Abstract
The trabecular bypass stent offers an alternative to filtration surgery. Patients who may be ideal candidates for considering this procedure are those with prior conjunctival surgery; for example, those who had a 360° peritomy from a scleral buckle might not do well with a trabeculectomy and there is no space for a tube. Highly myopic patients do not tolerate hypotony well, and the iSTB may be an option for some of these patients. I have used the iSTB in patients on anticoagulants who could not stop them, and they needed something beyond medications and laser to lower the IOP in subjects with open-angle glaucoma. Young patients, especially those with one eye, who need rapid visual recovery (for instance to return to work) may be good candidates to consider the iSTB as well. Because of the position used for clear corneal cataract surgery, the temporal approach is best for doing these. Therefore, if you are doing cataract surgery on someone who needs a lower IOP, you already are in the correct position to implant the devices. Patients may need some medications after the procedure to lower the IOP to the level desired. The results from Armenia are encouraging, given an IOP of 11.8 mmHg after 2 iSTB stents and taking daily travoprost. These results are difficult to reach even with a trabeculectomy. When selecting your fist patients, avoid those with the congested episcleral veins, look for patients with wide open angles, and if you can see aqueous veins at the slit-lamp it may indicate a viable outflow system. Probably avoid patients with IOPs over 35 mmHg. The micro-invasive trabecular bypass stents offer an alternative surgical intervention for select patients with open-angle glaucoma. Recent studies show that combining these micro-stents with medications can lead to as low of an intraocular pressure (IOP) as is achieved by many more invasive incisional surgeries. The technique is quite precise and learning the procedure is similar to clear corneal phacoemulsification followed by a goniotomy. Long-term data are starting to come in and the safety is favorable. The IOP success appears to be based on the patency of the outflow system for a given patient. Key factors in determining the success involve the placement of trabecular bypass devices into the canal of Schlemm and require a down-stream patency of the collector channel system and a low episcleral venous pressure. Because accessing the collector system may require placement by a patent channel, the placement of two stents, a longer stent with scaffolding or somehow imaging the outflow system may lead to the best control of the IOP.
Keywords: Glaucoma Surgery, Hydrus, iStent, Micro-Invasive Glaucoma Surgery, Trabecular Bypass Stent
INTRODUCTION
Glaucoma has been a difficult disease to treat. Most patients do not realize they have a serious problem. When providers prescribe medications or recommend surgery that could harm the patient, the patient feels caught in the middle between saving vision and potentially being harmed from a problem they do not “see” is causing a problem.1 Medications have improved over the years. Due to fewer side effects of the newer agents used to lower intraocular pressure (IOP) and we have once a day agents, patients are more likely to adhere to treatment recommendations.2 Laser treatment has progressed to the point that patients are fairly comfortable considering laser as a treatment option.3 Recommending glaucoma surgery, on the other hand, is more likely to cause concern for the patient and provider because of the legacy of glaucoma surgery causing issues for the patient and provider. Patients and doctors see the list of problems caused by surgery and look for something better.4 Recently, there has been a movement toward smaller device-based procedures with less risks.5 The new device-based procedures have been called various things and one more commonly used term is the micro-invasive glaucoma surgery (MIGS).6 The trade-off moving toward the MIGS procedures, however, is a concern about efficacy and not being able to lower the IOP enough with the surgery alone, so medications may need to be added to these device-based procedures to get the level of IOP desired.7 MIGS devices target, bypassing the angle structures. One group of MIGS devices bypass the trabecular meshwork (TM) and are called the trabecular bypass stents.
The trabecular bypass stent was designed to get away from the problems we see with our current filtration surgery. Trabeculectomy, the so-called gold standard in glaucoma surgery and other glaucoma filtering procedures bypass the sclera to create a new drainage site via a fistula with scleral and conjunctival coverage.8,9,10 The resulting fistula creates a filtering bleb in the conjunctiva. Blebs have been the bane of the glaucoma specialists' existence for the last three decades.11 This was especially noticeable after the use of antimetabolites became in vogue.12,13 Filtering procedures were mainly used for late stage glaucoma treatment and postoperatively lead to a high amount of complications. Due to the long-term healing issues antimetabolites have become routinely used for many glaucoma cases and have become a double-edged sword.
To avoid blebs, we tried the procedures usually reserved for children the trabeculotomy and goniotomy with varied success in adults; there are some indications that a 360° trabeculotomy may be more efficatious.14 Performing the limited 120° trabeculotomies were followed by a device-based procedure to remove the TM.15 There are some favorable results with the removal of the TM.16,17 However, some of the work by Johnstone indicates that the TM may serve a role in enhancing and maintaining the long-term outflow by pushing aqueous into the collector system.18 Hence, the desire to maintain the TM and not damage the canal of Schlemm has led to many surgeons considering the trabecular bypass as a surgical option for open-angle glaucoma.19
TRABECULAR AND THE CANAL OF SCHLEMM OUTFLOW SYSTEM
Aqueous humor is produced by the ciliary processes and provides nutrition of intraocular structures - such as lens and cornea. The aqueous exits, for the most part, through the “conventional outflow system:” The TM, into the canal of Schlemm (canal), into the collector channels and finally into the aqueous veins and ends up in the venous system via the episcleral veins. Some aqueous leaves via the unconventional (meaning nontrabecular-canal) outflow through the iris and uvea, also called the uveal-scleral outflow system. Both the trabecular (conventional) and unconventional outflow systems can have anatomic or pathological processes that lead to increased resistance and less outflow.20,21 The result from limited outflow is increased IOP and increased risk or definite damage from glaucoma.22 This paper will focus on targeting the trabecular outflow by MIGS stents and the history of these devices to achieve increased flow through the TM.
A lot of our understanding about trabecular outflow came from Grant and Trotter, for we now understand that the primary source of resistance to aqueous outflow is in the TM.23,24,25,26 In order to understand aqueous flow through the TM, different models were developed. In 2005, Johnson reviewed the various models for measuring the outflow.20 Whole fresh eyes seem to provide a good model for testing the outflow. In theory, 20 holes of 10 mm in diameter through the TM are adequate to restore normal outflow facility in open angle glaucoma. The site of the greatest outflow resistance within the TM is probably the juxtacanalicular tissue adjacent to the canal. If Schlemm's canal is open removing or bypassing this thin layer of tissue should reduce the outflow resistance in open angle glaucoma without the need of external filtration.27 Zhou and Smedley reported a theoretical model evaluating the effects of bypassing the TM.28 They further evaluated the effects of the canal. With increased dilation of the canal, there was a reduction in the IOP. In taking the canal from a breadth of 20 mm to a moderate dilatation of 40-50 mm there was an additional reduction in IOP. Bypassing the TM significantly increased the circumferential flow adjacent to the trabecular bypass. The implications are that increasing TM outflow should allow enough aqueous to dilate the canal and increase flow adjacent to the bypass and thus, decrease the IOP. One of the advantages of a trabecular bypass is that it may preserve the canal, and we do not see the collapse of the canal observed after trabeculectomy.29
FIRST TRABECULAR BYPASS STENTS
The thought about bypassing the TM has been around a while.30 In the early 1990's, Hill et al. published on the use of a laser to create a hole in the meshwork with the hopes of bypassing the TM.31 Others had attempted to use the laser or other thermal methods to bypass the TM with some success.32,33,34,35 Because of concerns about fibrosis or lack of holding the TM off of the back of the canal, Spiegel et al. developed a small stent to bypass the TM during the 1990's.36 This was first implanted in humans in the early 2000's using small tubes to bypass the TM, creating a direct route from the anterior chamber into the canal.37 First models were flexible silicone and several millimeters in length, and they were implanted ab externo during deep sclerectomy procedure. Initial reports indicated IOP lowered to 16.5 mmHg with medication use. This microtube had an inner lumen of 50 mm but could cause obstruction of the surgical site ostia and prevent flow into the collector channels adjacent to the implant and this could cause failure.38 Spiegel et al. proposed a smaller titanium stent and these were the ones tested by Zhou and Smedley and then the initial clinical experience from Germany was reported in 2007.39
TRABECULAR BYPASS STENTS AND MICRO-INVASIVE GLAUCOMA SURGERY 2014
The iStent® (Glaukos Corp., Laguna Hills, USA) is one example of a trabecular bypass stent and is the one describe above developed by Hill. It is approved in the United States by the US Food and Drug Administration (FDA) and is CE marked for use in Europe. We have begun implanting the iStent® trabecular bypass (iSTB) in Saudi Arabia. Competitor trabecular bypass implants are in clinical trial status. Ivantis Corporation (Irvine, CA, USA) has the Hydrus® implant with a little different design than the iStent® with a longer scaffold tube to help dilate the canal more than from the iSTB.40 Other MIGS devices involve targeting the suprachoroidal outflow41 or bypassing the angle altogether and creating a controlled fistula (a device controlled sclerostomy).42
The iSTB is made of nonferromagnetic titanium and is L-shaped with a curved open lumen. The tip is pointed to allow for insertion by penetrating through the TM and allow the body of the iSTB to rest in the canal. Just behind the tip is the trough that rests in the canal. Finally, there is the anterior chamber portion that is the “snorkel” that allows the aqueous to exit into the canal [Figure 1]. The device is heparin-coated. The canal portion is about 1 mm in length and has an outside diameter of 180 mm. The canal portion is half-pipe-shaped and designed to fit within the lumen of the canal (which averages about 225-250 mm).29 The half-pipe portion of the stent is placed with the convex side against the outer wall of the canal. This creates an arch to, hopefully, avoid blockage of the ostia of collector channels. Three barb-like ridges are on the side opposite the half-pipe and facilitate retention. The “snorkel” tube is hollow and goes through the TM connects with the anterior chamber. The weight of the stent is 60 mg. The hand piece for this trabecular bypass is disposable and has a “basket” that holds the “snorkel” of the stent with the sharper end with the trough to be placed through the TM and into the canal. The handle has a button that when depressed the “basket” opens and releases the stent into the TM [Figure 2].
Figure 1.

The iStent®. The snorkel, tough, and barbs can be seen
Figure 2.
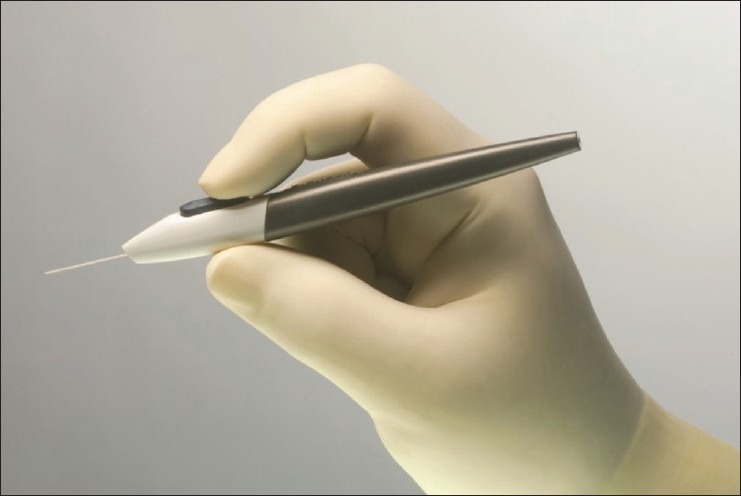
The handpiece for the iStent®, by pressing the button the stent is released
SURGICAL APPROACH TO PLACE MICRO-INVASIVE GLAUCOMA SURGERY DEVICES AND SPECIFICALLY THE ISTENT® TRABECULAR BYPASS
The surgical procedure is done with a temporal approach similar to the position used for phacoemulsification surgery. The view to see the angle is facilitated by a direct gonioscope and either tilting the microscope or turning the patient's head (or both) to see the TM through the gonioscope. A clear corneal incision of >1.5 mm is needed for insertion of the implant. If it is combined with cataract surgery, the phacoemulsification wound can be used. The anterior chamber is filled with viscoelastic material. The trabecular bypass stent is preloaded and inserted through the temporal clear cornea incision. Under the view through the gonioprism, the stent is preloaded on the applicator tip. There are left and right eye models that are only different for the direction of the trough from the snorkel. The right eye stent is labeled that way to allow for the nasal placement of the stent with the tip-and-trough pointing inferior. If the left stent were used in the right eye, the tip-and-tough would go superior if placed nasally (which is sometimes desired.) The stent is advanced to the opposite nasal anterior chamber angle [Figure 3]. The stent is positioned parallel to the TM and the leading edge of the device is slid through the TM and placed into the canal with the tip of the stent directed in the position dictated by the stent being right or left. Because the blood from the episcleral veins can backflow into the canal with a low IOP, blood reflux from the stent provides an indication being in the canal. The device is released by pushing a button on the applicator, and the final positioning is done by gently nudging the snorkel. Ideally, the stent should be parallel with the iris plane and the snorkel opening perpendicular to the meshwork [Figure 4]. After the iSTB is placed, the viscoelastic is evacuated by irrigation and aspiration.
Figure 3.
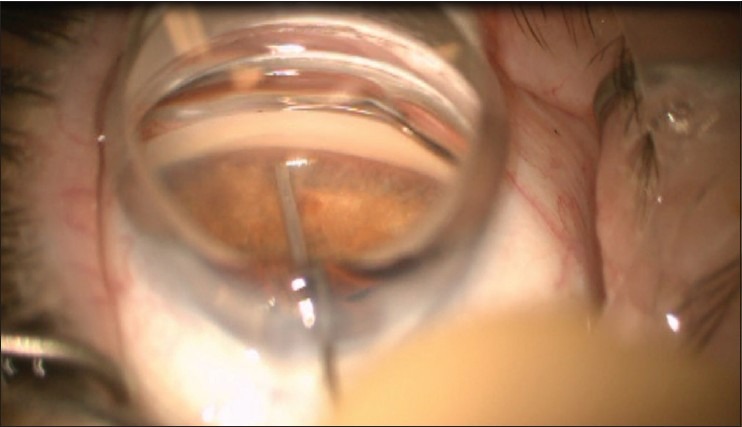
Goniolens to view. The stent is on the tip of the inserted, held by a “basket.”
Figure 4.
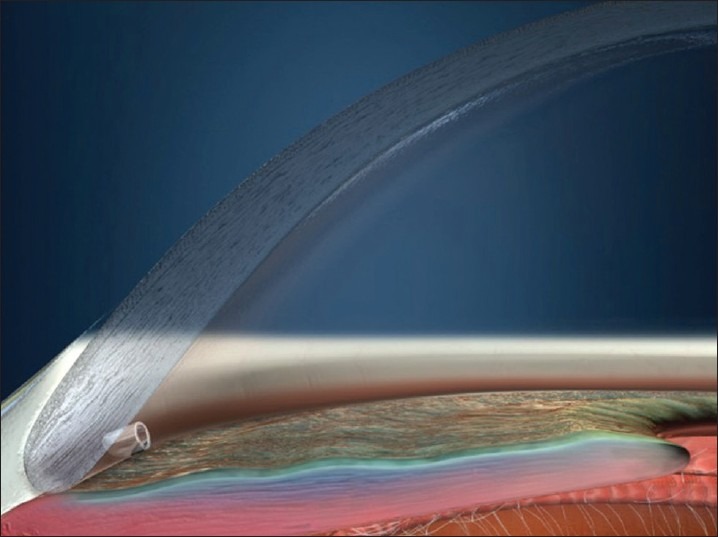
IStent® in place. The snorkel is into the anterior chamber, and the trough is against the back side of the canal
The Glaukos Corporation has also developed a second-generation stent called the iStent Inject®. The principles of how it works are very similar to the iStent® but two stents are loaded within the inserter itself. The inserters for the “Inject” are similar feeling to the iStent® but it has a small trocar that the “Inject” iStent's® are loaded onto [Figure 5]. The trocar penetrates the tissue and then a spring mechanism drives the “Inject” iStent into the TM by depressing the trigger on the handle, this delivers the stent and creates a “tube” into the canal.43 After one stent is injected into place a new location several clock hours away is picked, and a second stent is injected into place.
Figure 5.

IStent® Inject. The stent has another one behind it ready to be delivered so 2 stents can be injected. The trocar penetrates the trabecular meshwork
The Ivantis Corporation has a trabecular bypass stent that also involves a more canal dilating “pipe portion” of the stent. Their trabecular bypass device is called the Hydrus®. It is scaffolded with multiple vents to allow access to the collector channels.44 In theory, this should allow more dilation of the canal with better flow into the outflow system.45
WHAT DOES THE TRABECULAR BYPASS STENT ACHIEVE?
Success and adverse events with the trabecular bypass stents have been reported at 1 and 2 years for the patients participating in the US FDA pivotal trial.5,46 Interpreting the results has varied.47 The trial had potentially confounding issues (such as no validation of correct placement of the iSTB) but the effects of IOP reduction seemed to be sustained. It did appear that some individuals had a better response to the trabecular bypass than others.48 These have been called responders and/or potentially represent patients with placement that is correct and/or there is a downstream collector channel patency and low episcleral venous pressure allowing the aqueous to leave and the IOP to decrease from the device. The technique for finding the collector channels and determining the patency is still elusive.49,50,51,52 Most studies indicate the nasal portion of the eye has more collector channels so placing a trabecular bypass nasally would allow for better proximity; however, the complexity of the distal flow from the canal is potentially influenced by a system of struts and pores that facilitate the outflow from the canal, so other factors may be involved with getting a lower IOP even if the stents are patent and in the canal.18,53 Imaging the canal or collector system will be helpful when it is available.50,52,54,55,56,57
The mean IOP reported at year 2 for the iSTB pivotal trial was 17.1 ± 2.9 mm Hg. For the control group who had phacoemulsification alone, the IOP was 17.8 ± 3.3 mm Hg. The medication burden (how many IOP lowering medications the patient was on) was 0.3 ± 0.6 medications for the stent group and 0.5 ± 0.7 medications for the control phacoemulsification group.46 Yet physicians participating in the trial noticed that there were some patients with significant IOP reduction when the iSTB was used. When I reviewed the subjects who participated in the US FDA trial at our center after closure of the study, submission, and approval of the device, there were some patients with significant IOP reduction.58 Nine patients who had over a 20% reduction in IOP from baseline at the 1-month visit were included in this limited analysis. For this small group, the mean preop IOP after medication washout per protocol was 25.7 mmHg. At 1-month, this group had a mean IOP of 16.6 mmHg. This represented a mean decrease of 35.3%. At 24 months this group had a mean IOP of 13.9 mmHg (45.9% mean decrease in IOP) on no medication for any subject in the group. This may indicate that if the stent works at 1-month, there is either good placement, a good collector system, a low episcleral venous pressure. or a combination of all these factors allowing for a decreased IOP. These findings are similar to those of Arriola-Villalobos et al. who found a mean IOP of 16.26 mmHg at 53 months on the average out from surgery. About 42% of the patients were off meds and had the lower IOPs.59 Vandewalle et al. reported a group of 10 patients, 6 with concurrent phacoemulsification and 4 with stent alone at 1-year. The IOP range was range 9-25.60 This further supports some variability in the IOP drop.
MULTIPLE STENTS
Because of the concern with being able to “hit” the canal (correct placement,) some of the surgeons suggested using two iSTB stents to have a better likelihood of hitting the canal and/or getting near a segment of the canal and outflow collector channel system that was viable.61,62 The iStent Inject® is a two-stent system.63 The US FDA trial is nearly completing for the Inject® and some results with the device are being released.43,48 Voskanyan et al. have reported on the 1-year data for the Inject® being used for a postmarket trial in Europe.64 There were 99 patients with open-angle glaucoma included in the study. The major difference with this study compared to the US is this is a stand-alone study to use the Inject as a stand-alone procedure for lowering IOP. The baseline IOP was 26.3 mmHg ± 3.5 mmHg at baseline after washout. At month 12, the IOP was 15.7 mmHg ± 3.7 mmHg. The mean number of medications going into the study was 2.2 with various class of medications used preoperatively. At month 12, there were 65% of the patients off medications and the mean IOP for the medication-free group was 14.7 mmHg ± 3.7 mmHg. This will further support that if the stents access a patient downstream system and/or the stents are correctly placed, good IOP reduction is achieved.
HOW MANY STENTS DO WE NEED? THE NEED FOR A CANAL AND COLLECTOR CHANNEL TEST
Swaminathan et al. recently reviewed the outflow system with attention to the collector channels and canal of Schlemm.53 There is good evidence that the canal itself and the collector system may be a significant source of resistance to aqueous outflow. In addition, the effect of IOP on collapsing the canal may play a role in the success, and the outflow could be increased more with aqueous getting into the canal and increasing outflow.65 Hunter et al. evaluated the computational fluid dynamics and effects on IOP reduction from the stents themselves.66 One stent theoretically dropped the IOP 6 mmHg, and two stents dropped the IOP 8.09 mmHg. Hence, the effects of multiple stents when correctly placed are of limited value for further IOP reduction. Belovay et al. reported on the IOP effect of 2 and 3 iSTB stents on a limited number of subjects.61 The effect on the IOP beyond 2 stents is minimal from this limited study. Therefore, one or two correctly placed trabecular bypass stents that are probably all that are needed to achieve the best IOP reduction from this type of procedure because of limitations from the canal, collector system and episcleral venous pressure on aqueous outflow.
THE TRABECULAR BYPASS STENTS AND COMBINING WITH MEDICATIONS
Ahmed et al. reported on a series of patients that had 2 iSTB stents placed as a stand-alone procedure for subjects in Armenia with open angles who were on 2 medications at the time of entering the study. The subjects all had an IOP over 22 mmHg.7 The mean IOP was 22.2 mmHg ± 2.0 mmHg, and 100% of the subjects were on 2 medications. After washout, the mean IOP was 25.3 ± 1.8 mmHg. All patients (39 total) received 2 iSTB stents. Travoprost was started nightly from postoperative day 1 and continued throughout the study. The medicated IOP decreased to 13.0 mmHg ± 2.4 mmHg for these subjects receiving 2 iSTB stents and were on the travoprost at 12 months; after washing off of the travoprost for 1-month the IOP was 17.1 ± 2.2 mmHg at 13 months after surgery. After re-starting the travoprost at 18 months, the IOP was 11.8 mmHg ± 2.1 mmHg. For 43.6% of the eyes at 1-month, there was an IOP reduction of >50% with 84.6% of the patients had an IOP of < 15 mmHg. Again, this shows a significant response in some patients. However, all of the patients in the study at 12 months (on travoprost) had an IOP decrease of >20%.
PROS AND CONS
We see that the trabecular bypass has more than a 30% IOP reduction in over half of the patients from the various studies as mentioned above. So if a patient has a good response, it seems to be sustained for at least several years. Because the device is small, if a trabeculectomy or tube shunt were required, there should not be any limitations because of the prior MIGS procedure. Laser trabeculoplasty has been done after the stent was placed.46 Some of the patients do not have as much of a drop in the IOP, but no patients appeared to be significantly harmed.7,46,59 In the FDA trial, the subject who had the stent re-positioned did have a drop in the IOP after the re-positioning.46 Again, this indicates that the positioning is critical for success. The difficulty knows you are in the canal and if the collector channels and episcleral venous pressure will facilitate the flow enough to decrease the IOP. We do not know if prior angle closure will limit the success of these implants, but some of the patients in the Armenian study had a prior laser iridotomy.7
There is a learning curve to get accustomed to doing these procedures, but for most surgeons after 10 cases they start to feel more comfortable performing MIGS approaches. Patient cooperation and surgeon's steady hand are important for the precise placement into the narrow band of the TM. Sometimes, the placement is not deep enough [Figure 6] or it can be “over inserted” and too deep. Blood reflux at the time of surgery is fairly common and probably desirable because it tells you more likely than not that you are in the canal. There have been reports of delayed hyphema. After the stents are in place, there were a few patients who did develop synechia to the iSTB.46 These patients usually had the stent angled toward the iris, and the proximity was close, this may have contributed to the synechiae. There were no long-term cases of problematic iritis, loss of endothelial cells, or devices needing removal because of it harming the patient. Therefore, the device seems to be safe based on the experience thus far.
Figure 6.
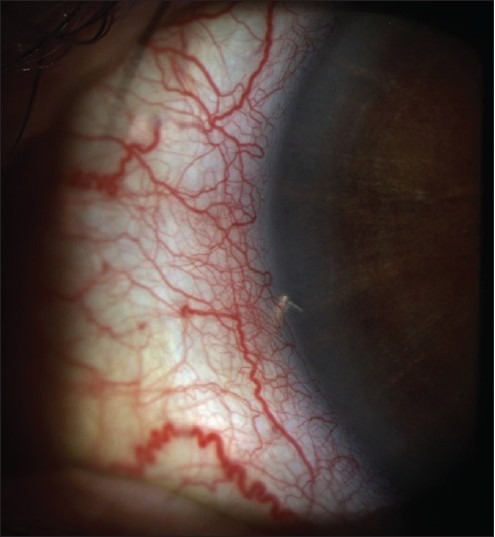
IStent® not flush with the trabecular meshwork and partly inserted. There was still an intraocular pressure reduction seen for this patient
SUMMARY AND CURRENT RECOMMENDATIONS
The trabecular bypass stent offers an alternative to filtration surgery. Patients who may be ideal candidates for considering this procedure are those with prior conjunctival surgery; for example, those who had a 360° peritomy from a scleral buckle might not do well with a trabeculectomy and there is no space for a tube. Highly myopic patients do not tolerate hypotony well, and the iSTB may be an option for some of these patients. I have used the iSTB in patients on anticoagulants who could not stop them, and they needed something beyond medications and laser to lower the IOP in subjects with open-angle glaucoma. Young patients, especially those with one eye, who need rapid visual recovery (for instance to return to work) may be good candidates to consider the iSTB as well. Because of the position used for clear corneal cataract surgery, the temporal approach is best for doing these. Therefore, if you are doing cataract surgery on someone who needs a lower IOP, you already are in the correct position to implant the devices. Patients may need some medications after the procedure to lower the IOP to the level desired. The results from Armenia are encouraging, given an IOP of 11.8 mmHg after 2 iSTB stents and taking daily travoprost.7 These results are difficult to reach even with a trabeculectomy. When selecting your fist patients, avoid those with the congested episcleral veins, look for patients with wide open angles, and if you can see aqueous veins at the slit-lamp it may indicate a viable outflow system. Probably avoid patients with IOPs over 35 mmHg.
There have been quite a few studies looking at the success from 1 or 2 stents, and one study looked at 3 stents. There is a little more effect on reducing the IOP with 2 iSTB stents. However, the IOP reduction seen with the second stent could be due to placement or proximity to collector channels in the clinical setting. There are good data that if one stent is placed correctly and the outflow system is viable, that is capable of achieving low IOP. It is probably reasonable to implant one stent for most patients. Two stents with the Inject® may help assure with the iStent® system a better likelihood of hitting the canal. If you have the funds available to implant 2 of the iSTB stents, that is a consideration. When interpreting your results, realize that the success is due to placement, a viable outflow system, and a low episcleral venous pressure. Recording in the operative note if you saw a blood reflux may serve useful as a check for placement at the time of surgery and postoperative gonioscopy is valuable. If the stent appears in the correct position and you still have an elevated IOP, it may be best to look for a different glaucoma surgery, such as a deep sclerectomy or trabeculectomy, to lower the IOP. Fortunately, IOP in the 11–14 mmHg range can be seen in about 50% of the patients who receive the iSTB, especially if one medication is added.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vuori ML, Nikoskelainen E. Evaluation of glaucoma patients referred to a university clinic during one year. Acta Ophthalmol Scand. 1997;75:692–4. doi: 10.1111/j.1600-0420.1997.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 2.Camras CB, Alm A, Watson P, Stjernschantz J. Latanoprost, a prostaglandin analog, for glaucoma therapy. Efficacy and safety after 1 year of treatment in 198 patients. Latanoprost Study Groups. Ophthalmology. 1996;103:1916–24. doi: 10.1016/s0161-6420(96)30407-7. [DOI] [PubMed] [Google Scholar]
- 3.Katz LJ, Steinmann WC, Kabir A, Molineaux J, Wizov SS, Marcellino G, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: A prospective, randomized trial. J Glaucoma. 2012;21:460–8. doi: 10.1097/IJG.0b013e318218287f. [DOI] [PubMed] [Google Scholar]
- 4.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–14.e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–67. doi: 10.1016/j.ophtha.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. doi: 10.1097/ICU.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed II, Katz LJ, Chang DF, Donnenfeld ED, Solomon KD, Voskanyan L, et al. Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma. J Cataract Refract Surg. 2014;40:1295–300. doi: 10.1016/j.jcrs.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968;66:673–9. [PubMed] [Google Scholar]
- 9.Watson PG, Barnett F. Effectiveness of trabeculectomy in glaucoma. Am J Ophthalmol. 1975;79:831–45. doi: 10.1016/0002-9394(75)90745-x. [DOI] [PubMed] [Google Scholar]
- 10.Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty-year follow-up study of trabeculectomy: Risk factors and outcomes. Ophthalmology. 2012;119:694–702. doi: 10.1016/j.ophtha.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Radhakrishnan S, Quigley HA, Jampel HD, Friedman DS, Ahmad SI, Congdon NG, et al. Outcomes of surgical bleb revision for complications of trabeculectomy. Ophthalmology. 2009;116:1713–8. doi: 10.1016/j.ophtha.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Edmunds B, Bunce CV, Thompson JR, Salmon JF, Wormald RP. Factors associated with success in first-time trabeculectomy for patients at low risk of failure with chronic open-angle glaucoma. Ophthalmology. 2004;111:97–103. doi: 10.1016/j.ophtha.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Singh K, Mehta K, Shaikh NM, Tsai JC, Moster MR, Budenz DL, et al. Trabeculectomy with intraoperative mitomycin C versus 5-fluorouracil. Prospective randomized clinical trial. Ophthalmology. 2000;107:2305–9. doi: 10.1016/s0161-6420(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Hirata A, Mizoguchi T. Outcomes of 360° suture trabeculotomy with deep sclerectomy combined with cataract surgery for primary open angle glaucoma and coexisting cataract. Clin Ophthalmol. 2014;8:1301–10. doi: 10.2147/OPTH.S64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis BA, See RF, Rao NA, Minckler DS, Baerveldt G. Ab interno trabeculectomy: Development of a novel device (Trabectome) and surgery for open-angle glaucoma. J Glaucoma. 2006;15:68–73. doi: 10.1097/01.ijg.0000196653.77836.af. [DOI] [PubMed] [Google Scholar]
- 16.Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22:205–8. doi: 10.1097/IJG.0b013e3182311b92. [DOI] [PubMed] [Google Scholar]
- 17.Jordan JF, Wecker T, van Oterendorp C, Anton A, Reinhard T, Boehringer D, et al. Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 2013;251:2753–60. doi: 10.1007/s00417-013-2500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone MA. The aqueous outflow system as a mechanical pump: Evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–38. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- 19.Nichamin LD. Glaukos iStent trabecular micro-bypass. Middle East Afr J Ophthalmol. 2009;16:138–40. doi: 10.4103/0974-9233.56227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DH. Trabecular meshwork and uveoscleral outflow models. J Glaucoma. 2005;14:308–10. doi: 10.1097/01.ijg.0000169397.32674.5e. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW. Intraocular pressure following peritomy and wet-field coagulation. Korean J Ophthalmol. 1990;4:92–102. doi: 10.3341/kjo.1990.4.2.92. [DOI] [PubMed] [Google Scholar]
- 22.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the collaborative initial glaucoma treatment study. Ophthalmology. 2011;118:1766–73. doi: 10.1016/j.ophtha.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant WM, Trotter RR. Tonographic measurements in enucleated eyes. AMA Arch Ophthalmol. 1955;53:191–200. doi: 10.1001/archopht.1955.00930010193003. [DOI] [PubMed] [Google Scholar]
- 24.Grant WM. Facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1955;54:245–8. doi: 10.1001/archopht.1955.00930020251012. [DOI] [PubMed] [Google Scholar]
- 25.Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958;60:523–33. doi: 10.1001/archopht.1958.00940080541001. [DOI] [PubMed] [Google Scholar]
- 26.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 27.Bahler CK, Smedley GT, Zhou J, Johnson DH. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthalmol. 2004;138:988–94. doi: 10.1016/j.ajo.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Smedley GT. A trabecular bypass flow hypothesis. J Glaucoma. 2005;14:74–83. doi: 10.1097/01.ijg.0000146360.07540.ml. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DH, Matsumoto Y. Schlemm's canal becomes smaller after successful filtration surgery. Arch Ophthalmol. 2000;118:1251–6. doi: 10.1001/archopht.118.9.1251. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann F. Mathematical consideration of aqueous outflow after trabeculo-electropunture (TEP) Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;204:87–93. doi: 10.1007/BF00414709. [DOI] [PubMed] [Google Scholar]
- 31.Hill RA, Baerveldt G, Ozler SA, Pickford M, Profeta GA, Berns MW. Laser trabecular ablation (LTA) Lasers Surg Med. 1991;11:341–6. doi: 10.1002/lsm.1900110405. [DOI] [PubMed] [Google Scholar]
- 32.Robin AL, Pollack IP. Q-switched neodymium-YAG laser angle surgery in open-angle glaucoma. Arch Ophthalmol. 1985;103:793–5. doi: 10.1001/archopht.1985.01050060053023. [DOI] [PubMed] [Google Scholar]
- 33.Jacobi PC, Dietlein TS, Krieglstein GK. Technique of goniocurettage: A potential treatment for advanced chronic open angle glaucoma. Br J Ophthalmol. 1997;81:302–7. doi: 10.1136/bjo.81.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobi PC, Dietlein TS, Krieglstein GK. Microendoscopic trabecular surgery in glaucoma management 11 the authors have no proprietary interest in the development or marketing of this or any competing equipment. Ophthalmology. 1999;106:538–44. doi: 10.1016/S0161-6420(99)90113-6. [DOI] [PubMed] [Google Scholar]
- 35.Wilmsmeyer S, Philippin H, Funk J. Excimer laser trabeculotomy: A new, minimally invasive procedure for patients with glaucoma. Graefes Arch Clin Exp Ophthalmol. 2006;244:670–6. doi: 10.1007/s00417-005-0136-y. [DOI] [PubMed] [Google Scholar]
- 36.Spiegel D, Kobuch K, Hill RA, Gross RL. Schlemm's canal implant: A new method to lower intraocular pressure in patients with POAG? Ophthalmic Surg Lasers. 1999;30:492–4. [PubMed] [Google Scholar]
- 37.Spiegel D, Kobuch K, Hill RA, Gross RL. Implant in Schlemm's canal. A new method for regulating intraocular pressure in patients with primary open-angle glaucoma? Ophthalmologe. 2001;98:94–6. doi: 10.1007/s003470170207. [DOI] [PubMed] [Google Scholar]
- 38.Spiegel D, Kobuch K. Trabecular meshwork bypass tube shunt: Initial case series. Br J Ophthalmol. 2002;86:1228–31. doi: 10.1136/bjo.86.11.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiegel D, Wetzel W, Haffner DS, Hill RA. Initial clinical experience with the trabecular micro-bypass stent in patients with glaucoma. Adv Ther. 2007;24:161–70. doi: 10.1007/BF02850004. [DOI] [PubMed] [Google Scholar]
- 40.Lorens K, Norbert P, Ramirez M, Scharioth G, Tets M, Vass C, et al. 6 Month Results from a Prospective, Multicenter Study of a Nickel-titanium Schlemm's Canal Scaffold for IOP Reduction in Open Angle Glaucoma. New York: American Glaucoma Society; 2012. [Google Scholar]
- 41.Hoeh H, Ahmed II, Grisanti S, Grisanti S, Grabner G, Nguyen QH, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39:431–7. doi: 10.1016/j.jcrs.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Varma R. Devices in development: AqueSys implant. Glaucoma Today. 2012;10:44–5. [Google Scholar]
- 43.Fea AM, Belda JI, Rekas M, Jünemann A, Chang L, Pablo L, et al. Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–82. doi: 10.2147/OPTH.S59932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hays CL, Gulati V, Fan S, Samuelson TW, Ahmed II, Toris CB. Improvement in outflow facility by two novel microinvasive glaucoma surgery implants. Invest Ophthalmol Vis Sci. 2014;55:1893–900. doi: 10.1167/iovs.13-13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camras LJ, Yuan F, Fan S, Samuelson TW, Ahmed IK, Schieber AT, et al. A novel Schlemm's Canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2012;53:6115–21. doi: 10.1167/iovs.12-9570. [DOI] [PubMed] [Google Scholar]
- 46.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J Cataract Refract Surg. 2012;38:1339–45. doi: 10.1016/j.jcrs.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Bacharach J. Efficacy of trabecular bypass stent through 2 years: Data from the United States pivotal trial. J Cataract Refract Surg. 2014;40:1325–6. doi: 10.1016/j.jcrs.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, García-Vidal SE, Fernández-Pérez C, García-Sánchez J, et al. Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. Br J Ophthalmol. 2013;97:1250–5. doi: 10.1136/bjophthalmol-2012-302394. [DOI] [PubMed] [Google Scholar]
- 49.Fernández-Barrientos Y, García-Feijoó J, Martínez-de-la-Casa JM, Pablo LE, Fernández-Pérez C, García Sánchez J. Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51:3327–32. doi: 10.1167/iovs.09-3972. [DOI] [PubMed] [Google Scholar]
- 50.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci. 2010;51:1498–504. doi: 10.1167/iovs.09-4327. [DOI] [PubMed] [Google Scholar]
- 51.Dashevsky AV, Lanzl IM, Kotliar KE. Non-penetrating intracanalicular partial trabeculectomy via the ostia of Schlemm's canal. Graefes Arch Clin Exp Ophthalmol. 2011;249:565–73. doi: 10.1007/s00417-010-1507-6. [DOI] [PubMed] [Google Scholar]
- 52.Johnson AW, Ammar DA, Kahook MY. Two-photon imaging of the mouse eye. Invest Ophthalmol Vis Sci. 2011;52:4098–105. doi: 10.1167/iovs.10-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swaminathan SS, Oh DJ, Kang MH, Rhee DJ. Aqueous outflow: Segmental and distal flow. J Cataract Refract Surg. 2014;40:1263–72. doi: 10.1016/j.jcrs.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith P, Samuelson D, Brooks D. Aqueous drainage paths in the equine eye: Scanning electron microscopy of corrosion cast. J Morphol. 1988;198:33–42. doi: 10.1002/jmor.1051980105. [DOI] [PubMed] [Google Scholar]
- 55.Bill A. Some aspects of aqueous humour drainage. Eye (Lond) 1993;7(Pt 1):14–9. doi: 10.1038/eye.1993.4. [DOI] [PubMed] [Google Scholar]
- 56.Kagemann L, Wollstein G, Ishikawa H, Bilonick RA, Brennen PM, Folio LS, et al. Identification and assessment of Schlemm's canal by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:4054–9. doi: 10.1167/iovs.09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Merwe EL, Kidson SH. The three-dimensional organisation of the post-trabecular aqueous outflow pathway and limbal vasculature in the mouse. Exp Eye Res. 2014;125:226–35. doi: 10.1016/j.exer.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Craven ER. Glaucoma 2012 Managing Challenging Glaucoma Problems - Merging Art and Science. Chicago, Il: American Academy of Ophthalmology; 2012. Late Breaking Development: How Will the Trabecular Micro-Bypass Approval Impact Glaucoma Management? [Google Scholar]
- 59.Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br J Ophthalmol. 2012;96:645–9. doi: 10.1136/bjophthalmol-2011-300218. [DOI] [PubMed] [Google Scholar]
- 60.Vandewalle E, Zeyen T, Stalmans I. The iStent trabecular micro-bypass stent: A case series. Bull Soc Belge Ophtalmol. 2009:23–9. [PubMed] [Google Scholar]
- 61.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38:1911–7. doi: 10.1016/j.jcrs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 62.Roelofs K, Arora S, Dorey MW. Implantation of 2 trabecular microbypass stents in a patient with primary open-angle glaucoma refractory to previous glaucoma-filtering surgeries. J Cataract Refract Surg. 2014;40:1322–4. doi: 10.1016/j.jcrs.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Bahler CK, Hann CR, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthalmol. 2012;153:1206–13. doi: 10.1016/j.ajo.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Voskanyan L, García-Feijoó J, Belda JI, Fea A, Jünemann A, Baudouin C, et al. Prospective, unmasked evaluation of the iStent ® inject system for open-angle glaucoma: Synergy trial. Adv Ther. 2014;31:189–201. doi: 10.1007/s12325-014-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen PK. Dependence of outflow facility on intraocular pressure? Invest Ophthalmol Vis Sci. 2014;55:1857. doi: 10.1167/iovs.13-13860. [DOI] [PubMed] [Google Scholar]
- 66.Hunter KS, Fjield T, Heitzmann H, Shandas R, Kahook MY. Characterization of micro-invasive trabecular bypass stents by ex vivo perfusion and computational flow modeling. Clin Ophthalmol. 2014;8:499–506. doi: 10.2147/OPTH.S56245. [DOI] [PMC free article] [PubMed] [Google Scholar]


