Abstract
Human umbilical cord mesenchymal stem cells, incorporated into an amnion carrier tubes, were assessed for nerve regeneration potential in a rat nerve defect model. Damaged nerves were exposed to human amnion carriers containing either human umbilical cord mesenchymal stem cell (cell transplantation group) or saline (control group). At 8, 12, 16 and 20 weeks after cell implantation, the sciatic functional index was higher in the cell transplantation group compared with the control group. Furthermore, electrophysiological examination showed that threshold stimulus and maximum stimulus intensity gradually decreased while compound action potential amplitude gradually increased. Hematoxylin-eosin staining showed that regenerating nerve fibers were arranged in nerve tracts in the cell transplantation group and connective tissue between nerve tracts and amnion tissue reduced over time. Gastrocnemius muscle cell diameter, wet weight and restoration ratio were increased. These data indicate that transplanted human umbilical cord mesenchymal stem cells, using the amnion tube connection method, promote restoration of damaged sciatic nerves in rats.
Keywords: umbilical cord mesenchymal stem cells, cell transplantation, amnion, sciatic nerve injury, restoration, stem cells, neural regeneration
Research Highlights
This study verifies that human amnion tissue, injected with human umbilical cord mesenchymal stem cells, contributes to sciatic nerve repair in a rat defect model, resulting in: (1) increased sciatic functional index; (2) gradual decrease in threshold stimulus and maximum stimulus intensity and gradual increase in compound action potential amplitude; (3) arrangement of regenerating nerve fibers into nerve tracts; (4) increased gastrocnemius muscle cell diameter, wet weight and restoration ratio.
INTRODUCTION
Human umbilical cord mesenchymal stem cells are derived from Wharton's jelly and perivascular tissue in the umbilical cord[1,2,3,4,5]. Umbilical cord mesenchymal stem cells are reported not to induce graft versus host reaction, making them favorable as an allogeneic stem cell source. The fibroblast colony-forming unit frequency, proliferation potential and differentiation capacity of umbilical cord mesenchymal stem cells are reported to be greater when compared with bone marrow mesenchymal stem cells. However, expression of HLA-1 and CD106 in umbilical cord mesenchymal stem cells is reported to be lower[6]. Amniotic epithelial cells and neural cells have been reported to exhibit homology and similarity, likely owing to the integration of amniotic epithelial cells into nerve tissues. Amniotic epithelial cells had been used as a tissue engineering scaffold and shown to play an important role in nerve repair, without leading to adverse host response in animal transplantation experiments. Furthermore, amniotic epithelial cells effectively prevented scar invasion and contributed to neural regeneration by inducing human umbilical cord mesenchymal stem cell differentiation into neural cells.
Human amnion tube tissue was used as a carrier for incorporation of human umbilical cord mesenchymal stem cells[7], to investigate their ability to repair peripheral nerve tissue in vivo.
RESULTS
Quantitative analysis of experimental animals
The right sciatic nerves of 80 Sprague-Dawley rats were exposed and a 6–8 mm segment on each nerve was excised. Sciatic nerve stumps were wrapped with human amnion to form a 10 mm amnion tube. Rats were randomly assigned to two groups. In the cell transplantation group, amnion tube was injected with human umbilical cord mesenchymal stem cells. In the control group, amnion tube was injected with saline. At 4, 8, 12, 16 and 20 weeks after cell transplantation (eight rats at each time point in each group), the sciatic functional index and gastrocnemius muscle wet weight were measured and electrophysiological and histomorphological examinations were conducted. A total of 80 rats were included in the final analysis.
Optimization of sciatic nerve injury rat model
Toe flexion and sluggish gait occurred on the damaged side of all rats from each group. The wound healed within 5 or 6 days. Two weeks after cell transplantation, red swelling and ulcers were visible in the weight-bearing area of the ankle joint on the damaged side. Two rats from each group developed ulcers. Three rats from the control group injured their own toes. By weeks 8–9, foot swelling disappeared and ulcers gradually healed on the damaged side of rats from both groups. The healing time was 8–9 days in the cell transplantation group and 12–15 days in the control group. Ulcers developed again after healing in some rats. The gastrocnemius muscle was atrophic to different degrees on the damaged side in both groups. Lameness was relieved at week 12 following cell transplantation. Motor function and gait were recovered and muscular atrophy was recovered at week 20 in the cell transplantation group. Lameness and gait were not markedly improved in the control group.
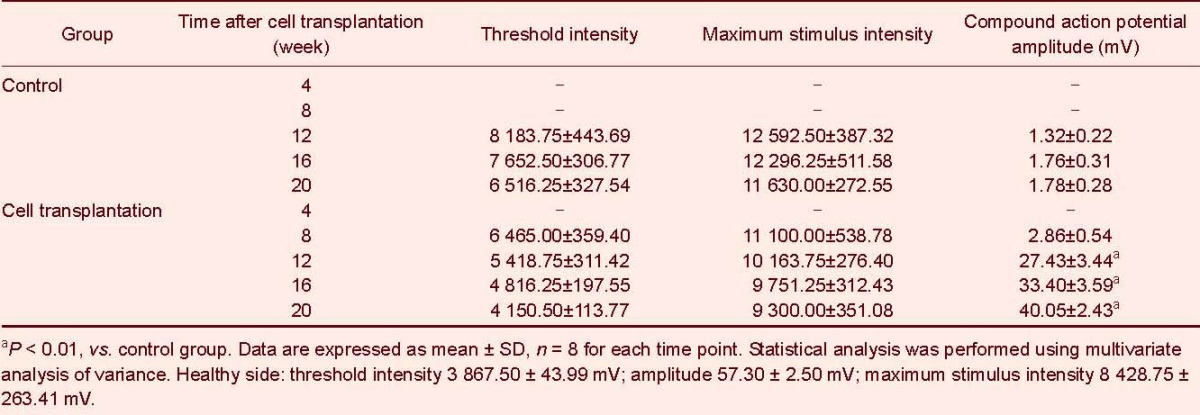
Changes in sciatic functional index of rats
Footprint measurement showed toe arrangement was unaltered in cell transplantation and control groups 4 weeks after intervention (Figure 1). At weeks 8, 12, 16 and 20, toe separation improved and footprint spacing became wide and clear in the cell transplantation group. No significant changes were detectable in the control group (Figure 1) in which toes were slightly separated. According to the Bain formula (see materials and methods) (Table 1), sciatic functional index was near complete injury in the cell transplantation and control groups (sciatic functional index =−100) (P > 0.05). Sciatic functional index reduced gradually in the cell transplantation group by weeks 8, 12, 16 and 20, at similar level to normal nerves (sciatic functional index = 0). A significant difference was detected between the cell transplantation and control groups (P < 0.01; Figure 1).
Figure 1.
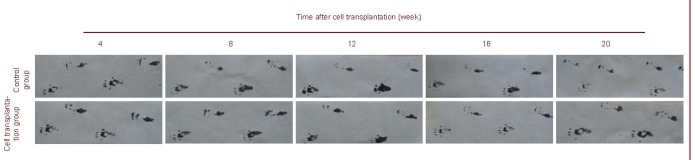
Rat footprint graph at 4, 8, 12, 16 and 20 weeks after cell transplantation.
The upper footprint represents the injured side. Toes were separated and toe spacing became wider and clearer from week 8 in the cell transplantation group. No significant change was detectable in the control group.
Table 1.
Sciatic functional index (SFI) in the sciatic nerve injury rats at 4–20 weeks following cell transplantation
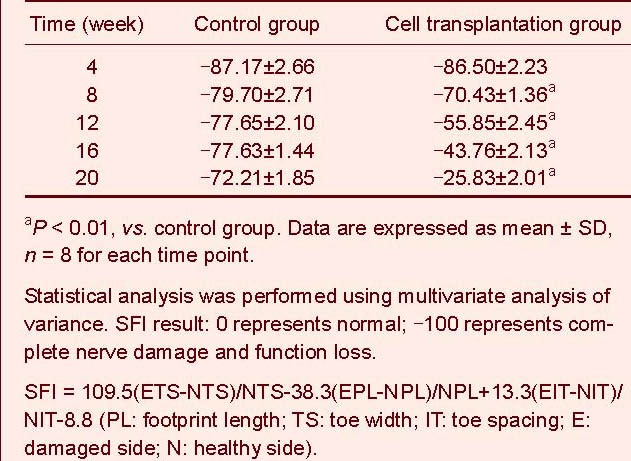
Changes in electrophysiological indices in the rat sciatic nerve
Threshold stimulus and maximum stimulus intensity were detected 8 and 12 weeks after implantation in the cell transplantation and control groups respectively. Threshold stimulus and maximum stimulus intensity gradually decreased over time, to near normal levels at week 20 in the cell transplantation group. Threshold stimulus and maximum stimulus intensity reduced slightly over time in the control group (Figure 2).
Figure 2.
Compound action potential amplitude after electrostimulation in rats (Y-axis: compound action potential amplitude mV; X-axis: electrostimulation time).
Compound action potential amplitude was smaller at weeks 12, 16 and 20 in the control group compared with week 8 in the cell transplantation group.
Compound action potential was visible by weeks 8, 12, 16 and 20 in the cell transplantation group. Compound action potential amplitude in this group increased over time.
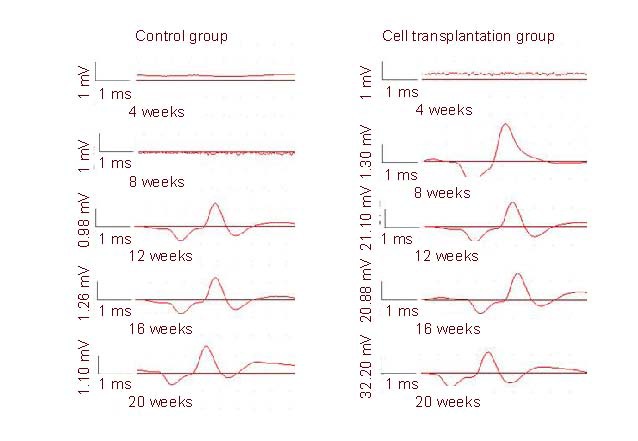
Significant difference in threshold stimulus and maximum stimulus intensity was determined between the cell transplantation and control groups at 12, 16 and 20 weeks (P < 0.01; Table 2).
Table 2.
Electrophysiological indices in rat sciatic nerves at 4–20 weeks after cell transplantation
Changes in gastrocnemius muscle wet weight
At 4 and 8 weeks after implantation, the gastrocnemius muscle on the damaged side was pink, soft and atrophic in both groups. By weeks 12, 16 and 20, the gastrocnemius muscle on the damaged side was hard, atrophic and thin with poor elasticity in the control group and pink, elastic, plump and slightly atrophic in the cell transplantation group.
The gastrocnemius muscle wet weight and restoration ratio gradually increased on the damaged side in the cell transplantation group, but gradually diminished in the control group. No significant difference in wet weight and restoration ratio was detectable between groups at 4 weeks (P > 0.05). The wet weight and restoration ratio were greater in the cell transplantation group compared with the control group at weeks 8, 12, 16 and 20 (P < 0.01; Table 3).
Table 3.
Changes in gastrocnemius muscle wet weight and restoration ratio in rats at 4–20 weeks following cell transplantation
General morphology of grafts and regenerated nerves
Grafts in both groups adhered slightly to the surrounding tissue at different time points and could be easily peeled off. At weeks 4 and 8 following implantation, blood vessels were visible on the surface of the human amnion and the graft thickened in the cell transplantation group. Acupuncture on the sciatic nerve of the distal end of the graft, resulted in a slight pain response in the cell transplantation group, which was absent in the control group. At week 12 following cell transplantation, numerous blood vessels were detected on the surface of the amnion.
Acupuncture on the sciatic nerve of the distal end of the graft, resulted in a stronger pain response in the cell transplantation group, which was absent in the control group. At weeks 16 and 20 following cell transplantation, partial amnion absorption had occurred and abundant capillary formation was visible on the surface of the blood vessels in the cell transplantation group. Furthermore, graft diameter was similar to nerve trunk diameter and the graft was integrated with the sciatic nerve. The morphology of the graft and regenerated nerve was similar to that of the normal nerve. Sutures had partially detached and the stoma was difficult to identify. In the control group, the stoma was integrated. However, the graft and regenerated nerve were thinner than the normal nerve. And no neuroma was formed. Acupuncture on the sciatic nerve trunk of the distal end of the graft resulted in a strong pain response and obvious muscle contraction of the posterior limb in the cell transplantation group. A minor pain response and hindlimb contraction was observed in the control group.
Histopathological assessment of the graft and regenerated nerve tissue
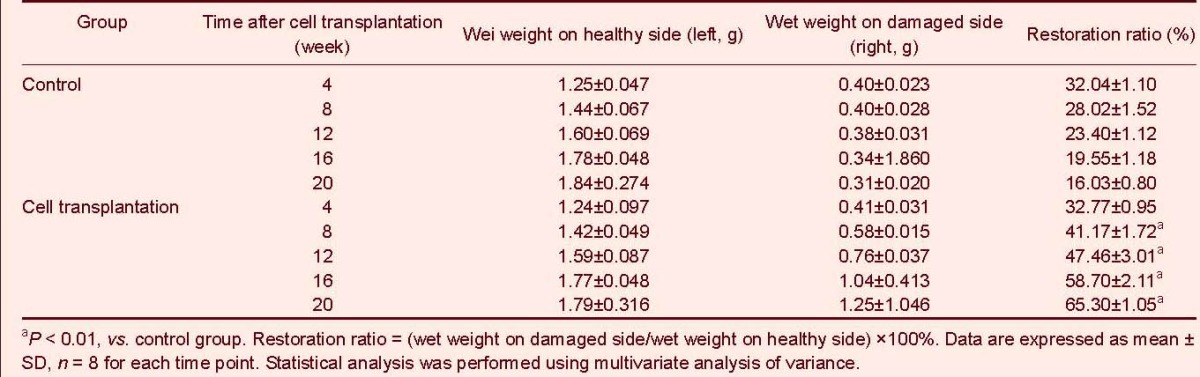
In the cell transplantation group, regenerating nerve fibers were arranged into nerve tracts. Fibrous tissue was detected among the nerve tracts and a thin perineurium with many capillary vessels was observed. Connective tissue between the nerve tracts reduced over time. Amnion tissue also reduced and neuron-like cells were observed under an optical microscope. In the control group, no obvious regenerating nerve fibers or capillaries were observed. In fact, the regeneration unit appeared disorganized comprising mainly of connective tissue (Figure 3).
Figure 3.
Hematoxylin-eosin staining of rat grafts at 4–20 weeks after cell transplantation (sagittal plane, × 400).
Regenerating nerve fibers are arranged into nerve tracts and abundant capillary vessels are observed.
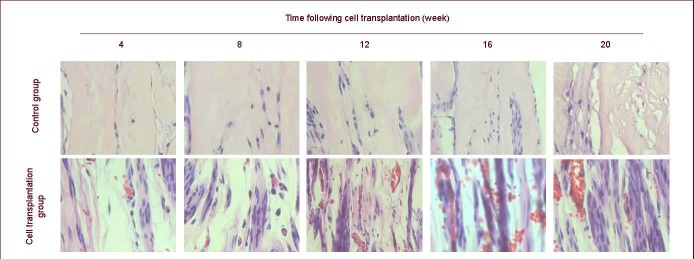
Histopathological change in gastrocnemius muscle
Gastrocnemius muscle was atrophic at weeks 4 and 8 in the cell transplantation and control groups. Atrophy was less in the cell transplantation group than in the control group. At 12, 16 and 20 weeks, atrophy reduced and cell diameter increased in the cell transplantation group, as observed using optical microscopy (Figure 4).
Figure 4.
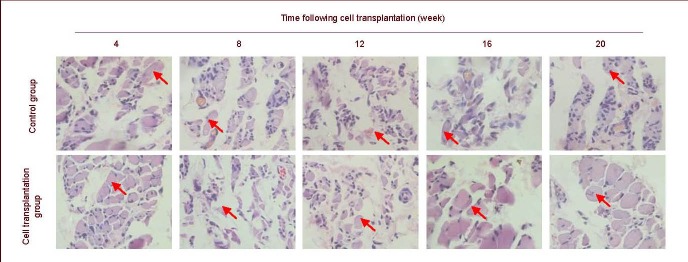
Hematoxylin-eosin staining in rat gastrocnemius muscle at 4–20 weeks after cell transplantation (coronal plane, × 400).
Red arrows show location of gastrocnemius muscle cells. Cells in the control group are atrophic at 4–20 weeks and cell structures are unclear at 12, 16 and 20 weeks. Cells in the cell transplantation group are atrophic at 4–20 weeks, but cell diameter becomes large and clear at 12, 16 and 20 weeks.
At weeks 12, 16 and 20, atrophy was severe and the tissue structures were unclear in the control group (Figure 4). Maximum long- and short-axis diameters of 10 randomly selected cells were measured to calculate the average value for quantitative analysis. Because structures were unclear in the control group at weeks 12, 16 and 20, the value could not be calculated. The gastrocnemius muscle cell diameters of both groups at various time points are listed in Table 4 and Figure 4.
Table 4.
Comparison of gastrocnemius muscle cell diameter (μm) at 4–20 weeks following cell transplantation
DISCUSSION
Sciatic functional index is considered as a valuable method to assess sciatic nerve regeneration and was measured over time following cell transplantation into a rat nerve damage model. Sciatic functional index was enhanced in the cell transplantation group compared with the control group from week 12. A 10-mm defect in the right sciatic nerve rat model induced nerve block. Nerve injury resulted in a series of histological and biochemical changes, including altered response to damage, Wallerian degeneration in the nerve fiber, vascular network rupture and hemorrhage of the nerve membrane, response to nerve cell body, axon and myelin sheath rupture, and corresponding changes to the end organs[8,9,10,11]. Muscles in the distal end of the damaged side were degenerated due to denervation resulting in prolonged, irreversible degeneration.
Results from this study demonstrate that the restoration ratio decreased at weeks 4 and 8 in the cell transplantation group, but increased from week 12, promoting the regeneration and function of Schwann's cells and causing prolongation of the regenerated axon and growth cone at the proximal end of the damaged nerve. Furthermore, increased muscle oxygen supply and nutrient substance, allowed synthesis of muscle glycogen and protein and reduced muscular atrophy by improving the microenvironment at the site of nerve injury. Normal nerves released nutrient substances at the nerve terminal to consistently adjust the metabolic activity of innervated tissues. Nerve transection affected the metabolic activity of the muscle, by reducing muscle glycogen synthesis and increasing protein decomposition and muscle atrophy. The self repair potential of the sciatic nerve was limited, leading to the death of abundant neurons in the control group. In the damaged region, both the axon and myelin sheath were ruptured and some ischemia occurred in the damaged nerve segment. Capillary permeability was increased and nerve edema was apparent. Wallerian degeneration of the nerve fibers, granular decomposition at the distal end of the axon, irregular swelling and damaged to the myelin sheath were detectable[12]. Electrophysiological changes were used as a sensitive measure to evaluate nerve injury and repair.
In the present study, sciatic nerve conduction was recovered over time in the cell transplantation group. Compound action potential amplitude was progressively increased, but threshold intensity and maximum stimulus intensity were gradually diminished over time in the cell transplantation group. Neurobehavioral manifestations were altered in the cell transplantation group, but no significant changes were detected in the control group. Electrophysiological indices demonstrated sciatic nerve conduction was progressively increased in rats from the cell transplantation group, but no significant changes were found in the control group, which may be directly associated with neurological recovery using human umbilical cord mesenchymal stem cells. However, the optimal seeding density of human umbilical cord mesenchymal stem cell warrants further investigation. Axon diameter is a crucial factor for nerve conduction velocity. Umbilical cord mesenchymal stem cells were successfully isolated, culture-expanded and multi-directionally differentiated in vitro [13,14,15]. This established them as good candidates for differentiating into neuron-like cells in vivo. Umbilical cord mesenchymal stem cells do not cause a host response in animal experiments[16], thus provide a suitable source for neural regeneration. Optimal matrix, cell and tube material were combined to connect damaged nerves, which improved functional rehabilitation following nerve injury[17].
In summary, human umbilical cord mesenchymal stem cells differentiated into neuron-like cells in vivo, promoting nerve fiber regeneration and repairing the damaged sciatic nerve of Sprague-Dawley rats. Nevertheless, the relationship between human umbilical cord mesenchymal stem cell concentration and functional rehabilitation, precise mechanism of differentiation, and optimal combination method for injecting amnion with human umbilical cord mesenchymal stem cells require further investigation.
MATERIALS AND METHODS
Design
Random controlled animal study.
Time and setting
Experiments were performed at the Laboratory of Anatomy and Department of Physiology, Liaoning Medical University, China from December 2008 to June 2009.
Materials
Animals: a total of 80 healthy adult Sprague-Dawley rats, male and female, weighing 208.76 ± 11.82 g, were supplied by the Experimental Animal Center, Liaoning Medical University, China (SCXY (Liao) 2003-2007). The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[18].
Placenta amnion: healthy, fresh, full-term fetal amnion, following spontaneous delivery was supplied by the Department of Gynaecology and Obstetrics, Jinzhou Central Hospital, China. Protocols and risks were informed to subjects and informed consent was given. Protocols were approved by the Hospital Ethics Committee in accordance with the Administrative Regulations on Medical Institution, issued by the State Council of China[19].
Methods
Management and preparation of human amnion
Under sterile conditions, amnion was isolated from the internal surface of the fetal membrane, immersed in 0.1% NH4OH, agitated for 20 minutes and washed three times in saline. Amnion epithelium on the basement membrane was removed using dry sterile Q-tips. The amnion was cut into 2.0 cm × 2.5 cm blocks, immersed in 500 mL PBS (pH 7.5, containing 4 × 105 U penicillin and 4 × 104 U gentamicin) and stored at −20°C (for a maximum of 0.5 months). Specimen were thawed at room temperature prior to use.
Passage and culture of human umbilical cord mesenchymal stem cells and preparation of cell suspension
Purified human umbilical cord mesenchymal stem cells (National Stem Cell Center, Tianda Science Park) were subcultured during the logarithmic phase of growth. After the medium was removed, human umbilical cord mesenchymal stem cells were washed in PBS, digested in 0.25% trypsin containing 0.02% ethylenediamine tetraacetic acid and observed under an inverted microscope. Human umbilical cord mesenchymal stem cells rounded and formed a single cell suspension. The reaction was terminated by adding medium containing 10% fetal bovine serum. Gentle tapping of the culture vessel caused the umbilical cord mesenchymal stem cells to detach from the flask and cells were collected and centrifuged at 1 000 r/min for 5 minutes. The supernatant was removed and new medium was added until the cell concentration was 1 × 106/mL. Penicillin and streptomycin were applied to prevent infection. Cells were subcultured at a ratio of 1:2. Cell suspensions of 1 × 105/mL at passages 3 to 4 were utilized for experimentation.
Establishment of models of sciatic nerve defect and amnion tube connection
Rats were intraperitoneally anesthetized with 10% chloral hydrate (0.35 mL/100 g). A 25-mm incision was made on the posterior femur. A blunt dissection was made between the biceps femoris and vastus lateralis. One white thick nerve was exposed at the interspace of the gluteus and lightly stimulated to induce contraction of the posterior limb. After exposure of the sciatic nerve, the amnion was spread out on the sciatic nerve trunk. Either edge of the amnion was sutured onto the nerve membrane with one stitch using 0/10 non-damaged medical suture (Ningbo Medical Suture Co., Ltd., Ningbo, China). 6–8 mm of the sciatic nerve was excised and the sciatic nerve was retracted, forming a 10-mm interspace amnion tube. A human umbilical cord mesenchymal stem cell suspension (concentration 1 × 105/mL, dose 50 μL)[20] was injected into the proximal end of the amnion tube using a microinjector (Shanghai Medical Laser Instrument, Shanghai, China). The seam was coated with glycerol to avoid cell suspension leakage. Gentamicin injection was added. A total of 50 μL was injected into the amniotic cavity in the control group.
General observation in rats
We measured wound healing, gait, ulcer occurrence and healing time, and gastrocnemius muscle changes.
Sciatic functional index examination in rats
A footprint walking box was developed (50 cm length, 10 cm breadth, 10 cm height). A blank sheet of paper was laid at the bottom of the box. Both rat hind feet were dipped into ink. Rats walked from one side to the other. The paper captured three or four footprints on each side (accurate to mm). (1) Podogram length: from heel to toe, the longest length was used; (2) toe spread: the distance from the first toe to the fifth toe, the longest was selected; (3) inter-toe distance: the distance from the second toe to the fourth toe, the longest was selected. Gait was observed at 4, 8, 12, 16 and 20 weeks following model induction and sciatic functional index was calculated[21].
Determination of electrophysiological indices in the rat sciatic nerve
A 20 mm sciatic nerve was exposed following anesthesia by intraperitoneal injection. A stimulating electrode was placed on the proximal nerve end, a leading electrode on the distal nerve end and a ground electrode in the middle, connected to a BL-420F biological experimental system (Taimeng Technology Corporation, Chengdu, China).
Stimulation parameters were: initial stimulus intensity of 3 000 mV, increments of 50 mV and stimulus interval 1 second. Threshold stimulus and maximum stimulus intensity were measured. The stimulus intensity exceeding 25% of the maximum stimulus intensity was selected to elicit compound action potential, to measure compound action potential amplitude and to observe the recovery of sciatic nerve conduction.
Measurement of wet weight of gastrocnemius muscle
At weeks 4, 8, 12, 16 and 20 following cell transplantation, the bilateral gastrocnemius muscle was obtained in accordance with a previously published method[21]. Wet weight was recorded using an electronic balance (Sartorius, Beijing, China). Restoration ratio was equal to wet weight on the damaged side/wet weight on the healthy side × 100%.
Histopathological changes in rat sciatic nerve observed by hematoxylin-eosin staining
At weeks 4, 8, 12, 16 and 20 following cell transplantation, cannulation of the ascending aorta was undertaken via the left ventricle. The specimen was fixed in 4% paraformaldehyde for 90 minutes. A 5 mm middle segment of the graft on the damaged side was fixed in 4% paraformaldehyde for 3 hours, washed in running water for 1 hour, dehydrated in gradient ethanol, permeabilized in cedar oil overnight, embedded in paraffin, sliced into 5-μm thick sections and stained using hematoxylin-eosin. The middle segment of the graft at the damaged and healthy sides was obtained at weeks 4, 8, 12, 16 and 20 following cell transplantation. Neuron cell numbers were recorded to determine recovery of neural function.
Statistical analysis
Data were expressed as mean ± SD, and analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Intergroup differences were analyzed utilizing multivariate analysis of variance. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Hui Zhao for assistance in culturing the umbilical cord stem cells.
Footnotes
Funding: This study was financially sponsored by the Natural Science Foundation of Liaoning Province, No. 20102138.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Liaoning Medical University, China.
(Edited by Li RX, Yang XY/Qiu Y/Song LP)
REFERENCES
- [1].Rönkkö H, Göransson H, Siironen P, et al. The capacity of the distal stump of peripheral nerve to receive growing axons after two and six months denervation. Scand J Surg. 2011;100(2):223–229. doi: 10.1177/145749691110000315. [DOI] [PubMed] [Google Scholar]
- [2].Sedaghati T, Yang SY, Mosahebi A, et al. Nerve regeneration with aid of nanotechnology and cellular engineering. Biotechnol Appl Biochem. 2011;58(5):288–300. doi: 10.1002/bab.51. [DOI] [PubMed] [Google Scholar]
- [3].Lavasani M, Gehrmann S, Gharaibeh B, et al. Venous graft-derived cells participate in peripheral nerve regeneration. PLoS One. 2011;6(9):e24801. doi: 10.1371/journal.pone.0024801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nekanti U, Mohanty L, Venugopal P, et al. Optimization and scale-up of Wharton's jelly-derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010;5(3):244–254. doi: 10.1016/j.scr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- [5].Sarugaser R, Lickorish D, Baksh D, et al. Human umbilical cord perivascul -ar(HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- [6].Farren TW, Giustiniani J, Liu FT. Differential and tumor-specific expression of CD160 in B-cell malignancies. Blood. 2011;118(8):2174–2183. doi: 10.1182/blood-2011-02-334326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu ZY, Hui GZ. Materials for neuro-transplantation and the amnion. Zhonghua Yixue Zazhi. 2006;119(16):1323–1326. [PubMed] [Google Scholar]
- [8].Jung J, Cai W, Lee HK, et al. Actin polymerization is essential for myelin sheath fragmentation during Wallerian degeneration. J Neurosci. 2011;31(6):2009–2015. doi: 10.1523/JNEUROSCI.4537-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leong ML, Gu M, Speltz-Paiz R, et al. Neuronal loss in the rostral ventromedial medulla in a rat model of neuropathic pain. J Neurosci. 2011;31(47):17028–17039. doi: 10.1523/JNEUROSCI.1268-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yu B, Zhou S, Wang Y, et al. Profile of microRNAs following rat sciatic nerve injury by deep sequencing: implication for mechanisms of nerve regeneration. PLoS One. 2011;6(9):e24612. doi: 10.1371/journal.pone.0024612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldenberg-Cohen N, Dratviman-Storobinsky O, Dadon Bar El S, et al. Protective effect of bax ablation against cell loss in the retinal ganglion layer induced by optic nerve crush in transgenic mice. Neuroophthalmol. 2011;31(4):331–338. doi: 10.1097/WNO.0b013e318227e4fb. [DOI] [PubMed] [Google Scholar]
- [12].Gaudet AD, Leung M, Poirier F, et al. A role for galectin-1 in the immune response to peripheral nerve injury. Exp Neurol. 2009;220(2):320–327. doi: 10.1016/j.expneurol.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [13].Bao C, Chen W, Weir MD, et al. Effects of electrospun submicron fibers in calcium phosphate cement scaffold on mechanical properties and osteogenic differentiation of umbilical cord stem cells. Acta Biomater. 2011;7(11):4037–4044. doi: 10.1016/j.actbio.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao L, Weir MD, Xu HH. Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials. 2010;31(14):3848–3857. doi: 10.1016/j.biomaterials.2010.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao L, Burguera EF, Xu HH, et al. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate-chitosan-biodegradable fiber scaffolds. Biomaterials. 2010;31(5):840–847. doi: 10.1016/j.biomaterials.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sakuragawa N, Thangavel R, Mizuguchi M, et al. Expression of markers for both neuronal and glial cells in human amniotic epithelial cells. Neurosci Lett. 1996;209(1):9–12. doi: 10.1016/0304-3940(96)12599-4. [DOI] [PubMed] [Google Scholar]
- [17].Kalbermatten DF, Kingham PJ, Mahay D, et al. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J Plast Reconstr Aesthet Surg. 2008;61(6):669–675. doi: 10.1016/j.bjps.2007.12.015. [DOI] [PubMed] [Google Scholar]
- [18].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [19].State Council of the People's Republic of Administrative Regulations on Medical Institution. 1994 Sep 01; [Google Scholar]
- [20].Mohammad J, Shernaq J, Rabinovsky E, et al. Modulation of peripheral nerve regeneration: a tissue-engineering approach. The role of amnion tube nerve conduit across a 1-centimetemerve gap. Plast Reconstr Surg. 2000;105(2):660. doi: 10.1097/00006534-200002000-00027. [DOI] [PubMed] [Google Scholar]
- [21].Kanaya F, Firrell JC, Breidenbach WC. Sciatic function index, nerve conduction tests, muscle contraction,and axon morphometry as indicators of regeneration. Plast Reconstr Surg. 1996;98:1264–1271. doi: 10.1097/00006534-199612000-00023. [DOI] [PubMed] [Google Scholar]


