Abstract
Aim:
To characterize the regression rate of posterior uveal melanoma following radioactive iodine-125 (I-125) plaque.
Materials and Methods:
We retrospectively analyzed 95 patients with posterior uveal melanoma who were treated with only radioactive I-125 plaque and had more than 3 years follow-up. All patients were treated with plaque radiotherapy using tumor dose of 85 Gy at the tumor apex, following COMS protocol. Regression rate was assessed with standardized A-scan ultrasonography. Associations with tumor regression were evaluated by means of mixed linear regression modeling.
Results:
Mean decrease in the tumor thickness (% original thickness) at 12, 24, and 36 months after radiotherapy for melanomas <3 mm in thickness was 29%, 38%, and 45%, for melanoma 3-8 mm in thickness was 32%, 44%, and 59%, and for melanoma more than 8 mm in thickness was 52%, 62%, and 68%, respectively. With a doubling of follow-up time (0.5-1 year, or 1-2 years of follow-up from treatment), tumors <3 mm in thickness at treatment showed a 0.5 mm decrease in tumor thickness, whereas melanomas 3-8 mm showed a 1 mm decrease, and melanomas >8 mm showed a 1.7 mm decrease. Uveal melanomas that developed systemic metastasis showed an additional 0.4 mm decrease with a doubling of follow-up time from treatment, compared with those that did not develop metastasis (P = 0.050).
Conclusions:
Posterior uveal melanomas with higher initial thickness show steeper and more reduction in tumor thickness following radioactive I-125 plaque. After the initial phases, the regression curve became similar for tumors with different thicknesses.
Keywords: Uveal Melanoma, Plaque Radiotherapy, Tumor Regression
INTRODUCTION
Tumor regression following radiotherapy depends not only on the radiosensitivity of tumor cells, but also the clearance rate of dead tumor cells, and dynamic balance between different tumor components such as fibroblasts, stromal cells and lymphocytes.1,2 Following radiotherapy, the correlation between tumor regression rate and tumor control or patient survival is a controversial topic.
Little has been reported, in literature, about the regression pattern of uveal melanoma following radioactive iodine-125 (I-125) plaque.3 In a review of 100 patients with posterior uveal melanoma treated with radioactive cobalt-60 plaque, Cruess et al.4 reported 20% decrease in tumor thickness by the end of the first 6 months of follow-up, 30% decrease by the end of the 1st year and 50% decrease by 54 months of follow-up. Later, in a larger series, Augsburger et al.5 reported that 45% of tumors showed <10% decrease in the tumor thickness at post-operative 3 months, between 10% and 25% decrease at post-operative 6 months and between 25% and 50% decrease at post-operative 12 months. Following proton beam radiotherapy, 63% of choroidal melanoma thicker than 8 mm showed more than 2 mm/year decrease in tumor thickness while 54% of tumors <5 mm decreased between 2 and 0.5 mm/year.3 There was a strong association between greater tumor thickness and rapid tumor regression. To the best of our knowledge, no publication has reported on the rate of regression and predictive factors for regression of uveal melanoma following radioactive I-125 plaque, most commonly used isotope in the United States and association with metastasis. In this study, we analyzed posterior uveal melanoma patients that were treated only with radioactive I-125 plaque and had minimum 3 years of follow-up with ultrasonography performed by the same ultrasonography technician. We characterized the extent and timing of tumor regression, predictive factors, and association with metastasis.
MATERIALS AND METHODS
A retrospective review was performed on the medical records of posterior uveal melanoma patients who were treated with radioactive I-125 plaque by one physician (Dr. Andrew K. Vine) at the W.K. Kellogg Eye Center, University of Michigan between January 1997 and June 2007. Patients with iris or ciliary body melanomas, or uveal melanomas with tumor apex anterior to the equator were excluded from the study to prevent any inconsistency in measurement with standardized A-scan ultrasonography. We analyzed 95 patients who had more than 3-years of follow-up. The institutional review board at the University of Michigan approved this study.
The patients’ age at the time of ocular diagnosis, gender and involved eye was noted. At the initial examination, the presence of subretinal fluid and orange pigmentation, tumor thickness and largest tumor diameter, location of tumor (juxtapapillary, macula, nasal, superior, inferior and temporal quadrants), growth pattern (diffuse, dome-shaped, mushroom-shaped) and retinal invasion were extracted from clinic notes, photographs, and ultrasound records. All patients were treated with plaque radiotherapy using tumor dose of 85 Gy at the tumor apex following COMS protocol. The plaque size was chosen with 2 mm margin beyond the base of the tumor on each side. Tumor regression was evaluated by measuring tumor thickness with the B-scan and standardized A-scan ultrasonography by the same ultrasonography technician every 4-6 months. Tumor thickness was measured as mean spikes height when the probe was perpendicular to the tumor's surface. Tumors are grouped into three categories based on tumor thickness; <3 mm, 3-8 mm and >8 mm similar to the categories used in COMS. Tumor thickness measurement by B-scan and standardized A-scan ultrasonography were performed at the same session and correlated with each other. We used tumor thickness rather than tumor volume to evaluate tumor regression. In evaluating the published methods of estimating choroidal melanoma volume, Singh et al.6 found that current choroidal volume calculation methods are inconsistent and might lead to incorrect volume estimations. For those who developed metastatic cancer during follow-up, the time of diagnosis of metastasis was recorded.
To provide a practical mathematical formula, associations of factors with tumor thickness over time were analyzed by mixed linear regression modeling (SAS 9.2, SAS Institute, Cary, NC). This model used a random subject intercept and slope. In order to deal with the non-linear trend of tumor thickness over time, a log2 transform of the time was applied. This transformation resulted in a linear relationship and interpretation is in terms of change in tumor thickness per doubling of follow-up time (example: 0.5-1 year, 1-2 years, 2-4 years, or 4-8 years of follow-up from treatment).
RESULTS
The demographic and clinical features of the patients were shown in Table 1. Regression rate following I-125 plaque radiotherapy was shown in Table 2. Following I-125 plaque radiotherapy, posterior uveal melanoma thickness regressed in the form of a downward sloping curve over time, with the initial regression being fast and then slowing thereafter. At any time point, tumors thicker than 8 mm showed the most decrease in tumor thickness, followed by tumors 3-8 mm and tumors <3 mm in tumor thickness. After a mean follow-up of 83 months (37-183 months), melanomas <3 mm showed a mean of 29% decrease in thickness (15-43%), melanomas 3-8 mm showed a mean of 37% decrease (0-93%) and melanomas >8 mm showed that a mean of 43% decrease (19-67%). With a doubling of follow-up time, uveal melanomas <3 mm in tumor thickness at treatment showed a 0.5 mm decrease in tumor thickness, whereas melanomas 3-8 mm showed a 1.0 mm decrease, and melanomas >8 mm showed a 1.7 mm decrease [Figure 1]. Over the follow-up time, the difference in decrease in tumor thickness between these three groups was found to be statistically significant (P < 0.0001). Multivariable analysis showed that none of the clinical features was predictive of tumor regression over time, but tumor location at presentation was significantly associated with tumor thickness. At any point in time, tumors located superiorly were on an average 1 mm thicker than those located in the nasal quadrant (P = 0.0032).
Table 1.
Demographic and clinical features of the patients with choroidal melanoma treated with iodine-125 plaque radiotherapy
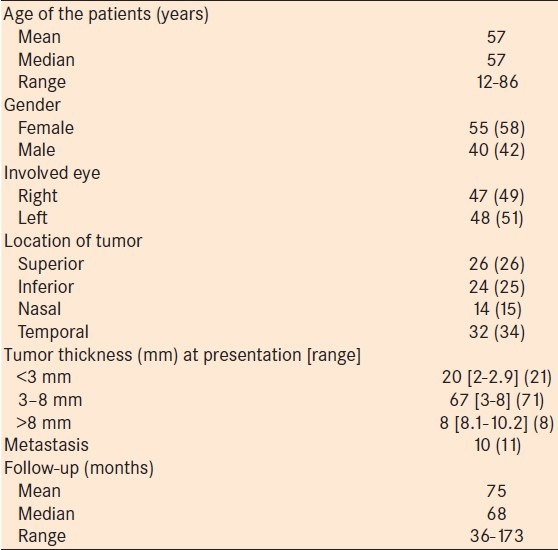
Table 2.
Regression rate of posterior uveal melanoma following plaque radiotherapy
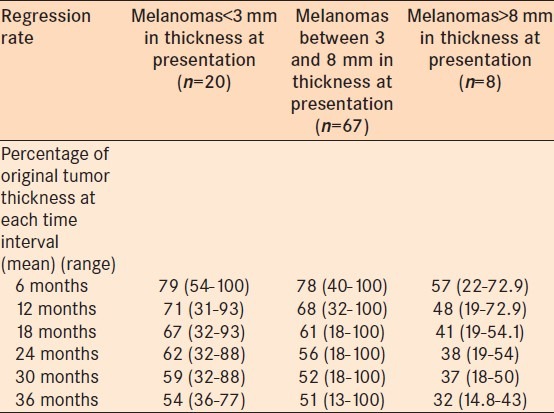
Figure 1.
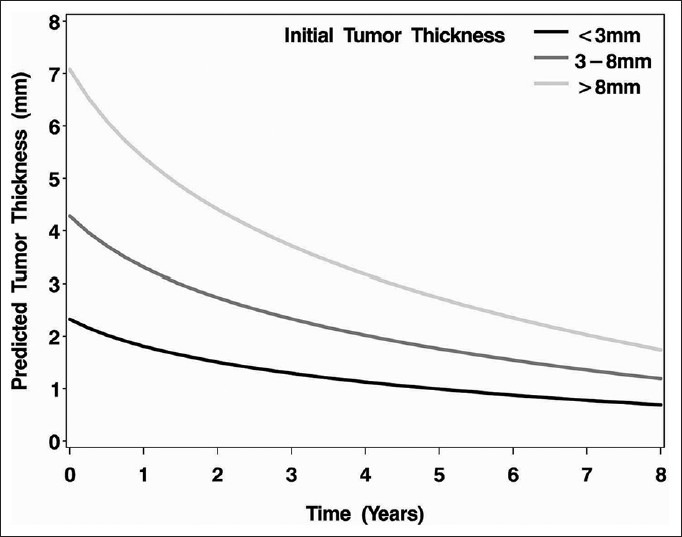
The predicted tumor thickness over time based on the initial tumor thickness (black line <3 mm, dark gray line 3-8 mm, light gray line >8 mm)
Regression rate of posterior melanomas that developed metastasis was shown in Table 3. When posterior uveal melanomas that developed systemic metastasis were evaluated, adjusting for uveal melanoma thickness at the time of treatment, they showed an additional 0.40 mm decrease with a doubling of time from treatment, compared to those that did not develop metastasis [Figure 2]. There was a significant difference in decrease in tumor thickness between melanomas that developed systemic metastasis and those that did not develop systemic metastasis (P = 0.05).
Table 3.
Comparison of regression rate between posterior uveal melanomas that developed metastasis and that did not develop metastasis
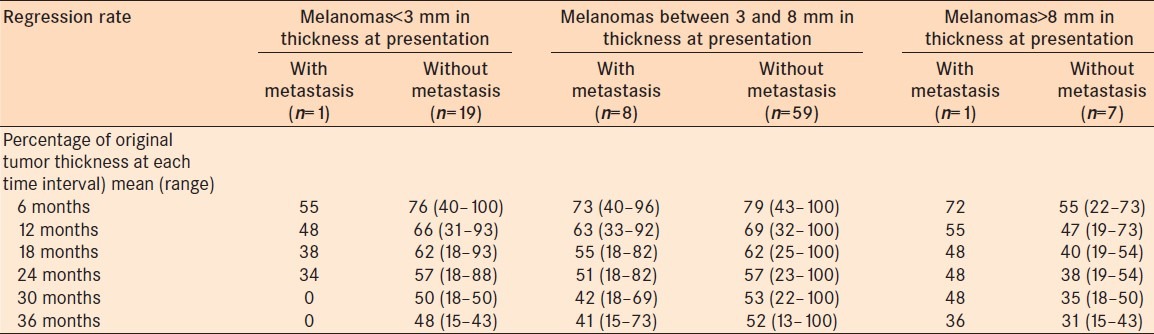
Figure 2.
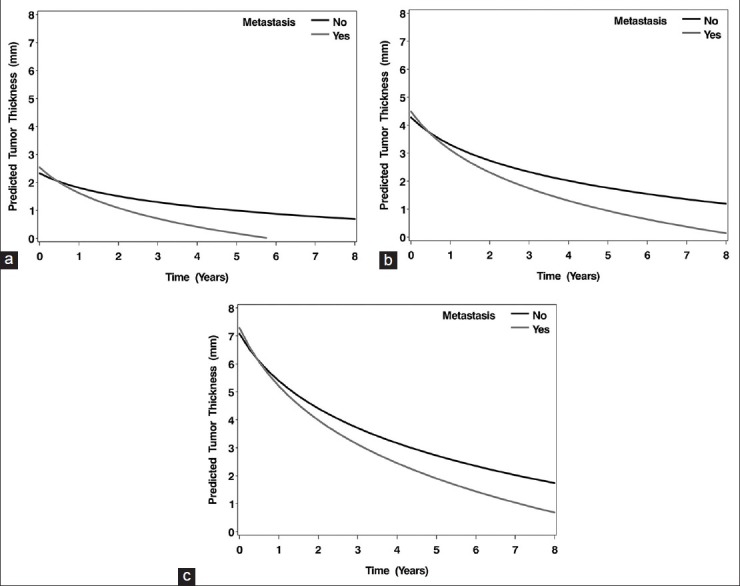
The predicted tumor thickness over time for uveal melanomas with tumor thickness that developed systemic metastasis (dark gray line) versus those that did not develop systemic metastasis (black line). Note, the predicted thickness is plotted for the average initial tumor thickness category, 3-8 mm. (a) initial tumor thickness <3 mm, (b) initial tumor thickness 3–8 mm, (c) initial tumor thickness >8 mm
DISCUSSION
Previous studies evaluating the regression patterns of uveal melanoma following cobalt-60 plaque radiotherapy, ruthenium-106 plaque radiotherapy and proton beam radiotherapy showed that tumors with higher initial thickness showed more reduction in thickness.3,4,5,7 Similarly, following I-125 plaque radiotherapy, we observed that the regression curve was steeper for tumors with greater tumor thickness, especially during the initial phases. After the initial phases, the regression curve became similar for tumors with different thicknesses. None of the clinical features was significantly associated with tumor regression rate in multivariate analysis. Recently, Shields et al.8 reported that uveal melanomas with chromosome 3 monosomy showed faster and greater tumor regression at 12 and 15 months following plaque radiotherapy and thermotherapy than melanomas with diosomy 3. Similar results were observed by Marathe et al.9 On the other hand, Chappell et al.10 found that there was no difference in the mean change in tumor thickness 24 months after proton beam radiotherapy between class 1 and 2 uveal melanomas. The relation between tumor regression and gene expression profiling needs to be evaluated in the patients with uveal melanoma.
The prognostic significance of uveal melanoma regression following plaque radiotherapy was evaluated for different isotopes including cobalt-60 and ruthenium-106. In a review of 100 posterior uveal melanoma patients treated with cobalt-60 plaque radiotherapy, Cruess et al.4 reported that posterior uveal melanoma regressed slowly and persisted as 50% of the thickness of the original tumor at 54 months follow-up. They did not find any difference in the rate and extent of tumor regression between the patients who developed metastasis and those who remained well systemically. Later, the same authors revisited their experience with 159 choroidal melanoma patients who were treated with radioactive cobalt-60 plaque and reported that the percentage of change in tumor thickness at 12 months was a borderline risk factor for death from metastatic melanoma.5 In a review of 147 eyes with choroidal melanoma treated with radioactive ruthenium-106 plaque, Kaiserman et al.7 found that the initial tumor thickness regression rate was significantly higher in patients who developed metastasis (6% per month) than the patients who did not (4% per month). They reported that tumors larger than 6 mm, tumors with an internal reflectivity <50%, and tumors with an initial rate of tumor height regression larger than 0.7 mm/month had a higher 5-year melanoma-related mortality. Kaplan-Meier survival analysis and the multivariable cox proportional hazards model showed tumor height and initial tumor regression rate were significant parameters for the development of metastasis. Similarly, Glynn et al.3 evaluated the change in tumor thickness following proton beam radiotherapy in 700 uveal melanoma patients and found that tumors regressing rapidly were significantly more likely to develop metastasis within 2 years of treatment, while tumors with slow regression were more likely to develop metastasis after 2 years of treatment. By using cox proportional hazards model, larger tumor diameter, older age at treatment, and ciliary body involvement was all associated with risk of metastasis during the first 2 years of treatment. Although our number of cases that developed metastasis is small, we observed that posterior uveal melanomas that develop metastasis showed more decrease in thickness than the ones that did not develop metastasis and our analysis showed that a decrease in thickness with a doubling of follow-up time after treatment that was 0.40 mm greater than those that did not develop metastasis. We observed that the highest number of uveal melanoma patients who developed systemic metastasis was in the tumor thickness 3-8 mm group [Table 3]. It is difficult to speculate any explanation for this observation, however, relatively higher number of patients in this group or presence of poor prognostic genetic or histopathologic features might play a role.
The effect of radiation on the tumor cells is related to the intrinsic radio sensitivity of cells as well as growth rate. Proliferating cells are more sensitive to ionizing radiation than non-proliferating cells.11 This is thought to be due to the fact that chromatin in proliferating cells is more susceptible to radiation-induced DNA strand-breakage than the dispersed chromatin of non-proliferating cells. However, regression pattern after radiotherapy is complex because of the multiple factors such as radio sensitivity of tumor cells, kinetics of death of the tumor cells, capacity of the tumor bed to remove the death tumor cells, tumor stroma and the host reaction against the residual tumor.12 Hence, tumor regression pattern and association with metastasis might vary according to the type of tumor and high decrease in tumor thickness might not necessarily show higher metastatic rate.
In summary, we found that tumor regression following radioactive I-125 plaque follows a downward sloping curve. The curve is steeper for thicker tumors and flatter for less thick tumors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Fang FM, Tsai WL, Go SF, Ho MW, Wu JM, Wang CJ, et al. Implications of quantitative tumor and nodal regression rates for nasopharyngeal carcinomas after 45 Gy of radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50:961–9. doi: 10.1016/s0360-3016(01)01531-0. [DOI] [PubMed] [Google Scholar]
- 2.Bartelink H. Prognostic value of the regression rate of neck node metastases during radiotherapy. Int J Radiat Oncol Biol Phys. 1983;9:993–6. doi: 10.1016/0360-3016(83)90386-3. [DOI] [PubMed] [Google Scholar]
- 3.Glynn RJ, Seddon JM, Gragoudas ES, Egan KM, Hart LJ. Evaluation of tumor regression and other prognostic factors for early and late metastasis after proton irradiation of uveal melanoma. Ophthalmology. 1989;96:1566–73. doi: 10.1016/s0161-6420(89)32685-6. [DOI] [PubMed] [Google Scholar]
- 4.Cruess AF, Augsburger JJ, Shields JA, Brady LW, Markoe AM, Day JL. Regression of posterior uveal melanomas following cobalt-60 plaque radiotherapy. Ophthalmology. 1984;91:1716–9. doi: 10.1016/s0161-6420(84)34087-8. [DOI] [PubMed] [Google Scholar]
- 5.Augsburger JJ, Gamel JW, Shields JA, Markoe AM, Brady LW. Post-irradiation regression of choroidal melanomas as a risk factor for death from metastatic disease. Ophthalmology. 1987;94:1173–7. doi: 10.1016/s0161-6420(87)33310-x. [DOI] [PubMed] [Google Scholar]
- 6.Singh AD, Terman S, Sculley L. Estimating choroidal melanoma volume: Comparison of methods. Ophthalmology. 2007;114:1212–4. doi: 10.1016/j.ophtha.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 7.Kaiserman I, Anteby I, Chowers I, Blumenthal EZ, Kliers I, Pe’er J. Post-brachytherapy initial tumour regression rate correlates with metastatic spread in posterior uveal melanoma. Br J Ophthalmol. 2004;88:892–5. doi: 10.1136/bjo.2003.036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shields CL, Bianciotto C, Rudich D, Materin MA, Ganguly A, Shields JA. Regression of uveal melanoma after plaque radiotherapy and thermotherapy based on chromosome 3 status. Retina. 2008;28:1289–95. doi: 10.1097/IAE.0b013e31817f7b3e. [DOI] [PubMed] [Google Scholar]
- 9.Marathe OS, Wu J, Lee SP, Yu F, Burgess BL, Leu M, et al. Ocular response of choroidal melanoma with monosomy 3 versus disomy 3 after iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2011;81:1046–8. doi: 10.1016/j.ijrobp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Chappell MC, Char DH, Cole TB, Harbour JW, Mishra K, Weinberg VK, et al. Uveal melanoma: Molecular pattern, clinical features, and radiation response. Am J Ophthalmol. 2012;154:227–32.e2. doi: 10.1016/j.ajo.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biade S, Stobbe CC, Chapman JD. The intrinsic radiosensitivity of some human tumor cells throughout their cell cycles. Radiat Res. 1997;147:416–21. [PubMed] [Google Scholar]
- 12.Suit HD, Walker AM. Assessment of the response of tumours to radiation: Clinical and experimental studies. Br J Cancer Suppl. 1980;4:1–10. [PMC free article] [PubMed] [Google Scholar]


