Abstract
Glaucoma is a potentially blinding disease that affects millions of people worldwide. The mainstay of treatment is lowering of intraocular pressure (IOP) through the use of medications, laser and/or incisional surgery. The trabecular meshwork (TM) is thought to be the site of significant resistance to aqueous outflow in open angle glaucoma. Theoretically, an incision through TM or TM removal should decrease this resistance and lead to a significant reduction in IOP. This approach, commonly referred to as goniotomy or trabeculotomy, has been validated in the pediatric population and has been associated with long-term IOP control. In adults, however, removal of TM tissue has been historically associated with more limited and short-lived success. More recent evidence, reveals that even adult patients may benefit significantly from removal of diseased TM tissue and can lead to a significant reduction in IOP that is long-lasting and safe. In this review, we discuss current evidence and techniques for ab interno trabeculectomy using various devices in the adult patient.
Keywords: Ab Interno Trabeculectomy, Glaucoma Surgery, Goniotomy, Trabecular Meshwork, Schlemm's Canal
INTRODUCTION
Current treatment of glaucoma focuses on the lowering of intraocular pressure (IOP) either pharmacologically or by laser or incisional surgery. The trabecular meshwork (TM), particularly the juxtacanalicular TM adjacent to Schlemm's canal, along with more distal outflow structures are thought to be the main sites of resistance to aqueous outflow.1,2,3 In theory, incising or removing TM should lower this resistance, leading to improved IOP control. Trabeculotomy and goniotomy are traditionally used in the treatment of congenital glaucoma and have a reasonable rate of success in this population.4,5,6 In adult patients, however, angle-based surgeries have a much lower success rate.7,8 The reasons for this are unclear but may be related to changes in TM composition with age or scarring either at the surgical site or a more distal location.9 Rather than a simple incision through TM, a more complete tissue removal or ablation could allow the surgically created cleft to remain open, leading to more sustained IOP control. In this review, we present current and future surgical options for ab interno trabeculectomy to treat adult open-angle glaucoma (OAG).
TRABECTOME™
The Trabectome™ (NeoMedix, Tustin, CA, USA) has been in use in the United States since its Food and Drug Administration approval in 2004 for the treatment of adult and juvenile OAG. The Trabectome consists of a disposable hand piece connected to a console that provides irrigation, aspiration, and electrocautery. As with modern phacoemulsification machines, a foot pedal controls these actions. The Trabectome was designed with the goal of permanently ablating and removing a strip of TM and the inner wall of Schlemm's canal.10 Theoretically, successful tissue removal with ablation of the cut edges helps prevent closure of the surgical cleft and postoperative fibrosis.
The handpiece is disposable with a 19.5 gauge tip designed to fit through a 1.6 mm or larger corneal incision. The tip of the handpiece has a footplate that is insulated with a proprietary multilayered polymer, designed to prevent thermal and electrical damage to surrounding tissues.10 The distal tip of the device is pointed to allow for insertion into Schlemm's canal, allowing the footplate to bring the tissues into contact with the bipolar electrodes. An aspiration port near the tip removes ablated tissue and debris, while the irrigation maintains pressure inside the eye and dissipates heat generated during cauterization.
The handpiece is connected to a console that contains the irrigation and aspiration unit as well as a high-frequency electrocautery generator. The electrocautery output power can be increased in 0.1 W increments up to a maximum of 10 W, although the recommended range for treatment is generally 0.5-1.5 W. This energy is applied in short bursts in order to cause tissue disruption and disintegration while minimizing buildup of thermal energy.
Surgical technique
Ab interno trabeculectomy using the Trabectome is performed under direct gonioscopic view. It is therefore suggested that the surgeon gain experience with this view prior to attempting the procedure, as careful technique and appropriate patient positioning are necessary to optimize the view of the anterior chamber angle. A temporal, clear corneal incision is made in the standard fashion, and the patient's head is rotated away from the surgeon. Intracameral anesthesia is usually sufficient, but other modes of anesthesia may be used, including retrobulbar, peribulbar, and sub-Tenon's injections. Cohesive viscoelastic is injected to maintain the anterior chamber and deepen the angle, and a direct gonioscopy lens is used to bring the nasal anterior chamber angle into view. The same viscoelastic may be placed on the corneal surface as a coupling agent for the gonioscopy lens. With the infusion on, the tip of the handpiece is visualized as it advances across the anterior chamber towards TM. The tip of the handpiece is inserted through TM into Schlemm's canal [Figure 1]. Aspiration and electrocautery are activated with the foot pedal as the surgeon slowly advances the device in either a clockwise or counterclockwise fashion, following the TM. Once the maximum treatment has been achieved in one direction, the handpiece is rotated 180° and treatment is performed in the opposite direction. It is not uncommon to see blood reflux from Schlemm's canal intraoperatively. The handpiece is then removed from the eye, and thorough removal of viscoelastic followed by wound closure should proceed according to surgeon preference.
Figure 1.
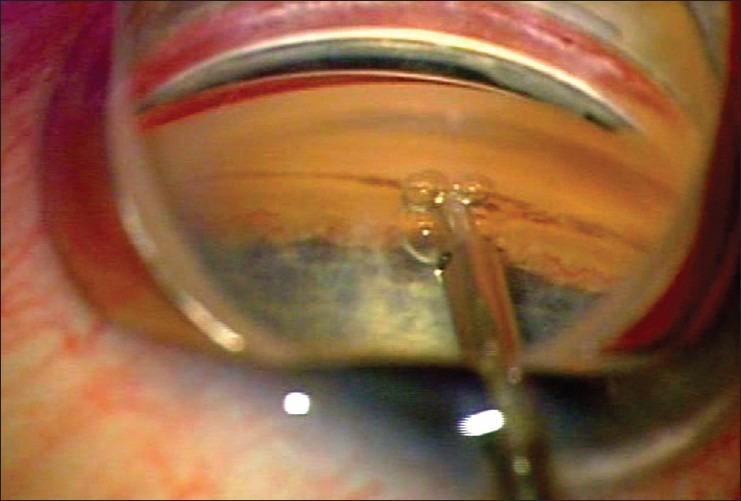
Intraoperative photo of the Trabectome being used for ab interno trabeculectomy. Note the direct gonioscopic view of the device inserted through trabecular meshwork into Schlemm's canal
Ab interno trabeculectomy using the Trabectome is approved as a standalone procedure or in conjunction with phacoemulsification cataract extraction (phaco). When combined with phaco, the Trabectome portion of the procedure can be performed either before or after removal of the cataract.
Clinical outcomes
The initial clinical report of ab interno trabeculectomy with the Trabectome followed 37 patients with uncontrolled OAG.11 Follow-up was variable and ranged from 3 to 13 months. Twenty-five patients had at least 6 months of follow-up, and mean IOP was reduced in this cohort by 38% from baseline. The use of IOP-lowering medications also decreased from an average of 1.2 ± 0.6 prior to surgery to 0.4 ± 0.6 at 6 months postoperatively. Blood reflux was noted in all patients at the time of surgery and all patients had a transient hyphema postoperatively, but this cleared at an average of 6.4 days after surgery. The same group reported longer-term results (up to 30 months postoperatively) of 101 patients treated with the Trabectome.12 Success, defined as IOP lower than 21 mmHg with or without medication and without additional glaucoma surgery, was reported to be 84%. Less than one percent of patients experienced early hypotony or a decrease in visual acuity of 2 or more Snellen lines.
A report of 679 consecutive patients who underwent treatment with the Trabectome showed similar results.13 This prospective study included patients with primary OAG (POAG) as well as pseudoexfoliation and pigmentary glaucomas. Seventy percent of these cases were Trabectome only, and the remaining 30% were combined Trabectome-phaco. The average decrease in IOP was 29% at 6 months postoperatively (n = 106), and this decrease was stable at 30% for those patients completing 24 months of follow-up (n = 30). The authors also reported a reduction in the use of adjunctive glaucoma therapy by an average of two medications.
Francis et al. reported results from 304 patients after combined Trabectome-phaco.14 The main outcome measures studied were IOP, glaucoma medication use, and surgical complications. Mean IOP was decreased from an average of 20.0 ± 6.3 mmHg preoperatively to 14.8 ± 3.5 mmHg at 6 months and 15.5 ± 2.9 mmHg at 1-year. Glaucoma medication use also decreased from a mean of 2.65 ± 1.13 prior to surgery to 1.76 ± 1.25 at 6 months and 1.44 ± 1.29 at 1-year. Complications were generally minor and transient. The most common complication was blood reflux, noted in 78.4% of patients; the authors noted that this resolved quickly in all cases. There were no cases of sustained hypotony and no patient lost 2 or more lines of Snellen visual acuity.
The largest reported series of Trabectome surgeries was a retrospective study by Minckler et al. published in 2008.15 The authors published results from 1127 cases, of which 738 (65%) were Trabectome alone and 366 (32%) were Trabectome combined with cataract surgery. The remaining Trabectome procedures were coupled with other procedures, such as penetrating keratoplasty (n = 1), endoscopic cyclophotocoagulation, (n = 1), or aqueous shunt implantation (n = 7). Trabectome-only cases had a decrease in IOP from an average of 25.7 ± 7.7 mmHg preoperatively to 16.6 ± 4.0 mmHg at 24 months (n = 46), a decrease of 40%. The cases of Trabectome combined with phacoemulsification showed a decrease in IOP from a preoperative mean of 20.0 ± 6.2 mmHg to a mean of 15.9 ± 3.3 mmHg at 12 months (n = 45), a decrease of 18%. These longer-term results should be interpreted with caution given the limited number of patients for whom 12- and 24-month data was available. Reflux bleeding was noted intraoperatively in 77.6% of patients. A small number of patients (n = 17) had hypotony (IOP < 5 mmHg) at the postoperative day 1 visit, but no patients were hypotonous by 1-month after surgery. There were no cases of choroidal effusions or hemorrhage.
A retrospective cohort study has compared ab interno trabeculectomy with the Trabectome to trabeculectomy with mitomycin-C (MMC) for the treatment of OAG.8 A total of 115 patients underwent Trabectome alone compared to 102 patients who underwent trabeculectomy with MMC. IOP and the number of adjunctive glaucoma medications decreased in both groups. In the Trabectome group, IOP decreased from a mean of 28.1 ± 8.6 mmHg at baseline to 15.9 ± 4.5 mmHg at 24 months postoperatively, a decrease of 43.5%. In the trabeculectomy group, IOP decreased from a baseline of 26.3 ± 10.9 mmHg to 10.2 ± 4.3 mmHg at 24 months, a reduction of 61.3%. At 24 months postoperatively, glaucoma medication use had decreased from a baseline of 3.3 ± 1.3 to 2.2 ± 1.6 in the Trabectome group and 3.4 ± 1.0 to 0.5 ± 1.0 in the trabeculectomy group. The trabeculectomy group had significantly lower IOP and less glaucoma medication use at all postoperative visits. Excluding hyphema, postoperative complications were more frequent in the trabeculectomy cohort. Postoperative hyphema was noted in 100% of the Trabectome group versus 2.9% in the trabeculectomy group. Early and persistent hypotony, wound leak, choroidal effusions, and cystoid macular edema all occurred in the trabeculectomy group but not in the Trabectome group. The authors concluded that both procedures are effective for lowering IOP. Patients who underwent trabeculectomy with MMC had a higher rate of success, albeit at the expense of a higher rate of postoperative complications.
Given that the mechanism of elevated IOP in exfoliation glaucoma is related to the accumulation of extracellular material in the anterior chamber angle and TM, incision and removal of TM is a logical treatment course. A report by Ting et al. compared outcomes after Trabectome-alone or Trabectome-phaco in patients with POAG versus exfoliation glaucoma.16 At 1-year postoperatively, IOP was significantly lower in all treatment groups. The reduction in IOP and success rate was greater in patients with exfoliation glaucoma, suggesting a role for ab interno trabeculectomy with the Trabectome in this patient population.
Jea et al. also published a retrospective study comparing the effect of a prior Trabectome on the success of a subsequent trabeculectomy.17 Thirty-four patients had undergone trabeculectomy after a prior Trabectome, compared to 42 patients who underwent trabeculectomy as a primary surgical treatment for glaucoma. They found no difference in IOP and success rates and concluded that a failed Trabectome does not compromise the outcome of future trabeculectomy.
Some have reported less promising long-term results with the Trabectome. An analysis of Trabectome procedures performed on 246 patients (both with and without concurrent phaco) by a single surgeon showed a reduction in mean IOP from 21.6 ± 8.6 mmHg preoperatively to 15.3 ± 4.6 mmHg at 24 months postoperatively, with a concurrent decrease in glaucoma medication use from a mean of 3.1 ± 1.1 to 1.9 ± 1.3.18 However, 66 patients (26.8%) were recommended to have additional glaucoma surgery at a mean of 10 months after initial ab interno trabeculectomy. Using success criteria of IOP ≤ 18 mmHg and a decrease of 20% or more from baseline, the success rate at 24 months was only 22%. The authors concluded that while the procedure is effective in lowering IOP and decreasing glaucoma medication use with an excellent safety profile, it is likely inappropriate for patients with a low target IOP.
The available evidence suggests that ab interno trabeculectomy using the Trabectome lowers IOP in a reliable fashion with few long-term adverse effects. It can be combined with cataract surgery or performed alone through a clear corneal incision, with the advantage of avoiding conjunctival dissection, allowing for future full-thickness glaucoma surgery if indicated. Because the IOP after Trabectome ultimately depends on physiologic aqueous outflow mechanisms downstream from the surgical site, it cannot be expected to lower IOP below that of episcleral venous pressure. Distal scarring and malfunction of outflow pathways may also limit the short- or long-term success of this procedure, and as such it may not be appropriate for patients with a low target IOP.
GONIOCURETTAGE
Other techniques to remove TM in adults have been reported. Jacobi et al. showed histologic and early clinical results regarding the use of an instrument they termed the “gonioscraper.”19 The device is shaped like a cyclodialysis spatula with a small bowl in the end with sharpened edges. Using intraoperative gonioscopy, goniocurettage is performed by using the gonioscraper to remove TM nasally. Histologic analysis of human eye bank eyes after treatment showed removal of TM as well as damage to intracanalicular septa and the posterior wall of Schlemm's canal. Early clinical results from four patients treated with the device showed a decrease in mean baseline IOP from 40.7 mmHg (range: 32–51 mmHg) to an average of 18.0 mmHg (range: 12–22 mmHg) at 6 months without significant complications.19
A prospective, nonrandomized trial of 25 eyes treated with goniocurettage followed this earlier report.20 Mean preoperative IOP was 34.7 mmHg (range: 29–48 mmHg) on an average of 2.2 glaucoma medications. Success, defined as an IOP of 19 mmHg or less on either one or zero glaucoma medications, was achieved in 15 eyes (60%) at last follow-up (mean 32.6 months, range: 30–45 months). Localized Descemet membrane detachments were noted in five eyes (20%) and anterior chamber bleeding in four eyes (16%).
GONIOSCOPY-ASSISTED TRANSLUMINAL TRABECULOTOMY
Grover et al. recently described a new technique for ab interno trabeculotomy.21 Termed gonioscopy-assisted transluminal trabeculotomy (GATT), the procedure seeks to create a 360° trabeculotomy from an ab interno approach. Briefly, a temporal corneal wound is made, and direct gonioscopy is used to visualize the nasal angle structures. A goniotomy is created in the nasal angle, and microsurgical forceps are then used to introduce a suture or illuminated microcatheter (iScience Interventional Corp, Menlo Park, CA, USA) into Schlemm's canal. The forceps are then used to advance the microcatheter or suture circumferentially until the distal tip is identified at the original goniotomy site and retrieved. Traction on the suture or catheter is then used to create a 360° trabeculotomy. The initial report of this procedure followed 85 patients for a minimum of 6 months postoperatively.21 In patients with POAG who underwent GATT, IOP decreased by an average of 11.1 mmHg from baseline at 12 months, with a corresponding average decrease of 1.1 glaucoma medications. The most common complication was a transient hyphema, which was noted in 30% of patients 1-week postoperatively. Although data regarding this procedure are limited, it is a promising approach that is minimally invasive and spares conjunctiva. It should be noted that GATT only achieves a trabeculotomy in contrast to other procedures in this review where TM tissue is both incised and removed. Additional studies are necessary to define a role for this procedure in the treatment of glaucoma.
A NOVEL DUAL-BLADE DEVICE
One disadvantage to the Trabectome system is a requirement for the electrocautery unit as well as the disposable handpieces. The cost associated with these supplies may limit the utility of this approach in resource-poor treatment areas. The Trabectome has also been shown to cause thermal damage to nearby tissues.22 As mentioned above, use of the manual gonioscraper caused injury to adjacent tissues as well, including splitting of the posterior wall of Schlemm's canal in one specimen.19 There remains a need for a device that can remove TM without collateral damage and without retaining significant TM leaflets, which can then occlude the outflow channels.
Recently, our group investigated the utility of a novel dual blade device (Kahook Dual Blade, New World Medical, Rancho Cucamonga, CA) for removal of TM while minimizing collateral damage [Figure 2].22 The dual blade device is designed with a taper at the tip to allow for smooth entry of the blade into Schlemm's canal. Once properly seated in the canal, the device is advanced along the TM. The ramp at the distal end of the instrument elevates TM tissue and guides it toward the blades on either side of the device, which then cleanly incise the tissue to allow for easy removal. By elevating the TM and placing it on stretch prior to cutting, the design allows for cleaner removal of tissue and minimizes damage to adjacent structures. The angle of the distal cutting surface and the size of the device shaft are engineered to allow for maximum clock hour treatment through a single clear corneal incision.
Figure 2.

Schematic of the dual blade device for ab interno trabeculectomy
In a preclinical study of human donor corneoscleral rims, the dual blade device was used to incise TM and then compared to similar treatment with a microvitreoretinal (MVR) blade and the Trabectome.22 Specimens were then fixed, sectioned, and examined histologically. Use of the MVR blade resulted in a full thickness incision through TM, but there was minimal tissue removal with large residual leaflets of TM on either side of the incision. In addition, there was damage to the adjacent sclera. The Trabectome produced a similar opening in TM, but again showed residual TM tissue as well as thermal damage to the residual TM leaflets. Analysis of specimens treated with the dual-blade showed more complete TM tissue removal and no significant damage to adjacent tissues.
Human whole eye perfusion studies were also undertaken to evaluate the IOP-lowering effect of this novel device. Use of the dual-blade, MVR blade, and Trabectome all resulted in a statistically significant reduction in IOP in this model. The dual-blade and Trabectome had a greater percentage decrease in IOP compared with the MVR blade, although this did not reach statistical significance. The number of degrees treated did not correlate with IOP lowering for any of the three devices. Although these results are preliminary, the dual-blade shows promise as an elegant and economical device for the treatment of glaucoma.
CONCLUSION
Although trabeculectomy remains the gold standard surgery for the treatment of glaucoma, new approaches for safer and more reproducible IOP lowering are constantly being explored. The development of minimally or micro-invasive glaucoma surgery (MIGS) devices and instrumentation aims to cultivate techniques to lower IOP surgically with improved rates of success and a greater margin of safety compared to traditional full thickness filtration surgeries. Many new implants, such as the iStent (Glaukos, Laguna Hills, CA, USA), seek to bypass outflow resistance at the level of TM and Schlemm's canal. Like most MIGS procedures, ab interno trabeculectomy can be performed through a clear corneal incision and combined with phacoemulsification if desired. Since the conjunctiva is not disrupted, bleb-based filtration surgery is still an option in the future. Success may be limited, however, by scarring and fibrosis either at the surgical site or further downstream the outflow pathway. Development of devices and techniques that lead to more complete removal of TM with minimal damage to nearby tissues has the potential to address some of these limitations. In the future, noninvasive techniques for in vivo imaging of aqueous outflow pathways may also allow for targeted TM removal to maximize postoperative aqueous drainage. Future studies will hopefully elucidate the most effective technique for ab interno trabeculectomy in the adult patient and help to better guide surgeons in the selection of the appropriate surgical intervention for each individual patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tamm ER. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp Eye Res. 2009;88:648–55. doi: 10.1016/j.exer.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Grant WM. Clinical measurements of aqueous outflow. AMA Arch Ophthalmol. 1951;46:113–31. doi: 10.1001/archopht.1951.01700020119001. [DOI] [PubMed] [Google Scholar]
- 3.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 4.deLuise VP, Anderson DR. Primary infantile glaucoma (congenital glaucoma) Surv Ophthalmol. 1983;28:1–19. doi: 10.1016/0039-6257(83)90174-1. [DOI] [PubMed] [Google Scholar]
- 5.Anderson DR. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmology. 1983;90:805–6. doi: 10.1016/s0161-6420(83)34484-5. [DOI] [PubMed] [Google Scholar]
- 6.Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J AAPOS. 2000;4:205–10. doi: 10.1067/mpa.2000.106201. [DOI] [PubMed] [Google Scholar]
- 7.Luntz MH, Livingston DG. Trabeculotomy ab externo and trabeculectomy in congenital and adult-onset glaucoma. Am J Ophthalmol. 1977;83:174–9. doi: 10.1016/0002-9394(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 8.Jea SY, Francis BA, Vakili G, Filippopoulos T, Rhee DJ. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology. 2012;119:36–42. doi: 10.1016/j.ophtha.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Horstmann HJ, Rohen JW, Sames K. Age-related changes in the composition of proteins in the trabecular meshwork of the human eye. Mech Ageing Dev. 1983;21:121–36. doi: 10.1016/0047-6374(83)90069-6. [DOI] [PubMed] [Google Scholar]
- 10.Francis BA, See RF, Rao NA, Minckler DS, Baerveldt G. Ab interno trabeculectomy: Development of a novel device (Trabectome) and surgery for open-angle glaucoma. J Glaucoma. 2006;15:68–73. doi: 10.1097/01.ijg.0000196653.77836.af. [DOI] [PubMed] [Google Scholar]
- 11.Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112:962–7. doi: 10.1016/j.ophtha.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Minckler D, Baerveldt G, Ramirez MA, Mosaed S, Wilson R, Shaarawy T, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc. 2006;104:40–50. [PMC free article] [PubMed] [Google Scholar]
- 13.Filippopoulos T, Rhee DJ. Novel surgical procedures in glaucoma: Advances in penetrating glaucoma surgery. Curr Opin Ophthalmol. 2008;19:149–54. doi: 10.1097/ICU.0b013e3282f4f49e. [DOI] [PubMed] [Google Scholar]
- 14.Francis BA, Minckler D, Dustin L, Kawji S, Yeh J, Sit A, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: Initial results. J Cataract Refract Surg. 2008;34:1096–103. doi: 10.1016/j.jcrs.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Minckler D, Mosaed S, Dustin L, Ms BF. Trabectome Study Group. Trabectome (trabeculectomy-internal approach): Additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:149–59. [PMC free article] [PubMed] [Google Scholar]
- 16.Ting JL, Damji KF, Stiles MC. Trabectome Study Group. Ab interno trabeculectomy: Outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38:315–23. doi: 10.1016/j.jcrs.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Jea SY, Mosaed S, Vold SD, Rhee DJ. Effect of a failed trabectome on subsequent trabeculectomy. J Glaucoma. 2012;21:71–5. doi: 10.1097/IJG.0b013e31820bcfda. [DOI] [PubMed] [Google Scholar]
- 18.Ahuja Y, Ma Khin Pyi S, Malihi M, Hodge DO, Sit AJ. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: The Mayo Clinic series in Rochester, Minnesota. Am J Ophthalmol. 2013;156:927–935.e2. doi: 10.1016/j.ajo.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Jacobi PC, Dietlein TS, Krieglstein GK. Technique of goniocurettage: A potential treatment for advanced chronic open angle glaucoma. Br J Ophthalmol. 1997;81:302–7. doi: 10.1136/bjo.81.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobi PC, Dietlein TS, Krieglstein GK. Goniocurettage for removing trabecular meshwork: Clinical results of a new surgical technique in advanced chronic open-angle glaucoma. Am J Ophthalmol. 1999;127:505–10. doi: 10.1016/s0002-9394(98)00448-6. [DOI] [PubMed] [Google Scholar]
- 21.Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology. 2014;121:855–61. doi: 10.1016/j.ophtha.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155:524–529.e2. doi: 10.1016/j.ajo.2012.09.023. [DOI] [PubMed] [Google Scholar]


