Blocking BE301622 gene expression by RNAi initiates differentiation of neural stem cells in rat. Cell Biochem Funct. 2007;25(6):775–779. doi: 10.1002/cbf.1367. [DOI] [PubMed] [Google Scholar]
- [7].Satoh M, Sugino H, Yoshida T. Activin promotes astrocytic differentiation of a multipotent neural stem cell line and an astrocyte progenitor cell line from murine central nervous system. Neurosci Lett. 2000;284(3):143–146. doi: 10.1016/s0304-3940(00)00981-2. [DOI] [PubMed] [Google Scholar]
- [8].Chen F, Guo Q, Yang Y, et al. Inhibition of AF116909 gene expression enhances the differentiation of neural stem cells. Neurol Res. 2005;27(5):557–561. doi: 10.1179/016164105X25162. [DOI] [PubMed] [Google Scholar]
- [9].Wen T, Gu P, Minning TA, et al. Microarray analysis of neural stem cell differentiation in the striatum of the fetal rat. Cell Mol Neurobiol. 2002;22(4):407–416. doi: 10.1023/a:1021059520618. [DOI] [PubMed] [Google Scholar]
- [10].Kim TE, Lee HS, Lee YB, et al. Sonic hedgehog and FGF8 collaborate to induce dopaminergic phenotypes in the Nurr1-overexpressing neural stem cell. Biochem Biophys Res Commun. 2003;305(4):1040–1048. doi: 10.1016/s0006-291x(03)00879-9. [DOI] [PubMed] [Google Scholar]
- [11].Omoteyama K, Takagi M. FGF8 regulates myogenesis and induces Runx2 expression and osteoblast differentiation in cultured cells. J Cell Biochem. 2009;106(4):546–552. doi: 10.1002/jcb.22012. [DOI] [PubMed] [Google Scholar]
- [12].Martinez-Ferre A, Martinez S. The development of the thalamic motor learning area is regulated by Fgf8 expression. J Neurosci. 2009;29(42):13389–13400. doi: 10.1523/JNEUROSCI.2625-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sato T, Joyner AL. The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development. 2009;136(21):3617–3626. doi: 10.1242/dev.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kalyani AJ, Mujtaba T, Rao MS. Expression of EGF receptor and FGF receptor isoforms during neuroepithelial stem cell differentiation. J Neurobiol. 1999;38(2):207–224. [PubMed] [Google Scholar]
- [15].Inglis-Broadgate SL, Thomson RE, Pellicano F, et al. FGFR3 regulates brain size by controlling progenitor cell proliferation and apoptosis during embryonic development. Dev Biol. 2005;279(1):73–85. doi: 10.1016/j.ydbio.2004.11.035. [DOI] [PubMed] [Google Scholar]
- [16].Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an àla carte menu of heparan sulfate motifs. Curr Opin Struct Biol. 2005;15(5):506–516. doi: 10.1016/j.sbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [17].Hung KW, Kumar TK, Kathir KM, et al. Solution structure of the ligand binding domain of the fibroblast growth factor receptor: role of heparin in the activation of the receptor. Biochemistry. 2005;44(48):15787–15798. doi: 10.1021/bi051030n. [DOI] [PubMed] [Google Scholar]
- [18].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [19].Tropepe V, Sibilia M, Ciruna BG, et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208(1):166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- [20].Tanaka A, Miyamoto K, Minamino N, et al. Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci U S A. 1992;89(19):8928–8932. doi: 10.1073/pnas.89.19.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Denaxa M, Sharpe PT, Pachnis V. The LIM homeodomain transcription factors Lhx6 and Lhx7 are key regulators of mammalian dentition. Dev Biol. 2009;333(2):324–336. doi: 10.1016/j.ydbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sunmonu NA, Li K, Guo Q, et al. Gbx2 and Fgf8 are sequentially required for formation of the midbrain-hindbrain compartment boundary. Development. 2011;138(4):725–734. doi: 10.1242/dev.055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fang ZF, Jin F, Gai H, et al. Human embryonic stem cell lines derived from the Chinese population. Cell Res. 2005;15(5):394–400. doi: 10.1038/sj.cr.7290307. [DOI] [PubMed] [Google Scholar]
- [24].Kubota K, Iseki S, Kuroda S, et al. Synergistic effect of fibroblast growth factor-4 in ectopic bone formation induced by bone morphogenetic protein-2. Bone. 2002;31(4):465–471. doi: 10.1016/s8756-3282(02)00852-9. [DOI] [PubMed] [Google Scholar]
- [25].Mattei MG, Pébusque MJ, Birnbaum D. Chromosomal localizations of mouse Fgf2 and Fgf5 genes. Mamm Genome. 1992;2(2):135–137. doi: 10.1007/BF00353862. [DOI] [PubMed] [Google Scholar]
- [26].Shan Y, Lambrecht RW, Donohue SE, et al. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20(14):2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]


