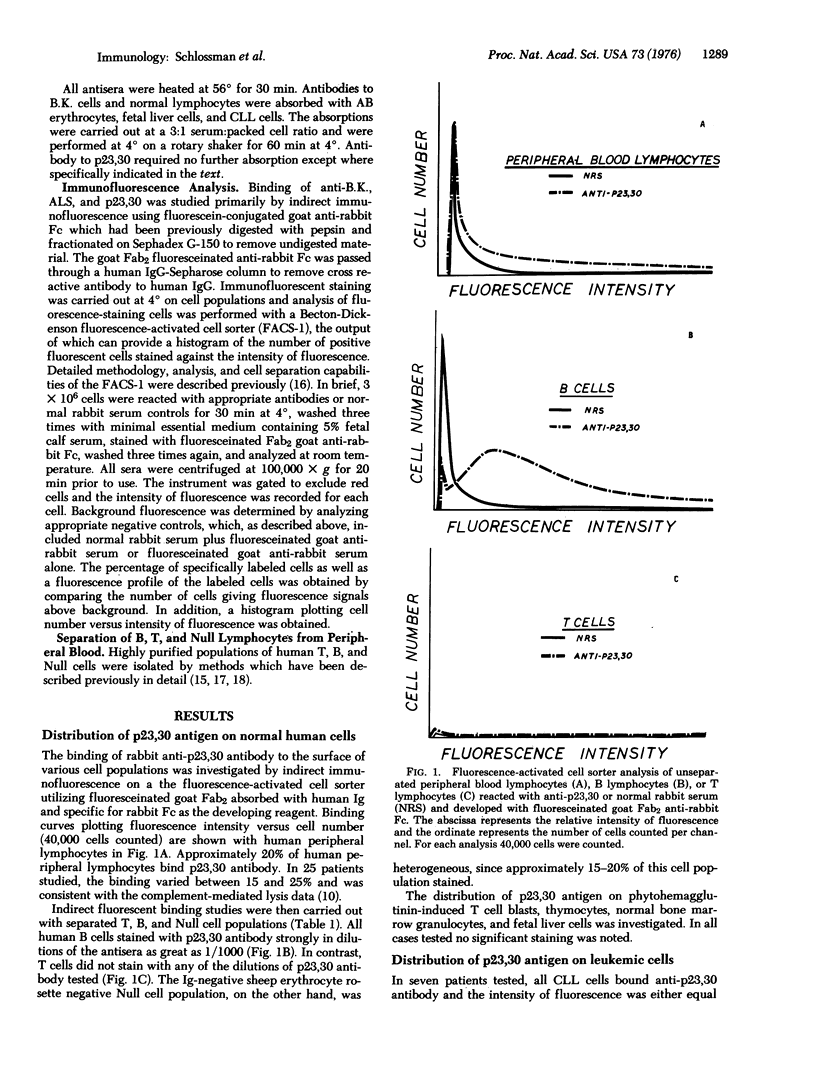
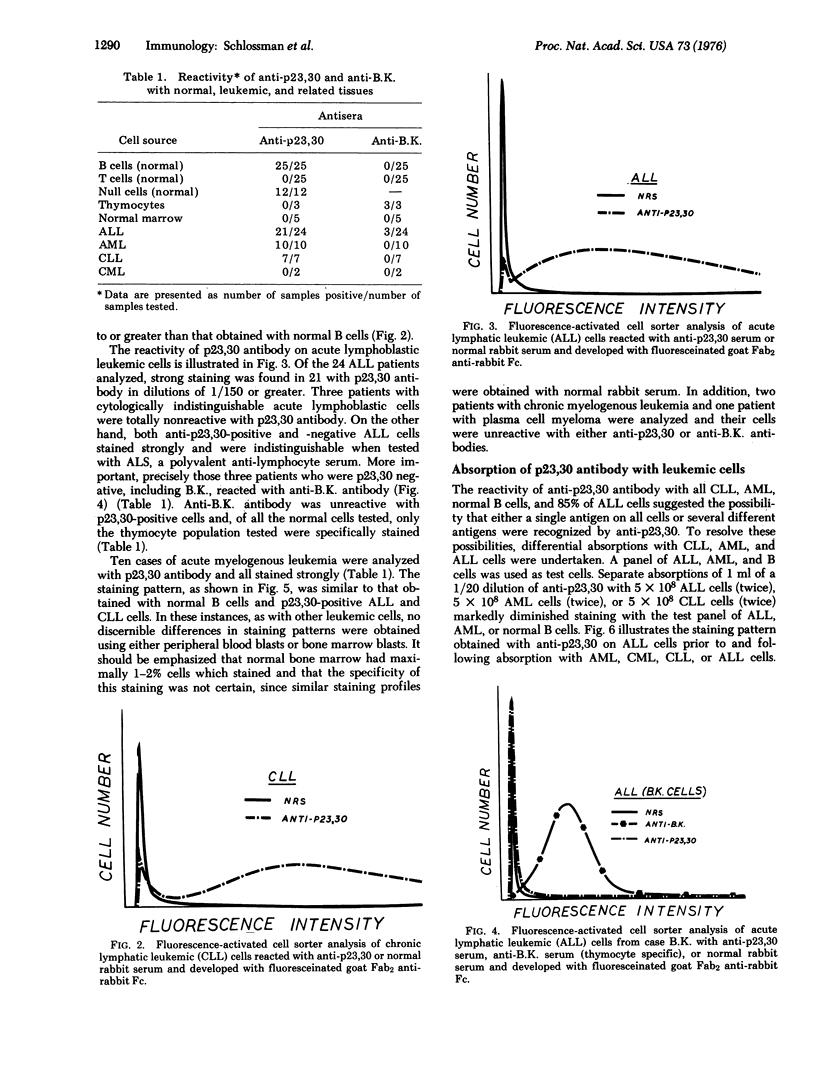
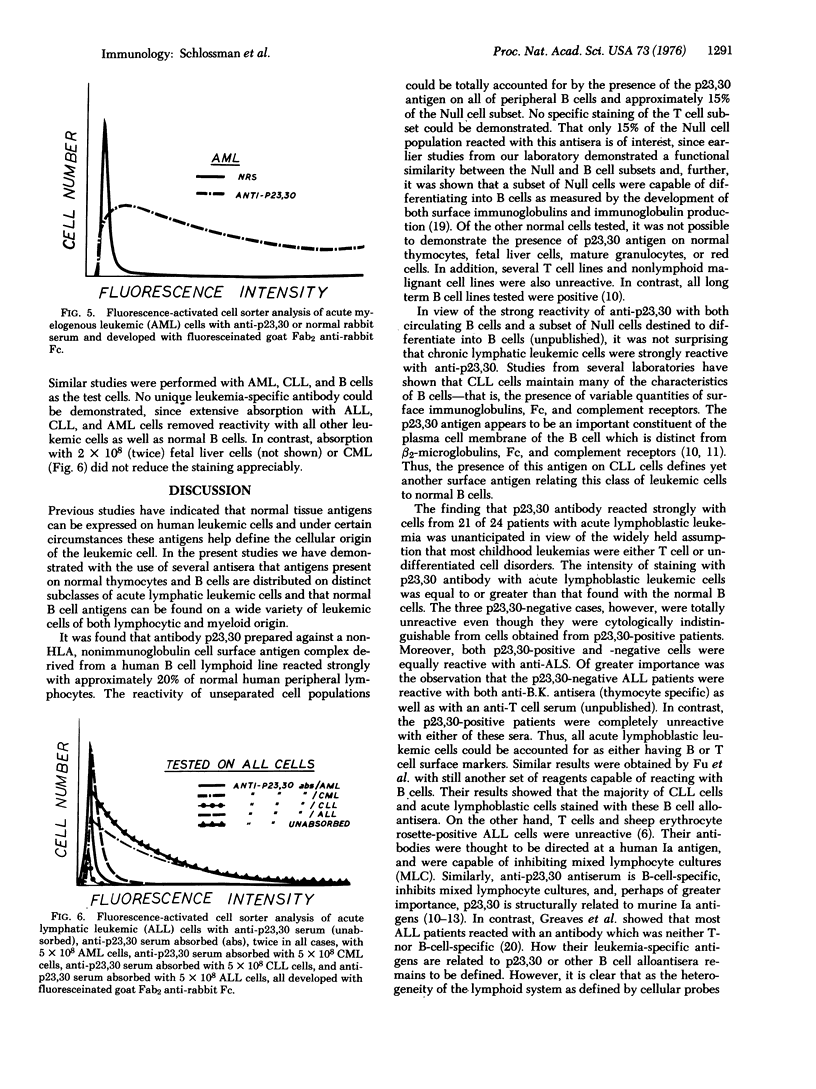
Abstract
Antiserum to a glycoprotein antigen complex of 23,000 and 30,000 dalton subunits (p23,30), isolated and purified from a human lymphoblastoid B cell line, was shown to be highly specific for human bursal-equivalent-processed (B) cells, reactive with 15-20% of human Null cells, but completely unreactive with human thymus-processed (T) cells. The p23,30 antigen is widely distributed on chronic lymphatic leukemic cells, 85% of acute lymphatic leukemic cells, all acute myelogenous leukemic cells, but not on chronic myelogenous leukemic cells. A rabbit antiserum specific for normal human thymocytes has also been prepared; it is reactive only with precisely that subset of acute lymphatic leukemic cells (15%) whose members do not have p23,30 on their surfaces.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J., Long J. C. Cell-surface immunoglobulins in chronic lymphocytic leukemia and allied disorders. Am J Med. 1973 Aug;55(2):184–191. doi: 10.1016/0002-9343(73)90167-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Borella L., Sen L. T cell surface markers on lymphoblasts from acute lymphocytic leukemia. J Immunol. 1973 Oct;111(4):1257–1260. [PubMed] [Google Scholar]
- Broome J. D., Zucker-Franklin D., Weiner M. S., Bianco C., Nussenzweig V. Leukemic cells with membrane properties of thymus-derived (T) lymphocytes in a case of Sézary's syndrome: morphologic and immunologic studies. Clin Immunol Immunopathol. 1973 Apr;1(3):319–329. doi: 10.1016/0090-1229(73)90049-4. [DOI] [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Indications of the thymus-derived nature of the proliferating cells in six patients with Sézary's syndrome. N Engl J Med. 1973 Aug 16;289(7):341–344. doi: 10.1056/NEJM197308162890703. [DOI] [PubMed] [Google Scholar]
- Brown G., Greaves M. F., Lister T. A., Rapson N., Papamichael M. Expression of human T and B lymphocyte cell-surface markers on leukaemic cells. Lancet. 1974 Sep 28;2(7883):753–755. doi: 10.1016/s0140-6736(74)90945-3. [DOI] [PubMed] [Google Scholar]
- Chess L., Levine H., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. VI. Further characterization of the surface Ig negative, E rosette negative (null cell) subset. J Immunol. 1975 Dec;115(6):1483–1487. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Dickler H. B., Siegal F. P., Bentwich Z. H., Kunkel H. G. Lymphocyte binding of aggregated IgG and surface Ig staining in chronic lymphocytic leukaemia. Clin Exp Immunol. 1973 May;14(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Kunkel H. G. The occurrence of the HL-B alloantigens on the cells of unclassified acute lymphoblastic leukemias. J Exp Med. 1975 Nov 1;142(5):1334–1338. doi: 10.1084/jem.142.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Brown G., Rapson N. T., Lister T. A. Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol. 1975 May;4(1):67–84. doi: 10.1016/0090-1229(75)90041-0. [DOI] [PubMed] [Google Scholar]
- Knapp W., Bolhuis R. L., Rádl J., Hijmans W. Independent movement of IgD and IgM molecules on the surface of individual lymphocytes. J Immunol. 1973 Oct;111(4):1295–1298. [PubMed] [Google Scholar]
- Mac Dermott R. P., Chess L., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. V. Isolation and functional analysis of a surface Ig negative, E rosette negative subset. Clin Immunol Immunopathol. 1975 Sep;4(3):415–424. doi: 10.1016/0090-1229(75)90010-0. [DOI] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Herberman R., Frank M. M., Green I. Receptors for complement and immunoglobulin on human leukemic cells and human lymphoblastoid cell lines. J Clin Invest. 1972 Aug;51(8):1933–1938. doi: 10.1172/JCI106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Wernet P., Kunkel H. G., Dupont B., Jersild C., Fu S. M. Recognition by pregnancy serums of non-HL-A alloantigens selectively expressed on B lymphocytes. J Exp Med. 1975 Apr 1;141(4):924–929. [PMC free article] [PubMed] [Google Scholar]



