Abstract
We are currently in the midst of a surge in interest in glaucoma surgery. Novel pathways for reducing intraocular pressure (IOP) have been tried with various levels of success over the last few years. While the trabecular bypass and suprachoroidal approaches have captured much of the attention, filtering aqueous into the sub-conjunctival space remains the gold standard for lowering IOP. This review attempts to focus on current research in surgical methods to enhance filtration by potentially improving on tried and tested methods like the trabeculectomy, deep sclerectomy, and tube surgeries.
Keywords: Deep Sclerectomy, Ex-PRESS, Glaucoma Surgery, Trabeculectomy
INTRODUCTION
It is safe to say that there has never been such interest in glaucoma surgery as what we are witnessing today.1 Glaucoma surgery that has been relatively stagnant for many decades is now being rejuvenated with the introduction of new technologies.2 Each of these new surgical methods comes with the promise of improved surgical efficiency and safety compared to the eternal gold standard, trabeculectomy.3 Claim is one thing though, and scientific reality is another. Most of these technologies are still undergoing the early stages of clinical testing, with the evident paucity of published peer-reviewed studies to support an established place for these technologies in the routine practices of glaucoma surgeons and general ophthalmologists. In fact, it is extremely hard at this point in time to make accurate evaluations of the utility of most of these technologies and devices. This chapter attempts to list what is commercially available, hitherto, to discuss the current literature and perhaps, to speculate on future trends and possible positioning of these new methods in the expanding armamentarium of the ophthalmic surgeon.
TERMINOLOGY
There have been attempts to group most of these devices and technologies under headings like minimally invasive, minimally effective, and conjunctiva-sparing surgery4,5,6 None of these terms are accurate for a number of reasons. Calling these methods and devices minimally invasive implies that they are less invasive than traditional and well-established glaucoma surgical methods like the trabeculectomy, nonpenetrating glaucoma surgery and tubes. These claims are not supported by evidence-based science, to date. In some of these novel methods, the conjunctiva is opened, thus compromising at least one quadrant, which would possibly go against the “noninvasive” terminology. Furthermore, each new methodology being introduced since the 19th century has always carried with it the claim of being less invasive, and as such the terminology is abused and exhausted.7,8
Calling it minimally effective would first group the technologies in one single “effectiveness” bracket, which would not be unbiased toward or against certain technologies, and would imply that we have enough knowledge on effectiveness of such devices and methods, when we in fact do not possess such knowledge.
CLASSIFICATION
In our opinion, a safe and relatively accurate way of classifying such technologies would be, to classify them according to their anatomic approach, and thus we can list the technologies as shown in the Figure 1.
Figure 1.

Classification of different approaches to the surgical management of intraocular pressure in glaucoma patients
SUBCONJUNCTIVAL FILTERATION STRATEGY
Ex-PRESS implant
The 2.64 mm stainless steel implant3,9,10,11,12 reduces pressure by diverting aqueous humor from the anterior chamber to the subscleral space after a dissection identical to a trabeculectomy with the exception of avoidance of surgical iridectomy13,14 [Figure 2]. Pressure release is initially the function of the implants length and inner diameter, until scarring starts in full force, and thus the pressure control is dependent on same forces as after trabeculectomy. Multiple studies have found superiority for Ex-PRESS in the initial postoperative period where its standardized filter may be a preventing factor for early hypotony with all its associated complications.15,16
Figure 2.
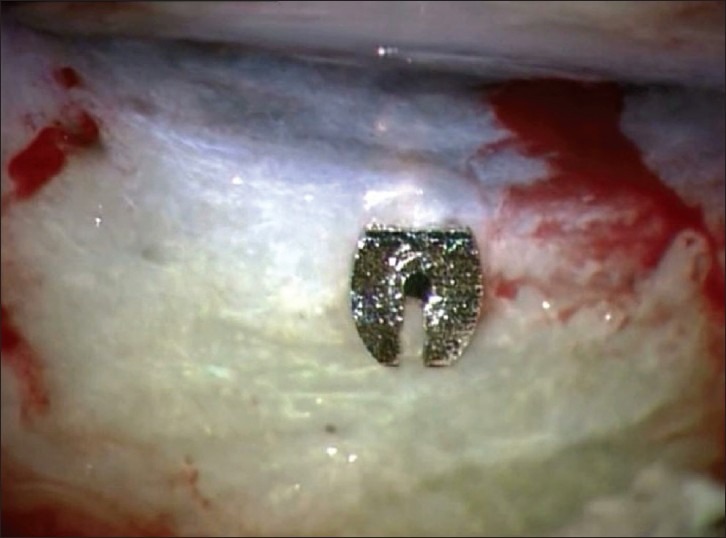
Implanted Ex-PRESS glaucoma drainage device
One other study also suggested that Ex-PRESS offers a faster visual rehabilitation to operated patients compared with trabeculectomy, which is a primordial factor that should always be taken into consideration.17
On the other hand, there are no long-term results reporting on the corneal endothelial effects of Ex-PRESS compared to trabeculectomy, taking in consideration the fact that glaucoma drainage tube studies have shown a progressive long-term reduction of endothelial cells with time.18
One additional factor that should always be taken into account is the economical repercussions of the introduction of a new device into the everyday surgical practice.9,19 Direct cost has a major impact. But is not the only factor that should be taken into consideration. Postoperative care in a procedure that has potential complications and the management of such complications should be factored in. Such studies need to be carried out on national levels as costs vary considerably among different countries and in many cases within the same country itself.
CO2 laser assisted sclerectomy surgery
When manually dissecting the deep corneo-scleral lamellae in deep sclerectomy, there is always the potential for either perforation into the anterior chamber or insufficient tissue removal.20 This is highly dependent on the surgeon's experience and skill.8 To overcome such challenges different kinds of lasers [Figure 3] were used to ablate the deep scleral tissue. Experimental and clinical studies using the excimer laser have also been reported with encouraging preliminary results.21 No comparative studies between laser assisted deep sclerectomy and trabeculectomy are available as yet.
Figure 3.
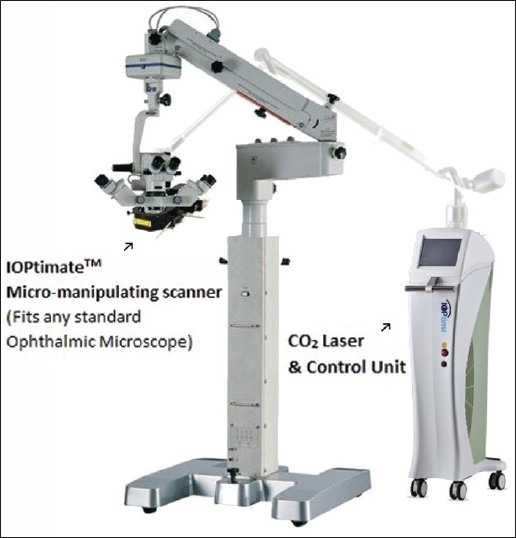
The ioptima CO2laser system
SURGICAL TECHNIQUE
A partial thickness (one-third to one-half) rectangular limbal-based 5 × 5 mm superior scleral flap is dissected at the limbus into the clear cornea [Figure 4].
Figure 4.
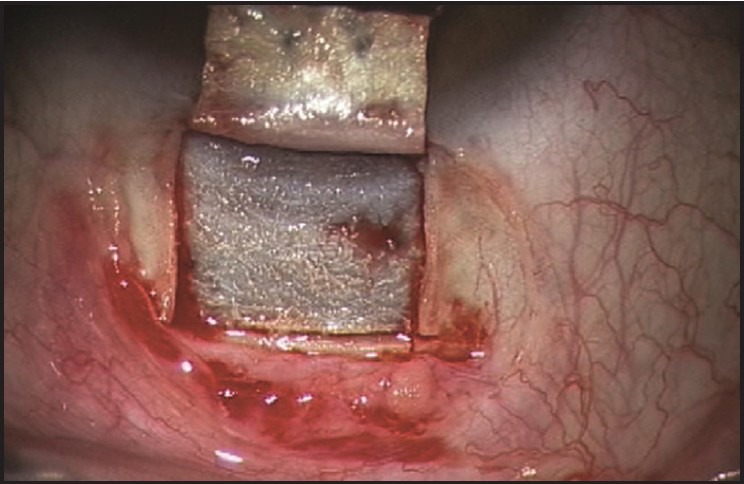
Partial thickness rectangular limbal-based 5 × 5 mm superior scleral flap
The desired scanning area and shape are set; the laser beam is focused, and the area to be treated is visually verified using a red laser aiming beam. The CO2laser beam is then applied over an area including the Schlemm's canal forming the scleral bed [Figure 5].
Figure 5.
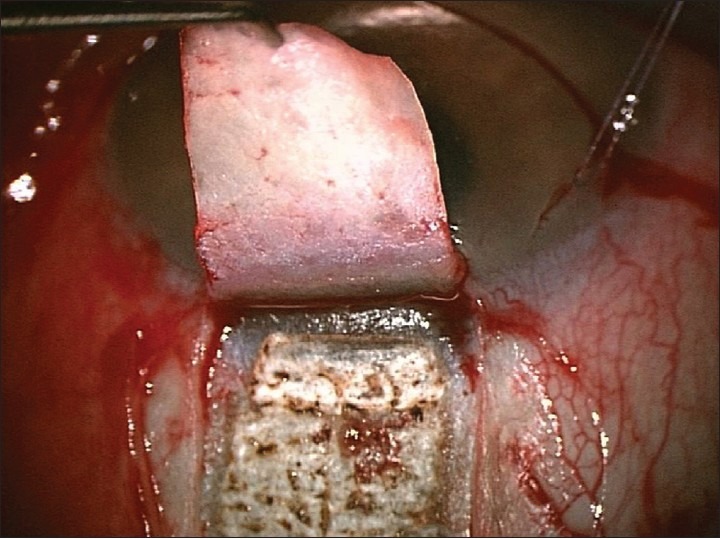
Applied CO2laser beam over scleral area including the Schlemm's canal
The residual charred tissue is wiped away with a sponge and ablation is continued until sufficient percolation is achieved [Figures 6 and 7]. Whereby the fluid absorbs laser's energy and prevents further ablation.
Figure 6.
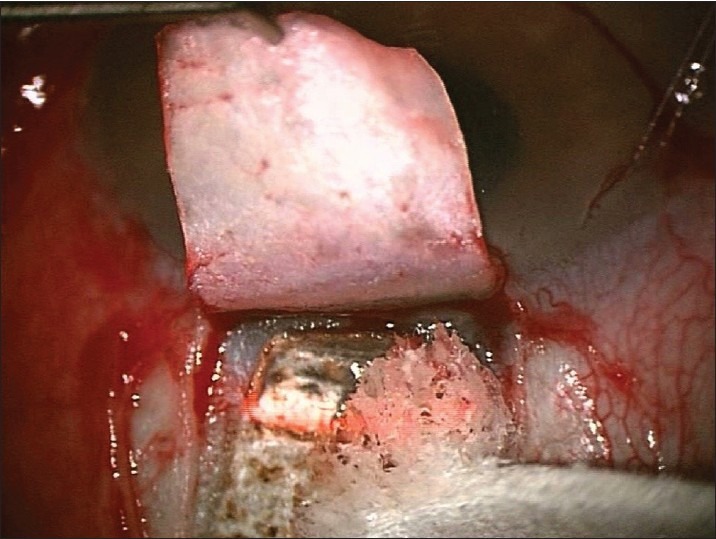
Residual charred tissue wiped away with a sponge
Figure 7.
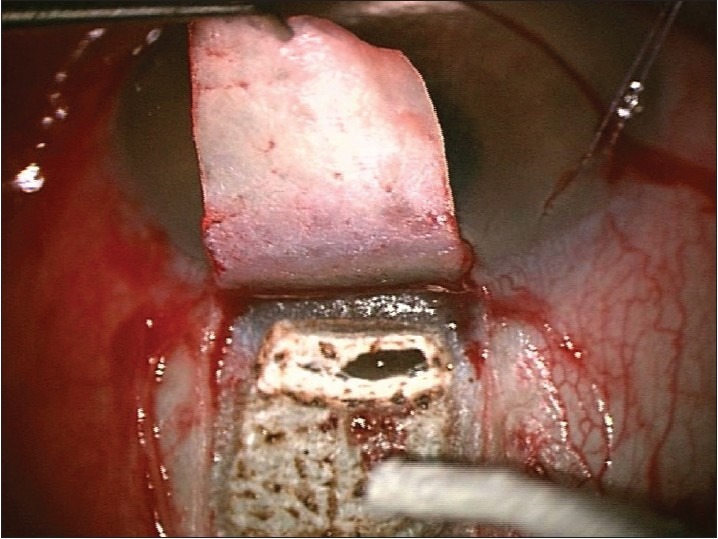
Starting of aqueous percolation after a sponge is used to remove ablated tissue
The scleral flap is repositioned and secured with 2 interrupted 10/0 nylon sutures and a high-molecular-weight ophthalmic viscosurgical material (Healon 5®, Abbott Medical Optics, Santa Ana, CA, United States) is applied beneath the flap or an intrascleral implant (Aquaflow collagen implant from STAAR, Nidau, Switzerland for example). The conjunctiva is adequately secured with 9/0 vicryl continuous suture.
The reported results are sufficiently promising to suggest that the CO2laser assisted sclerectomy surgery (CLASS) is an easily learned and simple surgical procedure to perform, which appears to be relatively safe and effective in the short and intermediate term.22,23 Randomized controlled trials comparing CLASS to manual technique are needed to improve our knowledge and understanding on how to place this new technology among our existent options.
The CO2laser has certain properties that offer significant advantages to facilitate deep sclerectomy. These include coagulation of bleeding vessels, photo-ablation of dry tissues, and absorption of the laser energy by percolating aqueous humor. As the emitted radiation is readily absorbed by the aqueous humor, the trabecular meshwork is potentially protected from the laser energy. Thus, perforation of the thin trabeculo - Descemet membrane during Descemet stripping (DS), which is the most frequent intraoperative complication of manual DS, is potentially minimized.
INNFOCUS MICROSHUNT
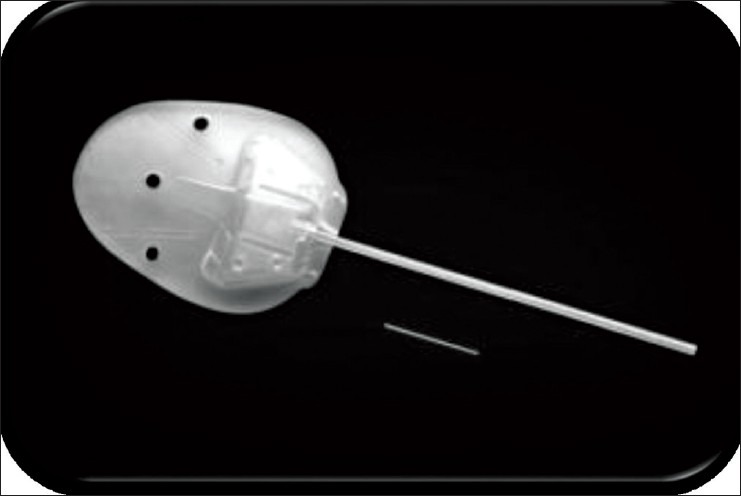
The InnFocus MicroShunt (InnFocus, Inc., Miami, Florida, United States) consists of a flexible tube with planar fins, which are located approximately halfway down the length of the tube that prevent the device from migrating into the anterior chamber24,26 [Figure 8] The fins also act as a “stopper” to minimize aqueous humor leakage around the tube and to reduce the likelihood of postoperative hypotony. The 70 mm diameter lumen of the device serves as a flow restrictor with the intention of avoiding hypotony yet dampening intraocular pressure (IOP) spikes postoperatively.
Figure 8.

Schematic of the InnFocus MicroShunt™. All units are in mm
A highly biocompatible biomaterial, poly (styrene-block-isobutylene-block-styrene) (SIBS),24 is possibly a key feature of the InnFocus MicroShunt and is one of the three elastomers, which have been approved by the US Food and Drug Administration for use in the fabrication of medical devices intended for long-term implant applications. The SIBS material is biostable, and its inert nature evokes minimal inflammation and scar formation. Initial studies of glaucoma drainage surgery in rabbit eyes compared the tissue response of tubes made from SIBS to silicone rubber tubes. Silicone rubber stimulates inflammation and promotes the development of a fibrotic capsule around the device that quickly becomes non-functional. In contrast, SIBS tubes demonstrated minimal encapsulation with continuous aqueous humor flow after 1-year.
Implantation of the InnFocus MicroShunt™ is relatively simple and requires only the dissection of a sub-conjunctival/Tenon's capsule flap and the development of a small scleral pocket to permit the passage of a needle tract into which the tube is inserted and the fins on the MicroShunt are wedged [Figure 9]. The procedure is performed ab externo and does not require the use of a gonioscope or viscoelastic fluid. Unlike trabeculectomy, neither sclerostomy nor iridectomy is necessary, and the only scleral trauma is the needle tract.
Figure 9.
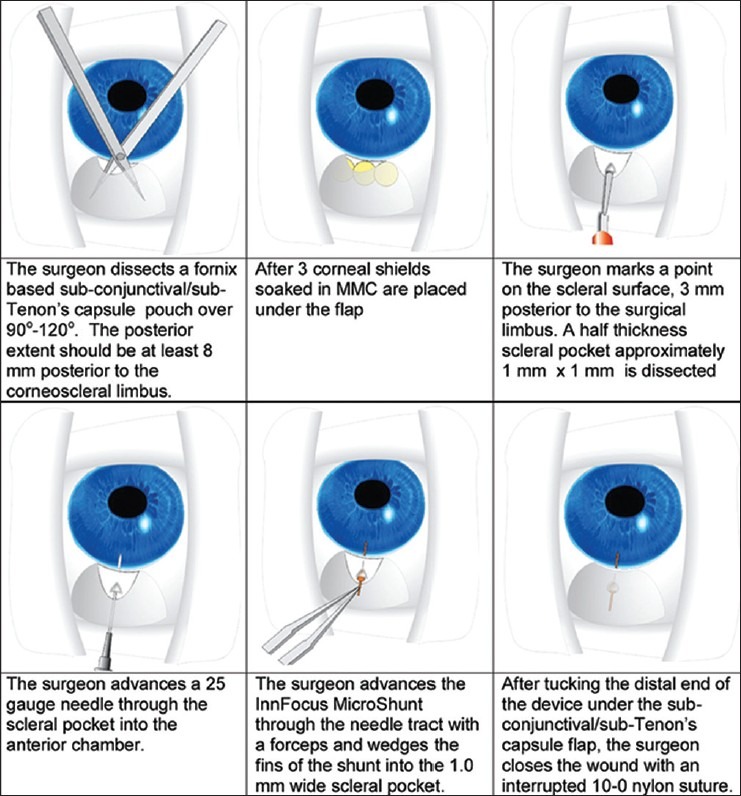
Implantation of the InnFocus MicroShunt
AB INTERNO APPROACH
The AqueSys XEN glaucoma implant
The XEN implant is a hydrophilic tube composed of a porcine gelatin and cross-linked with glutaraldehyde [Figures 10–12]. It decreases IOP by creating an outflow pathway from the anterior chamber to the sub-conjunctival space through which the aqueous humor can flow.
Figure 10.

The XEN implant is a hydrophilic tube composed of a porcine gelatin and cross-linked with glutaraldehyde
Figure 12.

The XEN device imaged by anterior segment optical coherence tomography revealing the sub-conjunctival location of the implant with extension into the anterior chamber anterior to the iris
Figure 11.
The XEN device adjacent to an Ahmad glaucoma drainage device for size comparison
During the implantation procedure, the implant hydrates and swells in place to become a soft nonmigrating drainage channel that is tissue conforming.
Procedure
The device is implanted ab interno through a small clear corneal incision and introduced through sclera and into the subtenon space with a small gauge needle [Figure 13]. The mechanism of action of the XEN glaucoma implant postimplantation is fundamentally consistent with other full thickness surgical treatments for glaucoma such as valved and non-valved tube shunts and full thickness trabeculectomies, which bypass all potential outflow obstructions. It maintains a micro-fistula between the anterior chamber and the sub-conjunctival space while the tissues surrounding the implant heal naturally. There is no need to perform an iridotomy and by introducing only a minimal amount of trauma, subsequent inflammation and fibrosis is potentially minimized and many of the complications associated with more invasive procedures such as trabeculectomy and tube shunt implantation can perhaps be avoided.
Figure 13.

The needle of the XEN inserter is designed to be minimally traumatizing by utilizing a small 25 gauge needle
CONCLUSION
There is undoubtedly a surge in interest in glaucoma surgery. As innovators try to explore routes for aqueous filtration, the sub-conjunctival route remains firmly on its throne. In fact, the new modalities that have been mentioned before might even help to enforce its position as a route of choice. Clinical research in the next few years will aim to include multicenter studies with larger numbers of patients and longer follow-up so as to substantiate the long-term safety and efficacy of these glaucoma surgical procedures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Loewen NA, Schuman JS. There has to be a better way: Evolution of internal filtration glaucoma surgeries. Br J Ophthalmol. 2013;97:1228–9. doi: 10.1136/bjophthalmol-2013-303237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salim S. Current variations of glaucoma filtration surgery. Curr Opin Ophthalmol. 2012;23:89–95. doi: 10.1097/ICU.0b013e32834ff401. [DOI] [PubMed] [Google Scholar]
- 3.Freedman J. What is new after 40 years of glaucoma implants. J Glaucoma. 2010;19:504–8. doi: 10.1097/IJG.0b013e3181ca7850. [DOI] [PubMed] [Google Scholar]
- 4.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. doi: 10.1097/ICU.0b013e32834ff1e7. [DOI] [PubMed] [Google Scholar]
- 5.Filippopoulos T, Rhee DJ. Novel surgical procedures in glaucoma: Advances in penetrating glaucoma surgery. Curr Opin Ophthalmol. 2008;19:149–54. doi: 10.1097/ICU.0b013e3282f4f49e. [DOI] [PubMed] [Google Scholar]
- 6.Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR, et al. Novel glaucoma procedures: A report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:1466–80. doi: 10.1016/j.ophtha.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Shaarawy T. Surgery for glaucoma in the 21 st century. Br J Ophthalmol. 2003;87:250. doi: 10.1136/bjo.87.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaarawy T, Flammer J. Pro: Non-penetrating glaucoma surgery - A fair chance. Graefes Arch Clin Exp Ophthalmol. 2003;241:699–702. doi: 10.1007/s00417-003-0715-8. [DOI] [PubMed] [Google Scholar]
- 9.Buys YM. Trabeculectomy versus ExPRESS shunt surgery in residency training. Surv Ophthalmol. 2012;57:375–8. doi: 10.1016/j.survophthal.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry M, Grover S, Baisakhiya S, Bajaj A, Bhatia MS. Artificial drainage devices for glaucoma surgery: An overview. Nepal J Ophthalmol. 2012;4:295–302. doi: 10.3126/nepjoph.v4i2.6547. [DOI] [PubMed] [Google Scholar]
- 11.Freidl KB, Moster MR. ExPRESS shunt surgery: Preferred glaucoma surgery in residency training? Surv Ophthalmol. 2012;57:372–5. doi: 10.1016/j.survophthal.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt W, Kastner C, Sternberg K, Allemann R, Löbler M, Guthoff R, et al. New concepts for glaucoma implants - Controlled aqueous humor drainage, encapsulation prevention and local drug delivery. Curr Pharm Biotechnol. 2013;14:98–111. [PubMed] [Google Scholar]
- 13.Salim S. Ex-PRESS glaucoma filtration device-surgical technique and outcomes. Int Ophthalmol Clin. 2011;51:83–94. doi: 10.1097/IIO.0b013e31821e5b82. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Zhou M, Huang W, Zhang X. Ex-PRESS implantation versus trabeculectomy in uncontrolled glaucoma: A meta-analysis. PLoS One. 2013;8:e63591. doi: 10.1371/journal.pone.0063591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salim S, Du H, Boonyaleephan S, Wan J. Surgical outcomes of the Ex-PRESS glaucoma filtration device in African American and white glaucoma patients. Clin Ophthalmol. 2012;6:955–62. doi: 10.2147/OPTH.S32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahan E, Ben Simon GJ, Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: A prospective, randomised study. Eye (Lond) 2012;26:703–10. doi: 10.1038/eye.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltran-Agullo L, Trope GE, Jin Y, Wagschal LD, Jinapriya D, Buys YM. Comparison of visual recovery following Ex-PRESS versus trabeculectomy: Results of a prospective randomized controlled trial. J Glaucoma. 2013 doi: 10.1097/IJG.0b013e31829e1b68. [DOI] [PubMed] [Google Scholar]
- 18.Mendrinos E, Dosso A, Sommerhalder J, Shaarawy T. Coupling of HRT II and AS-OCT to evaluate corneal endothelial cell loss and in vivo visualization of the Ahmed glaucoma valve implant. Eye (Lond) 2009;23:1836–44. doi: 10.1038/eye.2008.321. [DOI] [PubMed] [Google Scholar]
- 19.Buys YM. Trabeculectomy with ExPRESS: Weighing the benefits and cost. Curr Opin Ophthalmol. 2013;24:111–8. doi: 10.1097/ICU.0b013e32835907a6. [DOI] [PubMed] [Google Scholar]
- 20.Shaarawy T, Mansouri K, Schnyder C, Ravinet E, Achache F, Mermoud A. Long-term results of deep sclerectomy with collagen implant. J Cataract Refract Surg. 2004;30:1225–31. doi: 10.1016/j.jcrs.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 21.Argento C, Sanseau AC, Badoza D, Casiraghi J. Deep sclerectomy with a collagen implant using the excimer laser. J Cataract Refract Surg. 2001;27:504–6. doi: 10.1016/s0886-3350(00)00609-x. [DOI] [PubMed] [Google Scholar]
- 22.Geffen N, Ton Y, Degani J, Assia EI. CO2 laser-assisted sclerectomy surgery, part II: Multicenter clinical preliminary study. J Glaucoma. 2012;21:193–8. doi: 10.1097/IJG.0b013e3181f7b14f. [DOI] [PubMed] [Google Scholar]
- 23.Ton Y, Geffen N, Kidron D, Degani J, Assia EI. CO2 laser-assisted sclerectomy surgery part I: Concept and experimental models. J Glaucoma. 2012;21:135–40. doi: 10.1097/IJG.0b013e31820e2ccd. [DOI] [PubMed] [Google Scholar]
- 24.Pinchuk L, Wilson GJ, Barry JJ, Schoephoerster RT, Parel JM, Kennedy JP. Medical applications of poly (styrene-block-isobutylene-block-styrene) ("SIBS") Biomaterials. 2008;29:448–60. doi: 10.1016/j.biomaterials.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 25.Arrieta EA, Aly M, Parrish R, Dubovy S, Pinchuk L, Kato Y, et al. Clinicopathologic correlations of poly-(styrene-b-isobutylene-b-styrene) glaucoma drainage devices of different internal diameters in rabbits. Ophthalmic Surg Lasers Imaging. 2011;42:338–45. doi: 10.3928/15428877-20110603-01. [DOI] [PubMed] [Google Scholar]
- 26.Acosta AC, Espana EM, Yamamoto H, Davis S, Pinchuk L, Weber BA, et al. A newly designed glaucoma drainage implant made of poly (styrene-b-isobutylene-b-styrene): Biocompatibility and function in normal rabbit eyes. Arch Ophthalmol. 2006;124:1742–9. doi: 10.1001/archopht.124.12.1742. [DOI] [PubMed] [Google Scholar]


