Abstract
In this study, we examined 3-month-old female mice from the senescence-accelerated prone mouse 8 strain and age-matched homologous normal aging female mice from the senescence accelerated- resistant mouse 1 strain. Mice from each strain were housed in an enriched environment (including a platform, running wheels, tunnel, and some toys) or a standard environment for 3 months. The mice housed in the enriched environment exhibited shorter escape latencies and a greater percentage of time in the target quadrant in the Morris water maze test, and they exhibited reduced errors and longer latencies in step-down avoidance experiments compared with mice housed in the standard environment. Correspondently, brain-derived neurotrophic factor mRNA and protein expression in the hippocampus was significantly higher in mice housed in the enriched environment compared with those housed in the standard environment, and the level of hippocampal brain-derived neurotrophic factor protein was positively correlated with the learning and memory abilities of mice from the senescence-accelerated prone mouse 8 strain. These results suggest that an enriched environment improved cognitive performance in mice form the senescence-accelerated prone mouse 8 strain by increasing brain-derived neurotrophic factor expression in the hippocampus.
Keywords: Alzheimer's disease, enriched environment, cognition, brain-derived neurotrophic factor, neurotrophic factor, senescence-accelerated prone mouse, hippocampus, neural regeneration
Research Highlights
An enriched environment improved cognitive abilities and increased brain-derived neurotrophic factor expression in the hippocampus of mice from the senescence-accelerated prone mouse 8 strain.
Abbreviations
AD, Alzheimer's disease; BDNF, brain-derived neurotrophic factor; SAMP8, senescence-accelerated prone mouse 8; SAMR1, senescence accelerated-resistant mouse 1
INTRODUCTION
Alzheimer's disease (AD) is an irreversible, progressive neurodegenerative disorder characterized by cognitive decline. With the ongoing increase in the median age in many societies, the number of AD patients is persistently increasing. However, few therapeutic regimens improve the underlying pathogenic phenotypes of AD[1]. It has been previously suggested that an enriched environment improves learning and memory, enhances neurogenesis in the dentate gyrus of the hippocampus, increases brain mass and size, and enhances dendritic branching and new synapse formation in several areas of the brain in mice[2]. Many studies have shown that low levels of brain-derived neurotrophic factor (BDNF) may play a critical role in the etiology of AD[3,4,5] and BDNF may represent a potential neuroprotective agent[6,7,8]. The senescence-accelerated prone mouse 8 (SAMP8) strain of mice is a useful animal model to study aging or age-associated disorders, and it may be an excellent model for studying the earliest neurodegenerative changes associated with AD[9]. The senescence accelerated-resistant mouse 1 (SAMR1) strain of mice is often used as the control in studies of SAMP8 mice, because these mice have undergo a typical aging process. In the present study, we examined the effects of an enriched environment on cognitive behavior and BDNF expression in SAMP8 mice, and we investigated the association between delayed onset of AD in an enriched environment and BDNF expression in the hippocampus.
RESULTS
Quantitative analysis of experimental mice
Forty SAMP8 mice and forty SAMR1 mice were used, and they were equally and randomly assigned to either an enriched environment or a standard environment. All 80 mice were included in the final analysis.
Mice housed in an enriched environment performed better in the Morris water maze than mice housed in a standard environment
Acquisition of spatial memories
To assess the acquisition phase of spatial learning, the mice were tested every day for 4 consecutive days in the Morris water maze task. We found that mice housed in an enriched environment had shorter escape latencies than those mice housed in a standard environment. This occurred over 4 consecutive days in both the SAMP8 and SAMR1 strains (P < 0.01). SAMP8 mice had longer escape latencies than SAMR1 mice regardless of whether they were housed in an enriched (P < 0.05) or a standard environment (P < 0.01). Escape latencies significantly decreased over time in mice from each group, illustrating acquisition of spatial memories (Figure 1).
Figure 1.
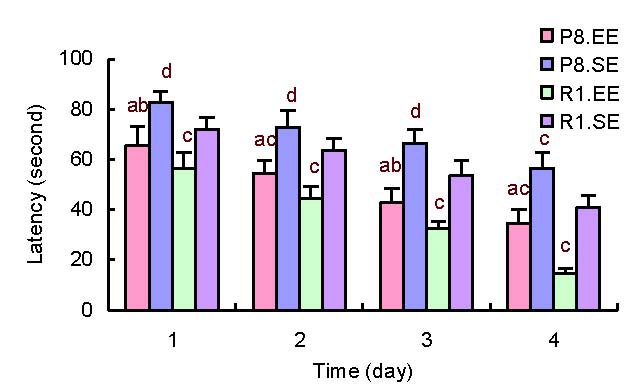
Comparison of average escape latencies in the Morris water maze task. aP < 0.01, vs. P8.SE; bP < 0.05, vs. R1EE; cP < 0.01,dP < 0.05, vs. R1. SE. The data are presented as mean ± SD.
Differences between the groups were analyzed using analysis of variance and paired t-tests.
P8: Senescence-accelerated prone mouse 8; R1: senescence accelerated-resistance mice 1; EE: enriched environment; SE: standard environment.
Memory retention
To evaluate memory retention, a probe trial was performed on the fifth day. The percentage of time in the target quadrant was longer in SAMP8 and SAMR1 mice housed in an enriched environment compared with SAMP8 and SAMR1 mice housed in a standard environment (P < 0.01). There was no difference in the percentage of time spent in the opposite quadrant between SAMP8 mice housed in an enriched environment or a standard environment (P = 0.09). The percentage of time spent in the opposite quadrant was smaller in SAMR1 mice housed in an enriched environment versus a standard environment (P < 0.05). SAMP8 mice housed in an enriched environment spent less time in the target quadrant (P < 0.05) and more time in the opposite quadrant (P < 0.01) than SAMR1 mice housed in an enriched environment. The percentage of time spent in the target quadrant was smaller in SAMP8 mice housed in a standard environment compared with SAMR1 mice housed in a standard environment (P < 0.01) and was larger than the percentage of time spent in the opposite quadrant by SAMR1 mice housed in a standard environment (P < 0.05). In probe trials (Figure 2B), we found that SAMP8 and SAMR1 mice housed in an enriched environment mainly stayed in the target quadrant, and this was more pronounced in SAMR1 mice. In contrast, mice housed in a standard environment spent similar amounts of time in every quadrant.
Figure 2.
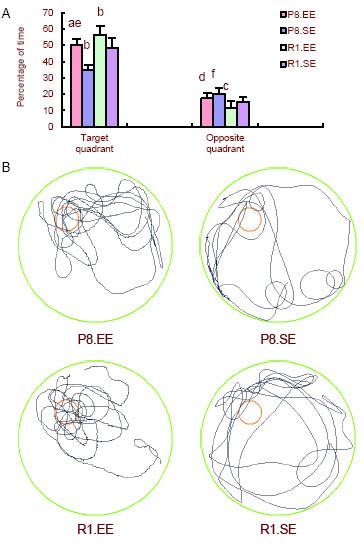
Comparison of swimming times in the target and opposite quadrants in the spatial probe test.
(A) Comparison of swimming times in the target and opposite quadrants in the spatial probe test. aP < 0.01, vs. P8.SE; bP < 0.01, cP < 0.05, vs. R1.SE;dP < 0.01, eP < 0.05, vs. R1.EE; fP < 0.05, vs. R1.SE.
The data are presented as mean ± SD. Differences between the groups were analyzed using analysis of variance and paired t-tests.
(B) Probe trials in the mice. Red circle: escape platform; black line: probe trial.
P8: Senescence-accelerated prone mouse 8; R1: senescence accelerated-resistant mouse 1; EE: enriched environment; SE: standard environment.
Mice housed in an enriched environment performed better in a step-down avoidance experiment compared with mice housed in a standard environment
Acquisition of step-down avoidance
On the first day, mice housed in an enriched environment had fewer errors than mice housed in a standard environment in both SAMP8 and SAMR1 (P < 0.01). SAMP8 mice made more errors than SAMR1 mice housing in either the enriched or standard environment (P < 0.01; Figure 3A).
Figure 3.
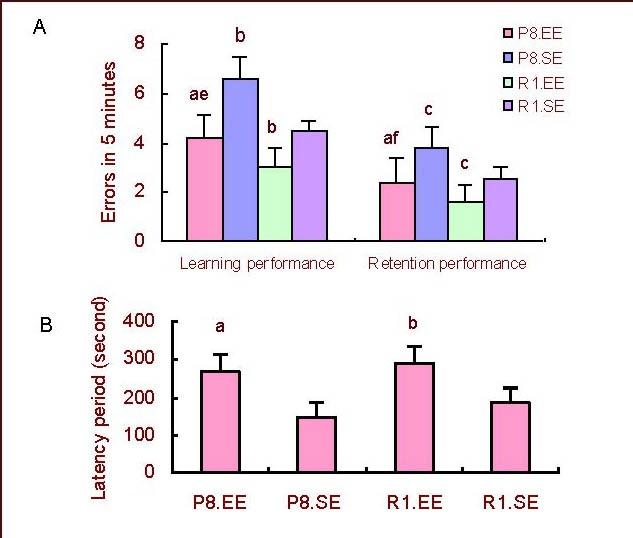
Comparison of learning and retention performance in the step-down avoidance experiment.
(A) Errors; (B) Latencies. aP < 0.01, vs. P8.SE; bP < 0.01, cP < 0.05, vs. R1.SE; eP < 0.01, fP < 0.05, vs. R1.EE. The data are presented as mean ± SD. Differences between the groups were analyzed using analysis of variance and paired t-tests.
P8: Senescence-accelerated prone mouse 8; R1: senescence accelerated-resistant mouse 1; EE: enriched environment; SE: standard environment.
Retention of step-down avoidance
On the second day, mice housed in an enriched environment had fewer errors than mice housed in a standard environment in both SAMP8 (P < 0.01) and SAMR1 mice (P < 0.05; Figure 3A). SAMP8 mice made more errors than SAMR1 mice regardless of whether they were housed in the enriched or standard environment (P < 0.05; Figure 3A). Mice housed in an enriched environment had longer latencies than mice housed in a standard environment in both SAMP8 and SAMR1 mice (P < 0.01; Figure 3B).
Effects of an enriched environment on BDNF mRNA and protein expression in the hippocampus of mice
Reverse transcription-PCR showed more BDNF mRNA expression in mice housed in an enriched environment compared with mice housed in a standard environment in both SAMP8 and SAMR1 mice (P < 0.01). SAMP8 mice had a lower relative level of BDNF mRNA than SAMR1 mice housed in either the enriched or standard environment (P < 0.05; Figure 4).
Figure 4.
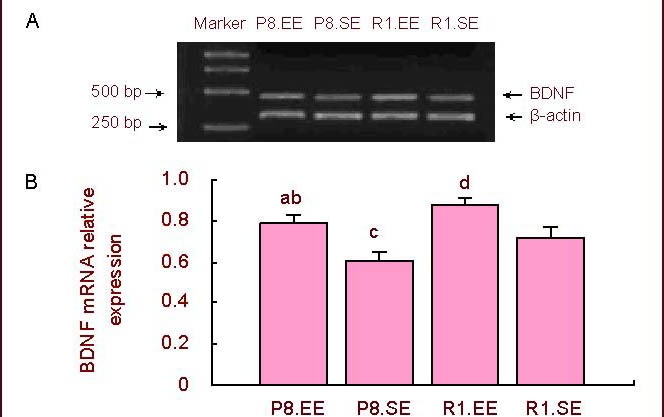
Expression of hippocampal BDNF mRNA.
(A) PCR results of BDNF.
(B) Comparison of the relative level of BDNF mRNA. aP < 0.01, vs. P8.SE; bP < 0.05, vs. R1.EE; cP < 0.05,dP < 0.01, vs. R1.SE. The data are presented as mean ± SD (absorbance ratio of BDNF mRNA/β-actin mRNA). Differences between the groups were analyzed using analysis of variance and paired t-tests.
BDNF: Brain-derived neurotrophic factor; P8: senescence-accelerated prone mouse 8; R1: senescence accelerated-resistant mouse 1; EE: enriched environment; SE: standard environment.
Mice housed in an enriched environment exhibited a higher relative level of BDNF protein compared with mice housed in a standard environment in both SAMP8 and SAMR1 mice (P < 0.01). SAMP8 mice had a lower relative level of BDNF protein than SAMR1 mice housed in either the enriched or standard environment (P < 0.05; Figure 5).
Figure 5.
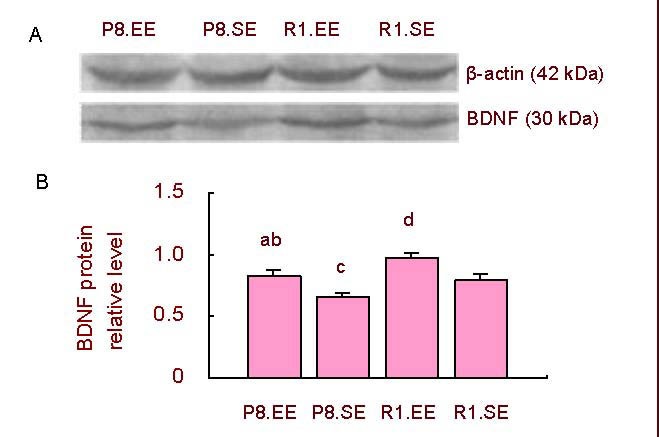
Hippocampal BDNF protein expression.
(A) Western blot results of BDNF.
(B) Comparison of the relative level of BDNF protein. aP < 0.01, vs. P8.SE; cP < 0.05,dP < 0.01, vs. R1.SE; bP < 0.05, vs. R1.EE. The data are presented as mean ± SD (absorbance ratio of BDNF/β-actin). Differences between the groups were analyzed using analysis of variance and paired t -tests.
BDNF: Brain-derived neurotrophic factor; P8: senescence-accelerated prone mouse 8; R1: senescence accelerated-resistance mice 1; EE: enriched environment; SE: standard environment.
Correlation between cognitive performance and BDNF protein expression
Linear correlation analysis showed a significant negative correlation between escape latencies in the first day of Morris water maze and the relative level of BDNF protein in SAMP8 mice housed in an enriched environment (r = −0.85, P < 0.01; Figure 6A).
Figure 6.
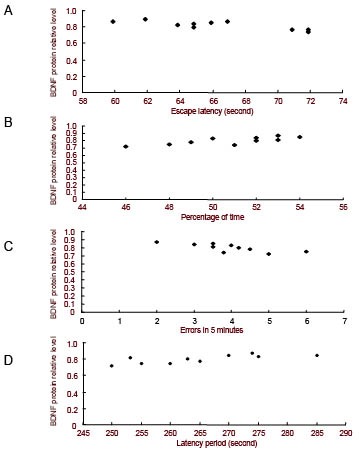
Correlation analysis between cognitive ability and the relative level of BDNF protein (linear correlation)
(A) A negative correlation was found (r = −0.85, P < 0.01) between escape latency on the first day and the relative level of BDNF protein (absorbance ratio of BDNF/β-actin) in SAMP8 mice.
(B) A positive correlation was found (r = 0.76, P < 0.01) between the percentage of time spent in the target quadrant on the fifth day and the relative level of BDNF protein in SAMP8 mice.
(C) A negative correlation was found (r = −0.79, P < 0.01) between errors on the first day in the step-down avoidance experiment and the relative level of BDNF protein in SAMP8 mice.
(D) A positive correlation was found (r = 0.81, P < 0.01) between latency on the second day in the step-down avoidance experiment and the relative level of BDNF protein in SAMP8 mice.
BDNF: Brain-derived neurotrophic factor; P8: senescence- accelerated prone mouse 8; R1: senescence accelerated- resistance mice 1; EE: enriched environment; SE: standard environment.
Likewise, there was a significant positive correlation between the percentage of time in the target quadrant on the fifth day and the relative level of BDNF protein in these same mice (r = 0.76, P < 0.01; Figure 6B). In addition, there was a significant negative correlation (r = −0.79, P < 0.01; Figure 6C) between the number of errors made on the first day in the step-down avoidance experiment and the relative level of BDNF protein in SAMP8 mice housed in an enriched environment. Likewise, there was a significant positive correlation (r = 0.81, P < 0.01; Figure 6D) between latencies on the second day and the relative level of BDNF protein in these same mice.
These results indicate that hippocampal BDNF protein relative level was positively related to learning and memory in SAMP8 mice.
DISCUSSION
The present study provided novel, direct evidence that an enriched environment improves cognitive abilities and increases the expression of BDNF in the hippocampus. An enriched environment refers to housing conditions in which groups of mice are kept in large cages containing tunnels, platforms, toys, and running wheels to increase social interactions, cognitive loads, and exercise relative to a standard environment[10,11]. Significant changes have been shown at the cellular, molecular, and behavioral levels particularly in the hippocampus of rodents living in an enriched environment[12]. At the behavioral level, enrichment enhances learning and memory[13,14] and reduces memory decline in aged mice[15]. Some evidence has also shown that an enriched environment can increase exploratory activity[16]. The impairment of cognitive functions, such as learning and memory, is considered to be one of the most striking impairment of the aging process. Neuroprotective effects of an enriched environment have been shown in our previous study[17]. In the present study, we assessed learning and memory in SAMP8 and SAMR1 using Morris water maze and step-down avoidance experiments. Three-month-old mice were randomly selected and housed in different environments. Behavior was evaluated when the SAMP8 mice were 6 months old. In the learning performance test, mice housed in an enriched environment exhibited shorter escape latencies in the Morris water maze and fewer errors in the step-down avoidance experiment compared with mice housed in a standard environment. In the retention performance test, mice housed in an enriched environment spent a larger percentage of time in the target quadrant in the Morris water maze and exhibited longer latency in the step-down avoidance experiment compared with mice housed in a standard environment. Moreover, in probe trials, we found that mice housed in an enriched environment mainly stayed in the target quadrant, and this was more pronounced in SAMR1 mice. However, mice housed in a standard environment spent similar amounts of time in every quadrant. All these behavioral results showed that mice housed in an enriched environment had better learning and retention performance than mice housed in a standard environment, which indicated that enriched environments may improve the cognitive abilities of mice, and delay decreased cognition with aging. Compared to mice housed in a standard environment, the larger space of the enriched environment provided more activity opportunities, and the larger number of mice in each cage offered increased social interaction and social stimulation. Mice could exercise by running wheels, which provided enhanced voluntary exercise. To enhance the novelty of the enriched environment, the present study changed the objects and the position of the objects twice a week. Exercise could reduce the risk of developing AD[18,19], reduce neuropathological changes, and repress neuronal cell death associated with AD[20,21].
We observed that SAMP8 mice housed in a standard environment had worse cognitive performance than SAMR1 mice housed in a standard environment when they were 6 months old. This may be related to the particular characteristics of this strain. Compared to SAMR1 mice, SAMP8 mice have shorter life spans (299 days vs. 568 days) and exhibit early manifestations of senescence, such as loss of activity, alopecia, lack of hair glossiness, and skin coarseness, which are similar to those manifestations that are often observed in older humans. At 6 months of age SAMP8 have entered senescence but SAMR1 are still young or middle-aged. We selected the age stage of intervention in SAMP8 from 3 months to 6 months, which is similar to humans from the young to aged stage. The enriched environment improved cognitive performance in both SAMP8 and SAMR1, which demonstrates the effects of enriched environments.
The age-related impairment of cognitive functions is probably due to the vulnerability of the hippocampus. BDNF in the hippocampus has been reported to decrease with age[22] and these decreases may contribute to the age-related cognitive impairments[23]. Enriched environments have also been shown to increase gene expression of neurotrophic factors such as nerve growth factor, BDNF, and glial cell-derived neurotrophic factor[24,25].
AD is accompanied by hippocampal neuronal loss and abnormal neurogenesis, both of which probably contribute to AD-related cognitive deficits[26]. In the present study, we examined the BDNF gene and protein expression in the hippocampus of mice housed in different environments. We found that mice housed in an enriched environment expressed more BDNF mRNA and more BDNF protein in the hippocampus compared with mice housed in a standard environment. In addition, hippocampal BDNF protein relative level was positively related to learning and memory in SAMP8 mice. Changes in BDNF expression may contribute to the changes in cognitive performance in SAMP8 mice. Studies have shown that the effects of enhanced motor activity, through access to running wheels or forced running on treadmills, can increase BDNF levels[27]. In particular, exercise can benefit neuronal function, promote learning and memory[28], ameliorate cognitive deterioration, and protect synaptic changes[29]. Exercise has also been shown to maintain and reduce the cognitive decline associated with aging[30]. BDNF is a powerful modulator of synaptic plasticity that can support neuronal survival and differentiation[31], modify neuronal excitability and synaptic transmission[32], and play a key role in positive cognitive effects[33]. This study provides the first indication that the protective effects of an enriched environment against cognitive decline is associated with significant effects on BDNF in the hippocampus.
In summary, an enriched environment improves learning and memory performance of SAMP8 mice, upregulates BDNF gene and protein expression in the hippocampus, and delays AD progression. These results provide a theoretical foundation to prevent and delay AD in human through the use of enriched environments. However, further studies are needed to investigate the impact of enriched environments on other neurotrophic factors and synaptic structures.
MATERIALS AND METHODS
Design
A randomized, controlled, animal study.
Time and setting
The experiment was performed at the Central Laboratory of the First Hospital of Hebei Medical University, Brain Aging and Cognitive Neuroscience Laboratory of Hebei Province, China from March 2010 to May 2011.
Materials
Mice
Forty 3-month-old female SAMP8 and forty 3-month-old female SAMR1 mice were purchased from The Chinese University of Hong Kong. The mice were housed in a room maintained at 22–25°C and 55 ± 5% humidity with 12-hour light/dark cycles. They were allowed free access to food and water, and these conditions were maintained for 3 months until the behavioral tests were conducted. All experimental use of the mice was performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[34].
Cages
The enriched environment cages (52 cm × 37 cm × 22 cm) consisted of two running wheels, a platform, several tunnels, a variety of toys, and nesting material. The standard environment cages (32 cm × 20 cm × 15 cm) only consisted of nesting material (supplementary Figure 1 online).
Methods
Feeding
SAMP8 and SAMR1 mice were randomly and equally assigned to an enriched environment (10–12 mice per cage) group or a standard environment (5–6 mice per cage) group.
Morris water maze test
After mice were housed in the enriched or standard environment for 3 months, all mice were tested using the SLY-WMS Morris water maze analysis apparatus (Anhui Huaibei Zhenghua Biological Equipment, Anhui, China), for spatial learning and memory acquisition. The swimming pool (120 cm in diameter, 50 cm in height) was divided into four quadrants. An escape platform (14 cm in diameter, 20 cm in height) was fixed 1.5 cm under the water surface during the course of the Morris water maze procedure and was defined as the target quadrant (supplementary Figure 2 online). The water, maintained at 22 ± 2°C, was made opaque by placing black ink dye to prevent the mice from seeing the platform. A 4-day test paradigm was used. The mice were placed into the tank facing the wall from every quadrant by turns and the mice could randomly alter their trace. The trial was continued until the mice found the platform or for a maximum duration of 120 seconds. If the mice failed to find the platform, they were gently guided to the platform. At the end of each trial, the mice were allowed to rest on the platform for 10 seconds. The time to reach the platform (escape latency) was recorded for each mouse. To assess spatial memory retention, a probe trial was performed for 1 day. The platform was removed from the pool, while all other factors remained the same. Mice were allowed to swim for 120 seconds, during which time the percentage of time spent in each quadrant was recorded and their swim paths were automatically recorded by a video tracking system[35].
Step-down avoidance experiment
Approximately 3 hours after the Morris water maze test, a step-down avoidance experiment was performed using the YLS-3T step-down avoidance apparatus (Anhui Huaibei Zhenghua Biological Equipment). The apparatus consists of five 13 cm × 13 cm × 20 cm black boxes, with a floor composed of parallel copper bars (3 mm in diameter) spaced 0.8 cm apart. A platform (5 cm in diameter, 5 cm in height) was placed on the floor of the box against the right wall (supplementary Figure 3 online). Mice were placed on the platform and their latency to step down on the grid with all four paws was measured with an automatic device. Immediately after stepping down on the grid, the mice received a 0.3 mA foot shock. In the first day, the times mice were shocked within 5 minutes, which were named errors, were used to assess learning. In the second day, the latencies to the first step down onto the platform and the errors were used to assess retention[36].
Reverse transcription-PCR
After the behavioral tests were finished, 10 mice in each group were sacrificed. Fresh hippocampus tissue was dissected[37] on ice. RNA was purified from fresh tissue using the Trizol reagent (Fermentas Company, Shenzhen, China) and was reverse transcribed. Primers were synthesized by Shanghai Bioasia Daily Chemical Co., Ltd. (Shanghai, China):
The primers are as follows:
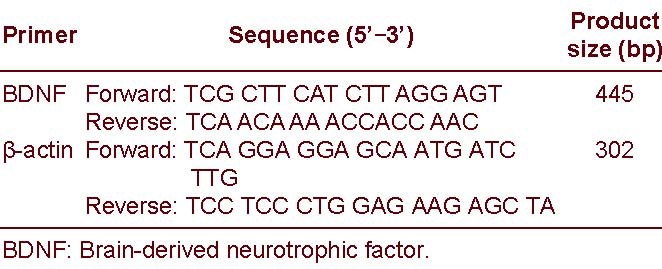
The cDNA subsequently was used for PCR. For BDNF and β-actin amplifications, the number of cycles used were 30 and 27, respectively. The images were scanned and the data were analyzed with a JEDA 801E Gel imager analyzer (Nanjing University company, Nanjing, China). PCR products were quantitatively analyzed. The results are expressed as the ratio of the absorbance value of BDNF to β-actin.
Western blot assay
After the behavioral tests were completed, another 10 mice in each group were sacrificed. According to George Paxinos mouse brain atlas[38], protein of fresh hippocampus tissue was extracted and the supernatant was collected. Proteins (60 μg) from each sample were subjected to polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. The membrane was incubated with rabbit anti-BDNF polyclonal antibody (1:500; Chemicon Biotechnology, Temecula, CA, USA) and rabbit anti-β-actin polyclonal antibody (1:200; Zhongshan Golden Bridge Biotechnology, Beijing, China) for 1 hour at room temperature. Then, they were incubated overnight at 4°C, followed by addition of horseradish peroxidase- conjugated goat anti-rabbit secondary antibody (1:1 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 hours. The products were developed by Super ECL Plus luminescence fluid (Applygen Technologies Inc., Beijing, China). Following film scanning, visualized bands were analyzed utilizing the Quantity One-4.6.2 software (Bio-rad, Hercules, CA, USA). β-actin served as the internal reference. The results are expressed as the ratio of the absorbance value of BDNF to β-actin.
Statistical analysis
The data are presented as mean ± SD. SPSS 11.5 software (SPSS, Chicago, IL, USA) was used to analyze the measured results. Differences between groups were analyzed using analysis of variance and paired t-tests. The correlation between BDNF protein levels and cognitive performance was analyzed using linear correlation analysis. Values were considered significantly different when the P value < 0.05.
Acknowledgments:
The authors thank Yew David, Department of Anatomy, The Chinese University of Hong Kong, for his gift of the SAMP8 and SAMR1 mice.
Footnotes
Funding: The study was supported by the Program of Health Department of Hebei Province, No. 20090338; the Natural Science Foundation of Hebei Province, No. C2009001242; Funding Project for Introduced Abroad Study Personnel of Hebei Province.
Conflicts of interest: None declared.
Ethical approval: All animal experiments were approved by the Animal Ethics Committee of Hebei Medical University, China.
Supplementary information: Supplementary data associated with this article can be found, in the online version, by visiting www.nrronline.org.
(Edited by Li NX, Mao SP/Su LL/Song LP)
REFERENCES
- [1].Hoveida R, Alaei H, Oryan S, et al. Treadmill running improves spatial memory in an animal model of Alzheimer's disease. Behav Brain Res. 2011;216(1):270–274. doi: 10.1016/j.bbr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [2].Hamilton DA, Kolb B. Differential effects of nicotine and complex housing on subsequent experience-dependent structural plasticity in the nucleus accumbens. Behav Neurosci. 2005;119(2):355–365. doi: 10.1037/0735-7044.119.2.355. [DOI] [PubMed] [Google Scholar]
- [3].Peng S, Wuu J, Mufson EJ, et al. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93(6):1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- [4].Laske C, Stransky E, Leyhe T, et al. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J Neural Transm. 2006;113(9):1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- [5].Shin MK, Kim HG, Kim KL. A novel trimeric peptide, Neuropep-1-stimulating brain-derived neurotrophic factor expression in rat brain improves spatial learning and memory as measured by the Y-maze and Morris water maze. J Neurochem. 2011;116(2):205–216. doi: 10.1111/j.1471-4159.2010.07078.x. [DOI] [PubMed] [Google Scholar]
- [6].Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Angelucci F, Spalletta G, di lulio F, et al. Alzheimer's disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res. 2010;7(1):15–20. doi: 10.2174/156720510790274473. [DOI] [PubMed] [Google Scholar]
- [8].Laske C, Stellos K, Hoffmann N, et al. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 2011;14(3):399–404. doi: 10.1017/S1461145710001008. [DOI] [PubMed] [Google Scholar]
- [9].Pallas M, Camins A, Smith MA, et al. From aging to Alzheimer's disease: unveiling “the switch” with the senescence-accelerated mouse model (SAMP8) J Alzheimers Dis. 2008;15(4):615–624. doi: 10.3233/jad-2008-15408. [DOI] [PubMed] [Google Scholar]
- [10].Mohammed AH, Zhu SW, Darmopil S, et al. Environmental enrichment and the brain. Prog Brain Res. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- [11].van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- [12].Rampon C, Jiang CH, Dong H, et al. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci U S A. 2000;97(23):12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schrijver NC, Bahr NI, Weiss IC, et al. Dissociable effects of isolation rearing and environmental enrichment on exploration, spatial learning and HPA activity in adult rats. Pharmacol Biochem Behav. 2002;73(1):209–224. doi: 10.1016/s0091-3057(02)00790-6. [DOI] [PubMed] [Google Scholar]
- [14].Lee EH, Hsu WL, Ma YL, et al. Enrichment enhances the expression of sgk, a glucocorticoid-induced gene, and facilitates spatial learning through glutamate AMPA receptor mediation. Eur J Neurosci. 2003;18(10):2842–2852. doi: 10.1111/j.1460-9568.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- [15].Bennett JC, McRae PA, Levy LJ, et al. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85(2):139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [16].Friske JE, Gammie SC. Environmental enrichment alters plus maze, but not maternal defense performance in mice. Physiol Behav. 2005;85(2):187–194. doi: 10.1016/j.physbeh.2005.03.022. [DOI] [PubMed] [Google Scholar]
- [17].Yuan ZY, Gu P, Liu L, et al. Neuroprotective effects of enriched environment in MPTP-treated SAMP8 mice. Neurosci Lett. 2009;454(1):6–10. doi: 10.1016/j.neulet.2009.02.058. [DOI] [PubMed] [Google Scholar]
- [18].Scarmeas N, Luchsinger JA, Brickman AM, et al. Physical activity and Alzheimer disease course. Am J Geriatr Psychiatry. 2011;19(5):471–481. doi: 10.1097/JGP.0b013e3181eb00a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Radak Z, Hart N, Sarga L, et al. Exercise plays a preventive role against Alzheimer's disease. J Alzheimers Dis. 2010;20(3):777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- [20].Nation DA, Hong S, Jak AJ, et al. Stress, exercise, and Alzheimer's disease: a neurovascular pathway. Med Hypotheses. 2011;76(6):847–854. doi: 10.1016/j.mehy.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Um HS, Kang EB, Koo JH, et al. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer's disease. Neurosci Res. 2011;69(2):161–173. doi: 10.1016/j.neures.2010.10.004. [DOI] [PubMed] [Google Scholar]
- [22].Hattiangady B, Rao MS, Shetty GA, et al. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- [23].Gooney M, Messaoudi E, Maher FO, et al. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiol Aging. 2004;25(10):1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [24].Mohammed AH, Zhu SW, Darmopil S, et al. Environmental enrichment and the brain. Prog Brain Res. 2002;138:109–133. doi: 10.1016/S0079-6123(02)38074-9. [DOI] [PubMed] [Google Scholar]
- [25].Pham TM, Winblad B, Granholm AC, et al. Environmental influences on brain neurotrophins in rats. Pharmacol Biochem Behav. 2002;73(1):167–175. doi: 10.1016/s0091-3057(02)00783-9. [DOI] [PubMed] [Google Scholar]
- [26].Herring A, Ambrée O, Tomm M, et al. Environmental enrichment enhances cellular plasticity in transgenic mice with Alzheimer-like pathology. Exp Neurol. 2009;216(1):184–192. doi: 10.1016/j.expneurol.2008.11.027. [DOI] [PubMed] [Google Scholar]
- [27].Farmer J, Zhao X, van Praag H, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- [28].Kramer AF, Colcombe SJ, McAuley E, et al. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26(Suppl 1):124–127. doi: 10.1016/j.neurobiolaging.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [29].García-Mesa Y, López-Ramos JC, Giménez-Llort L, et al. Physical exercise protects against Alzheimer's disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24(3):421–454. doi: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- [30].Laurin D, Verreault R, Lindsay J, et al. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- [31].Zhang L, Fang Y, Zeng Z, et al. BDNF gene polymorphisms are associated with Alzheimer's disease-related depression and antidepressant response. J Alzheimers Dis. 2011;26(3):523–530. doi: 10.3233/JAD-2011-110113. [DOI] [PubMed] [Google Scholar]
- [32].Kafitz KW, Rose CR, Thoenen H, et al. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401(6756):918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- [33].Foster PP, Rosenblatt KP, Kuljiš RO. Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer's disease. Front Neurol. 2011;2:28. doi: 10.3389/fneur.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [35].Hrubá L, Schutová B, Pometlová M, et al. Effect of methamphetamine exposure and cross-fostering on cognitive function in adult male rats. Behav Brain Res. 2010;208(1):63–71. doi: 10.1016/j.bbr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- [36].Ji DB, Ye J, Li CL, et al. Antiaging effect of Cordyceps sinensis extract. Phytother Res. 2009;23(1):116–122. doi: 10.1002/ptr.2576. [DOI] [PubMed] [Google Scholar]
- [37].Lee DH, Jeon BT, Jeong EA, et al. Altered expression of sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 in mouse hippocampus after kainic acid treatment. Biochem Biophys Res Commun. 2010;393(3):476–480. doi: 10.1016/j.bbrc.2010.02.027. [DOI] [PubMed] [Google Scholar]
- [38].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


