Abstract
Surgical options for glaucoma have expanded in recent years. This article provides an evidence-based update on the novel or emerging surgical techniques for the treatment of open-angle glaucoma that are based on the Schlemm's canal (SC). Canaloplasty is an ab externo approach and was developed as an alternative to traditional filtering surgeries. The Hydrus microstent (Ivantis Inc., Irvine, CA) is a so-called SC scaffold that directly bypasses the trabecular meshwork to drain aqueous humor into the SC, which it keeps dilated over approximately one quadrant. Canaloplasty has also been shown to lower intraocular pressure (IOP) by up to 40% and combined with cataract surgery. IOP was lowered 44% at 24 months while maintaining a favorable safety profile. The Hydrus device has been proposed as an adjunct to cataract extraction surgery. To date, no published evidence from clinical trials is available on its in vivo safety and efficacy. Schlemm's canal based glaucoma procedures show promise as alternative treatments to traditional glaucoma surgery. Surgeons must be comfortable with angle anatomy. A prerequisite for functionality of these techniques is the integrity of the distal outflow system. At present, however, it is not possible to conclude whether these novel procedures will be viable alternatives to standard filtering surgery over the long-term.
Keywords: Canaloplasty, Glaucoma, Hydrus Microstent, Intraocular Pressure, Scaffold, Schlemm's Canal, Surgery
INTRODUCTION
At present, elevated intraocular pressure (IOP) is the only modifiable risk factor for glaucoma.1 Surgery is widely considered to be the most efficient method to lower IOP to levels considered as safe.2,3 Yet, the gold standard technique, trabeculectomy, suffers from a suboptimal safety profile and is as a consequence frequently reserved for glaucoma patients with progressive disease. After decades of relative immobility, the field of glaucoma surgery has in recent years benefited from an innovative push. This chapter discusses innovative approaches to increase drainage of aqueous humor to and from Schlemm's canal (SC).
Schlemm's canal is named after German anatomist Friedrich Schlemm, who in 1830 discovered the vein at the chamber angle that collects aqueous humor from the anterior chamber and delivers it into the bloodstream. It took until the late 20th century for glaucoma surgeons to directly address this structure. Krasnov,4 in his sinusotomy technique, unroofed the external wall of SC but left the inner wall untouched. With the advent of operative microscopes, nonpenetrating glaucoma surgery (NPGS) techniques that unroofed both walls of SC were pioneered. In deep sclerectomy, a deep layer of corneo-scleral tissue is excised to leave behind a thin trabeculo-descemet's membrane (TDM) to provide some resistance to outflow. In NPGS, by removing the juxtacanalicular component of the trabecular meshwork (TM), the highest point of resistance in the conventional outflow pathway is believed to eliminate.5 The surgically created intrascleral space is usually maintained patent through the placement of an implant. Although filtering blebs in deep sclerectomy are less prominent than in trabeculectomy, this remains a bleb-dependent procedure. In viscocanaloplasty, SC is further dilated by the injection of high-viscosity viscoelastic material into both ostia. By attempting to stimulate the physiological outflow pathway, this procedure is meant to be independent of filtering blebs. In fact, although blebs are significantly smaller than in deep sclerectomy, their presence has been described and may play a role in the functionality of this procedure.6 In the last decade, SC-based surgery has experienced a renaissance.
AB EXTERNO PROCEDURES
Canaloplasty
Canaloplasty is an ab externo procedure, which was designed to enhance the outflow of aqueous humor by dilating SC, establishing circumferential flow and stretching out the TM. For this purpose, SC is cannulated with a fiber optic probe (iScience International, Menlo Park, California, USA). A suture is then introduced 360° and tightened until the TM is put on adequate stretch to improve aqueous humor outflow. Conceptually, it is an extension of viscocanalostomy with the addition of catheter-aided dilation, the placement of a permanent suture under tension (or stent, see below) in SC, and the creation of an intrascleral filtering space or “lake.” It has the CE-mark in Europe and is Food and Drug Administration-approved in the USA.
Surgical technique
The initial steps are almost identical to standard NPGS: After creating a conjunctival flap, a scleral flap of approximately half-scleral thickness and 5 mm in width and length is dissected. A deep scleral flap is then dissected down. The correct depth is reached when ciliary body/choroid becomes visible underneath a remaining thin scleral layer. This is carefully advanced anteriorly until SC is unroofed. The deep flap dissection is then further anteriorly into clear cornea to expose a small segment of Descemet's membrane to create the TDM. The ostia of the canal are then carefully dissected, identified (sometimes visco-dilated) to allow easy access to the flexible microcatheter (iTrack, iScience International, Menlo Park, California, USA).
The beacon tip of the microcatheter (iTrack) is illuminated using a laser-diode based micro illumination system ( i Lumin, iScience International, Menlo Park, California, USA). The catheter is 250 mm in diameter at the tip with a shaft diameter of 200 mm [Figure 1a and b]. Its lumen allows the injection of an ocular viscoelastic device (OVD) to inflate the canal. The device also contains an optical fiber with a lighted beacon at the tip to indicate the course of the catheter. The microcatheter is then cannulated 360° through SC until it exits from the other ostium. A 10-0 prolene suture is tied to its distal end, and the microcatheter is withdrawn into the canal in the opposite direction from which it was introduced, thereby threading the suture through SC. This step should be performed in a smooth continuous movement. As the tip and suture are retracted, OVD is injected every two clock hours through a one-eighth turn of the inbuilt viscoelastic injector. The suture in the canal is tightened, causing distention of the TM inwards and a further distention of the canal. This can be verified intraoperatively using the custom-built high-resolution ultrasound system (iUltrasound, iScience International, Menlo Park, California, USA). After satisfactory suture tightening, the deep scleral flap is excised creating a “scleral lake,” and the ends of the suture are tied with enough tension to distend the TM inside the eye. The superficial flap is then closed tightly.
Figure 1.
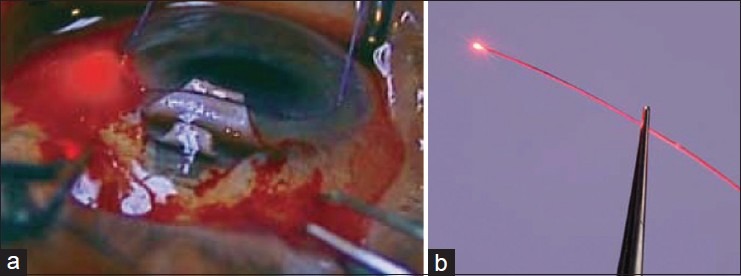
(a) Canaloplasty. Circumferential dilation of Schlemm's canal with the microcatheter; (b) note the blinking light of the Cather's tip (a)
Similar to other NPGS techniques, canaloplasty is contraindicated in patients with neovascular glaucoma, angle closure glaucoma, and in patients with previous surgery, which would prevent 360° catheterization of SC.
Efficacy
Lewis et al.,7 published the first large series on canaloplasty. In this noncomparative, prospective, multicenter study, 94 eyes with open-angle glaucoma (OAG) were treated by canaloplasty or canaloplasty combined with cataract surgery. The mean preoperative IOP was 24.7 ± 4.8 mmHg and mean number of medications used for treatment was 1.9 ± 1.0. Eyes with canaloplasty alone had a postoperative IOP of 16.3 ± 3.7 mmHg on 0.6 ± 0.8 medications. Eyes with combined canaloplasty – cataract surgery had a mean postoperative IOP of 13.4 ± 4.0 mmHg on 0.2 ± 0.4 medications. Successful catheterization was achieved in 83 (88%) eyes, and a tension suture was placed in 74 (78.7%). In about 21% the suture could not be placed.
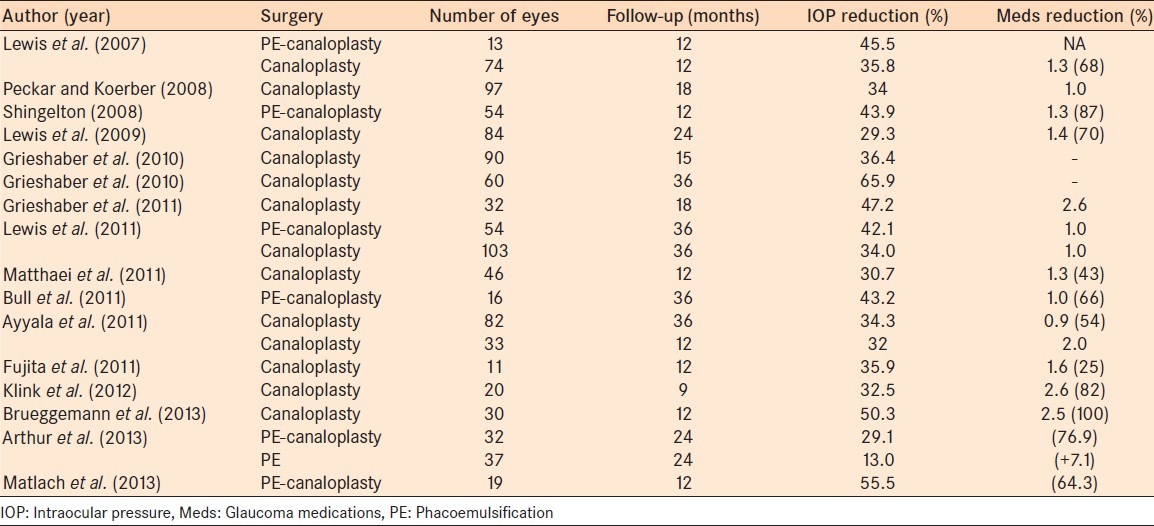
Shingleton et al.,8 conducted another noncomparative, prospective, multicenter trial of 54 eyes with up to 1-year of follow-up and showed a mean IOP reduction of 43.8%. A prospective trial of 60 black African eyes with a medium-term follow-up of 36 months showed complete and qualified success rates of 77.5% and 81.6%, respectively.9 Of note, in these at-risk eyes for surgical failure, mean preoperative IOP was 45.0 mmHg. Another, smaller study found an average 55% drop in IOP after 12 months.10 This study also was weakened by not having a phacoemulsification alone control group. In the only study to date comparing phacoemulsification – canaloplasty to phacoemulsification alone, Arthur et al.,11 reported a more profound IOP-lowering effect of the combined procedure throughout 24 months, although half to two-thirds of patients were lost to follow-up. The study was retrospective, so there was no randomization, and the starting IOPs were relatively low in each group (16.2 in the phacoemulsification group and 18.2 in the phacoemulsification – canaloplasty group). Although there was no statistically significant difference in IOP at baseline or 24 months, patients in the combined group were on fewer medications and were less likely to fail. Another retrospective study on 46 eyes found a mean postoperative IOP of 12.6 mmHg at 1-year (18.2 mmHg preoperatively) with a reduction of medications from 2.3 to 1.0. Table 1 provides an overview of efficacy studies with canaloplasty.12,13,14,15,16,17,18
Table 1.
Overview of efficacy studies with canaloplasty
In a series of studies, Grieshaber et al.,19 proposed measures to evaluate and predict surgical success after canaloplasty. In a study of 28 eyes of black African patients undergoing canaloplasty the presence of blood reflux into SC, was evaluated with provocative gonioscopy and episcleral vein filling with a fluorescein tracer. They found that the level of postoperative IOP correlated with the grade of both blood reflux and episcleral vein filling. The same group also performed channelography by injecting fluorescein dye into SC using a flexible microcatheter. This technique allowed the differentiation between episcleral veins directly connected to the aqueous outflow system from ciliary veins.20 Grieshaber et al.,21 observed the presence of microhyphema on the first postoperative day in canalosplasty eyes that went on to achieve lower IOPs over an average of 21 months of follow-up. They postulated that this would be a sign of a restored and patent physiological outflow system and might be of positive predictive value for a successful surgery.
Surgical complications
The safety profile is similar to rates reported after classic NPGS.22 Lewis et al.,7 in their large international series, reported hyphema (3 eyes), elevated IOP > 30 mmHg,3 Descemet's tear,1 hypotony,1 choroidal effusion,1 and exposed closure suture with epiphora and eyelid edema and erythema.1 The most common adverse effect is the presence of microhyphema on the first postoperative day, followed by IOP spikes over 30 mmHg (reported in 1.6-8.7%), and descemet's detachment (1.6-6.1%). Palmiero et al.,23 reported a case of bilateral descemet's membrane detachment in a 70-year-old man with primary OAG immediately following canaloplasty surgery in both eyes performed 1-week apart. The descemet's membrane spontaneously reattached in both eyes within 3 months. Overall, the incidence of this complication has been reported to be as much as 7.4%, with some cases leading to corneal decompensation.24,25 Fewer cases have been described with erosion of the prolene suture into the anterior chamber because of constant tension on the TM.
Conclusion
Overall, canaloplasty alone as well as combined phacoemulsification - canaloplasty has been shown to lower IOP by approximately 40% in numerous studies. The lack of comparative phacoemulsification alone group in the canaloplasty studies is a substantial limitation of the available evidence. The safety profile of this procedure is uncontestably favorable, although comparative randomized trials to trabeculectomy are lacking.
The procedure is technically challenging: The external dissection is more difficult and time-consuming than other angle-based procedures. The learning curve for obtaining the right degree of suture tensioning can be long and arduous. Yet, it is an important step to master as the distension of the SC inner wall is thought to increase trans-trabecular aqueous egress. It further has the additional disadvantage of causing conjunctival scarring, which makes subsequent glaucoma surgery technically more difficult and increases the risk of failure. Other potential risks are related to the use of antifibrotic agents by some surgeons and bleb formation in a minority of patients. Also, canaloplasty does not provide direct access to collector channels (CCs), and the reduction of IOP is limited by SC resistance and episcleral venous pressure. In addition, the long-term effects of a foreign body in SC are presently unknown. Finally, an additional disadvantage is the expense of the operative equipment (use of fiber optics to confirm placement, the use of ultrasound biomicroscopy to confirm suture tension).
Stegmann canal expander
The SCE expander (Ophthalmos GmbH, Schaffhausen, Switzerland) was designed to overcome one of the major difficulties with canaloplasty: The placement and tightening of the tension suture, as well as complications of “cheese-wiring.” The new flexible “stents” are made of polyimide and with a length of 9.0 mm. Each is designed to occupy a quarter of SC circumference maintaining dilatation and allowing aqueous humor to access the CCs through its fenestrations [Figure 2].
Figure 2.
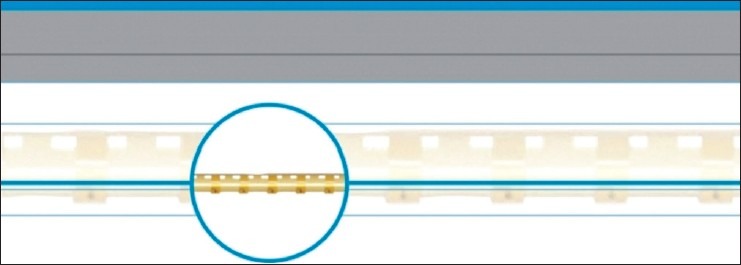
Stegmann canal expander
They are placed following dilatation with the same type of microcatheter as is used in the tension suture technique (iTrack) using a 6/0 carrier to position the expander. After completed dilation, the microcatheter is withdrawn, and the SCE is placed inside both SC ostia. With the intra-canalicular stents, it is easier to overcome angles over the SC that are difficult to pass with the prolene tip. The advantage of the SCE is that although it may not possible in some cases to use a tension suture, it would still usually be possible to thread the SCE into each side.
The device received the CE mark in 2013. Clinical trials are forthcoming.
Ab interno procedures
Schlemm's canal scaffold (Hydrus microstent)
The Hydrus microstent (Ivantis Inc., Irvine, CA) has been designed to bypass the TM as a so-called nonluminar “intracanalicular scaffold.” It has received CE-mark in Europe and is currently under clinical investigations in the US. The device has a circular shape designed to correspond to the curvature of SC. It has a length of 8 mm. It is open posteriorly and contains three windows along its length [Figure 3]. It is made of nitinol®, a nickel-titanium alloy, rendering it elastic and biocompatible. The device is designed to increase the outflow by bypassing the TM, thus bypassing the main site of outflow resistance and dilating 3–4 clock hours of SC to increase circumferential flow. It creates a maximum SC dilation of 241 mm or approximately 4–5 times the natural cross-sectional area of SC.
Figure 3.
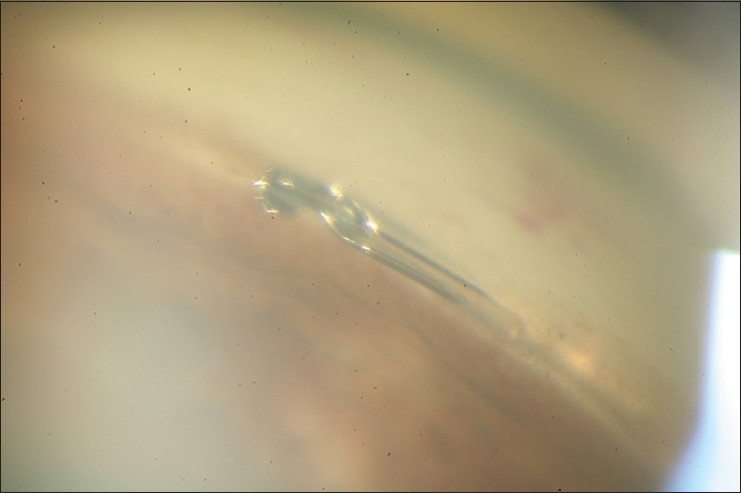
Hydrus microstent. Final placement of the device in Schlemm's canal
Rationale
The rationale behind the Hydrus microstent is to allow aqueous to circumvent the TM as the putative major site of outflow resistance in glaucoma and to drain directly to SC. It is further supported by Johnstone and Grant's finding that elevated IOP causes SC to collapse and causes lasting changes in TM and SC structures.26,27 It has been shown previously that circumferential surgical dilation of SC leads to increased aqueous outflow and lower IOP. Contrary to canaloplasty where the entire SC lumen is dilated, the Hydrus microstent dilates approximately one quadrant of SC. The nasal quadrant is the most common site for Schlemm canal surgery due to its easy access through clear corneal temporal incisions. This site also coincides with the area of the highest concentration of CCs. However, bypassing the TM might not be enough in all patients as these distal components of the conventional outflow pathway also offer resistance.
Provocative testing can assist the surgeon by suggesting the location of unobstructed CCs and aqueous veins.28 Another useful method to assess optimal intraoperative confirmation of accurate device placement may be through the observation of the episcleral venous fluid wave while performing canal-based glaucoma surgery (ab-interno trabeculotomy). This has been postulated to correlate with the extent of surgically induced canal cleavage, and may be a prognostic indicator for surgical success.29 The wave-seen as blanching of injected balanced salt solution through the venous plexus was confined to the surgical site or to the type of device, thus confirming in vivo the long-held belief based on laboratory work by Chandler, Grant, Epstein, and others that there is very little circumferential flow in SC. Therefore, the episcleral venous fluid wave may confirm that a canal-based procedure has been successful in creating communication to the CCs and shows the CCs are at least anatomically functioning in that area. It needs to be demonstrated how well the extent of the episcleral fluid wave could predict clinical success. Also, Grant's work was done on normal eyes and whether his findings apply to eyes with glaucoma is controversial.5 At present, the importance of having open and functioning CCs that drain aqueous out of SC has not been well explored within this hypothesis.
Surgical technique
Using a manual inserter, the device is delivered through the TM into SC with an ab-interno gonioscopically-guided approach. Once the cannula penetrates the TM, the microstent is advanced smoothly along the circumference of SC. When approximately 1 mm of the proximal end protrudes from the TM into the AC the microstent was released from the delivery system.
The proximal 1 mm inlet of the microstent remains outside SC in the anterior chamber in order to provide the TM bypass, permitting direct communication with SC while the portion within SC provides persistent canal dilation and access to CC ostia.
Safety and efficacy
Scarce published data are available at present. Saheb et al. (communication) used scanning electron microscopy (SEM) to perform structural assessment of the effect of the Hydrus device (iStent, Glaukos Corp., Laguna Hills, CA) implantation on the outer wall of SC. SEM images showed patency of CC ostia and minor changes in the outer wall of SC. In an ocular biocompatibility study, Grierson et al.,30 implanted the Hydrus device in the eyes of eight rabbits and the SC of two monkeys. They found minimal inflammatory response compared to sham eyes after up to 26 weeks post implantation.
Camras et al.,31 compared changes in outflow facility of human anterior segments after implantation of a Hydrus microstent to control eyes at 4 different pressure levels (10, 12, 30, 40 mmHg). In nine eyes, they reported a 92% increase in outflow facility from 0.24 ± 0.02 to 0.46 ± 0.07 uL/min/mmHg after implantation of the device compared to no change in outflow facility in control eyes (P = 0.03). Of interest, the only pressure level at which they did not find a change in outflow facility was the 10 mmHg one. In a subsequent study, Hays et al. (IOVS 2014), and using the same methodology, the same group compared outflow facility after implantation of the Hydrus device vs. 2 trabecular micro-bypasses (iStent, Glaukos Corp., Laguna Hills, CA) in the fellow eye. They reported a greater outflow facility increase with the Hydrus (73%) versus the iStent (34%).
Johnstone et al.,32 evaluated the immediate morphological changes on the outer wall of SC and CC ostia after implantation of two different sizes of the Hydrus microstent using SEM in dissected 12 human eyes. They found that eyes receiving the 8 mm and 15 mm device both maintained CC ostia patency but a smaller area of external wall contact was evident from insertion of the 8 mm microstent. They concluded that the higher profile 15 mm design, which was in more contact with the outer wall of SC, would have the greater potential to obstruct CC ostia.
Ahmed et al., (communication) presented 6-month outcomes of 28 eyes with mild-to-moderate OAG after combined phacoemulsification and Hydrus implantation. Baseline IOP was 17.9 ± 4.1 mmHg on 2.4 ± 1.0 glaucoma drops, and washed-out IOP was 29.9 ± 5.8 mmHg on the day of surgery. IOP and number of glaucoma drops were significantly reduced to 15.3 ± 2.3 mmHg and 0.1 ± 0.4, respectively.
Surgical complications
Ahmed et al. in 28 eyes at 6 months of follow-up reported subconjunctival hemorrhage (n = 1), hyphema (n = 1), and peripheral anterior synechiae (n = 2). Based on these early reports, the Hydrus device seems to be a relatively safe procedure. The majority of complications are related to malposition of the implant and stent obstruction.
Conclusion
No published clinical data are available for the Hydrus device. Performed together with cataract surgery it seems to be a safe procedure, but it remains unclear how much of an additional IOP-lowering effect it offers compared to cataract surgery alone. Its main advantage appears to be the relatively simple surgical technique as well as in the reduction in hypotensive medication use.
CONCLUSION AND FUTURE DIRECTIONS
Although trabeculectomy and to a lesser extent, glaucoma drainage devices remain the standard incisional glaucoma surgical procedures, the search continues apace for a procedure that can effectively and safely lower IOP. The surgical options for concurrently managing cataract and glaucoma have expanded in recent years. Canal-based techniques and devices are still in the initial stage of clinical experience and lacking widespread use. Despite a wide body of evidence for canaloplasty, there is currently no published randomized comparing this procedure with trabeculectomy. Clinical trials for the Hydrus device are currently lacking.
These new procedures appear to be less effective than trabeculectomy. Therefore, their role in glaucoma management remains the subject of debate. While there is enough long-term data to suggest that canaloplasty may be worthwhile as a stand-alone and safe glaucoma surgery, the Hydrus device, however, may most appropriately be combined with phacoemulsification in patients with mild glaucomatous damage in whom low levels of IOP are not needed.
Future studies need to address factors related to the optimal extent of SC surgery, such as the number of inserted devices, the length and placement site of a device and other modifiable factors that can obtain the optimal increase in conventional outflow. Another unknown is whether SC-based procedures may help a patient whose CCs are atrophied and not functioning. New imaging devices may provide valuable information in this regard. Finally, as longer-term results become available for canal-based techniques, their cost-effectiveness profile and quality-of-life impact needs also to be evaluated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: A review. JAMA. 2014;311:1901–11. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansouri K, Medeiros FA, Weinreb RN. Global rates of glaucoma surgery. Graefes Arch Clin Exp Ophthalmol. 2013;251:2609–15. doi: 10.1007/s00417-013-2464-7. [DOI] [PubMed] [Google Scholar]
- 3.Mansouri K, Orguel S, Mermoud A, Haefliger I, Flammer J, Ravinet E, et al. Quality of diurnal intraocular pressure control in primary open – Angle patients treated with latanoprost compared with surgically treated glaucoma patients: A prospective trial. Br J Ophthalmol. 2008;92:332–6. doi: 10.1136/bjo.2007.123042. [DOI] [PubMed] [Google Scholar]
- 4.Krasnov MM. Externalization of Schlemm's canal (sinusotomy) in glaucoma. Br J Ophthalmol. 1968;52:157–61. doi: 10.1136/bjo.52.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 6.Shaarawy T, Nguyen C, Schnyder C, Mermoud A. Five year results of viscocanalostomy. Br J Ophthalmol. 2003;87:441–5. doi: 10.1136/bjo.87.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, et al. Canaloplasty: Circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: Two-year interim clinical study results. J Cataract Refract Surg. 2009;35:814–24. doi: 10.1016/j.jcrs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Shingleton B, Tetz M, Korber N. Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: One-year results. J Cataract Refract Surg. 2008;34:433–40. doi: 10.1016/j.jcrs.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 9.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Canaloplasty for primary open-angle glaucoma: Long-term outcome. Br J Ophthalmol. 2010;94:1478–82. doi: 10.1136/bjo.2009.163170. [DOI] [PubMed] [Google Scholar]
- 10.Matlach J, Freiberg FJ, Leippi S, Grehn F, Klink T. Comparison of phacotrabeculectomy versus phacocanaloplasty in the treatment of patients with concomitant cataract and glaucoma. BMC Ophthalmol. 2013;13:1. doi: 10.1186/1471-2415-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arthur SN, Cantor LB, WuDunn D, Pattar GR, Catoira-Boyle Y, Morgan LS, et al. Efficacy, safety, and survival rates of IOP-lowering effect of phacoemulsification alone or combined with canaloplasty in glaucoma patients. J Glaucoma. 2014;23:316–20. doi: 10.1097/IJG.0b013e3182741ca9. [DOI] [PubMed] [Google Scholar]
- 12.Matthaei M, Steinberg J, Wiermann A, Richard G, Klemm M. Canaloplasty: A new alternative in non-penetrating glaucoma surgery. Ophthalmologe. 2011;108:637–43. doi: 10.1007/s00347-010-2305-6. [DOI] [PubMed] [Google Scholar]
- 13.Lewis RA, von Wolff K, Tetz M, Koerber N, Kearney JR, Shingleton BJ, et al. Canaloplasty: Three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37:682–90. doi: 10.1016/j.jcrs.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 14.Ayyala RS, Chaudhry AL, Okogbaa CB, Zurakowski D. Comparison of surgical outcomes between canaloplasty and trabeculectomy at 12 months' follow-up. Ophthalmology. 2011;118:2427–33. doi: 10.1016/j.ophtha.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Bull H, von Wolff K, Körber N, Tetz M. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol. 2011;249:1537–45. doi: 10.1007/s00417-011-1728-3. [DOI] [PubMed] [Google Scholar]
- 16.Fujita K, Kitagawa K, Ueta Y, Nakamura T, Miyakoshi A, Hayashi A. Short-term results of canaloplasty surgery for primary open-angle glaucoma in Japanese patients. Case Rep Ophthalmol. 2011;2:65–8. doi: 10.1159/000324808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klink T, Panidou E, Kanzow-Terai B, Klink J, Schlunck G, Grehn FJ. Are there filtering blebs after canaloplasty? J Glaucoma. 2012;21:89–94. doi: 10.1097/IJG.0b013e3182027905. [DOI] [PubMed] [Google Scholar]
- 18.Brüggemann A, Despouy JT, Wegent A, Müller M. Intraindividual comparison of Canaloplasty versus trabeculectomy with mitomycin C in a single-surgeon series. J Glaucoma. 2013;22:577–83. doi: 10.1097/IJG.0b013e318255bb30. [DOI] [PubMed] [Google Scholar]
- 19.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Clinical evaluation of the aqueous outflow system in primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis Sci. 2010;51:1498–504. doi: 10.1167/iovs.09-4327. [DOI] [PubMed] [Google Scholar]
- 20.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Channelography: Imaging of the aqueous outflow pathway with flexible microcatheter and fluorescein in canaloplasty. Klin Monbl Augenheilkd. 2009;226:245–8. doi: 10.1055/s-0028-1109305. [DOI] [PubMed] [Google Scholar]
- 21.Grieshaber MC, Schoetzau A, Flammer J, Orgül S. Postoperative microhyphema as a positive prognostic indicator in canaloplasty. Acta Ophthalmol. 2013;91:151–6. doi: 10.1111/j.1755-3768.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- 22.Mansouri K, Tran HV, Ravinet E, Mermoud A. Comparing deep sclerectomy with collagen implant to the new method of very deep sclerectomy with collagen implant: A single-masked randomized controlled trial. J Glaucoma. 2010;19:24–30. doi: 10.1097/IJG.0b013e3181a2fa46. [DOI] [PubMed] [Google Scholar]
- 23.Palmiero PM, Aktas Z, Lee O, Tello C, Sbeity Z. Bilateral Descemet membrane detachment after canaloplasty. J Cataract Refract Surg. 2010;36:508–11. doi: 10.1016/j.jcrs.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Jaramillo A, Foreman J, Ayyala RS. Descemet membrane detachment after canaloplasty: Incidence and management. J Glaucoma. 2014;23:351–4. doi: 10.1097/IJG.0b013e318279ca7f. [DOI] [PubMed] [Google Scholar]
- 25.Brandao LM, Orgul S, Grieshaber MC. Hemorrhagic descemet membrane detachment after classic canaloplasty. Klin Monbl Augenheilkd. 2014;231:348–50. doi: 10.1055/s-0034-1368273. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75:365–83. doi: 10.1016/0002-9394(73)91145-8. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone MA, Grant WM. Microsurgery of Schlemm's canal and the human aqueous outflow system. Am J Ophthalmol. 1973;76:906–17. doi: 10.1016/0002-9394(73)90080-9. [DOI] [PubMed] [Google Scholar]
- 28.Ashton N, Smith R. Anatomical study of Schlemm's canal and aqueous veins by means of neoprene casts. III. Arterial relations of Schlemm's cana. Br J Ophthalmol. 1953;37:577–86. doi: 10.1136/bjo.37.10.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellman RL, Grover DS. Episcleral venous fluid wave: Intraoperative evidence for patency of the conventional outflow system. J Glaucoma. 2014;23:347–50. doi: 10.1097/IJG.0b013e31827a06d8. [DOI] [PubMed] [Google Scholar]
- 30.Grierson I, Saheb H, Kahook MY, Johnstone MA, Ahmed II, Schieber AT, et al. A Novel Schlemm's canal scaffold: Histologic observations. J Glaucoma. 2013 doi: 10.1097/IJG.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 31.Camras LJ, Yuan F, Fan S, Samuelson TW, Ahmed IK, Schieber AT, et al. A novel Schlemm's Canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2012;53:6115–21. doi: 10.1167/iovs.12-9570. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone MA, Saheb H, Ahmed II, Samuelson TW, Schieber AT, Toris CB. Effects of a Schlemm canal scaffold on collector channel ostia in human anterior segments. Exp Eye Res. 2014;119:70–6. doi: 10.1016/j.exer.2013.12.011. [DOI] [PubMed] [Google Scholar]


