Abstract
OBJECTIVE:
To evaluate the treatment effects and safety of topiramate in migraine prophylaxis.
DATA RETRIEVAL:
We searched the Medline database, EMbase, Cochrane Library and China National Knowledge Infrastructure database for articles published between January 1995 and May 2011, using the key words “migraine”, “topiramate”, and “prophylaxis”.
SELECTION CRITERIA:
We selected randomized controlled trials of migraine patients, in which the experimental group was orally administered topiramate, and the control group was given placebo. Odds ratios (ORs) and mean differences (MDs) were calculated using a fixed effects model/random effects model. Quality evaluation and data extraction were performed independently by two researchers utilizing RevMan 5.0 software.
MAIN OUTCOME MEASURES:
Efficacy was recorded as the responder rate (response defined as at least a 50% reduction in average monthly migraine frequency) and change in mean monthly number of migraine days. Adverse events were recorded as the number of subjects exhibiting at least one adverse event.
RESULTS:
Eight randomized controlled trials were found to be appropriate, and had available data. The meta-analysis results revealed that topiramate (100 or 200 mg/d) was more effective than placebo in responder rate (OR = 2.97, 95% confidence interval (CI): 2.17–4.08, P < 0.01; OR = 2.35, 95%CI: 1.77–3.12, P < 0.01). Topiramate (100 mg/d) was more effective than placebo in terms of the change in mean monthly migraine days (MD: –1.14, 95%CI: –1.69 to –0.59, P < 0.01). The total incidence rate of adverse events for topiramate was higher than in the placebo group (P < 0.01), but most adverse events were mild to moderate.
CONCLUSION:
Overall, topiramate obtained good outcomes and safety in migraine prophylaxis.
Keywords: topiramate, migraine, prophylaxis, efficacy, meta-analysis, neural regeneration
Research Highlights
Meta-analysis results revealed that topiramate exhibited good outcomes and safety in migraine prophylaxis.
Abbreviations
OR, odds ratio; MD, mean difference; CI, confidence interval
INTRODUCTION
Migraine is characterized by recurrent unilateral/bilateral throbbing headache. Studies have documented the high prevalence and high socio- economic and personal impacts of migraine. The World Health Organization now ranks migraine as 19th among all diseases causing disability[1]. Although symptomatic migraine therapy is helpful in relieving pain temporarily, for patients with frequent episodes of migraine, severe intensity of headache or poor response to acute-care medications, preventing the headache attack is extremely important. Topiramate is a commonly used drug in prophylaxis of migraine. As such, summarizing and evaluating the efficacy and safety of the drug in time will be useful in the preventive treatment of migraine. Although some previous studies[2,3] have examined the efficacy of topiramate in migraine prophylaxis, most referred to only three major trials[4,5,6]. A meta-analysis performed in 2009 determined the decrease of average monthly migraine frequency, and revealed a significant improvement in the topiramate group[7].
We searched for randomized controlled clinical trials published between January 1995 and May 2011, and performed a meta-analysis including some high quality smaller trials to determine the relative effectiveness and safety of topiramate compared with placebo for the prevention of migraine attacks.
DATA AND METHODOLOGY
Data retrieval
We conducted a search of randomized clinical trials published between January 1995 and May 2011 in the Medline database (PubMed), EMbase, Cochrane Library and China National Knowledge Infrastructure database. All studies that included the following terms in their key words were considered: “migraine”, “topiramate”, and “prophylaxis”. The references included in the published articles identified in these searches were used as an additional source to identify other clinical trials. All included articles were written in English or Chinese.
Inclusion and exclusion criteria
Study type: Randomized controlled clinical trials.
Subjects: Migraine patients diagnosed by the criteria of ICHD-I[8] (diagnose criteria 1.2.3 Treatment): the experimental group was orally administered topiramate 100–200 mg per day, and the control group was given placebo. For studies comparing more than two groups of anti-migraine drugs, we selected only the two groups we needed to analyze.
Quality evaluation
Two researchers collected the quality assessment of the included studies in terms of improved Jadad scores[9], 1–3 scores represent low quality, and 4–7 scores represent high quality studies.
Data extraction
Two researchers read the titles and abstracts of each study independently, excluded trials that did not meet the inclusion criteria, and then crosscheck was done. In the case of divergence, the two researchers undertook discussion to decide whether to include or exclude the study. The obtained data included information on report identification, methodology, subjects and treatment.
Outcome measures
The primary endpoint was the responder rate (response defined as at least a 50% reduction in average monthly migraine frequency). The secondary endpoint was change of mean monthly migraine days. Adverse events were recorded as the number of subjects with at least one adverse event.
Statistical analysis
We performed all analyses using RevMan version 5.1 (The Cochrane Collaboration, Copenhagen, Denmark). Odds ratios (ORs) and mean differences (MDs) were calculated to represent categorical data and variable data, respectively, and 95% confidence intervals (CI) were computed to represent the efficacy and adverse events. We computed Chi-square tests with a significance level of α = 0.05 to compare topiramate and placebo for efficacy and adverse events. We used I2 to test the heterogeneity of multiple tests, and the significance level was set at 50%. If I2< 50%, there was no significant heterogeneity among the tests, and the data were calculated using a fixed effects model. If I2> 50%, the heterogeneity among the tests was considered significant, and the data were calculated using a random effects model.
RESULTS
Data retrieval
From 357 citations, 49 clinical trials were identified, of which eight were deemed appropriate and had available data[4,5,6,10,11,12,13,14] (Figure 1).
Figure 1.
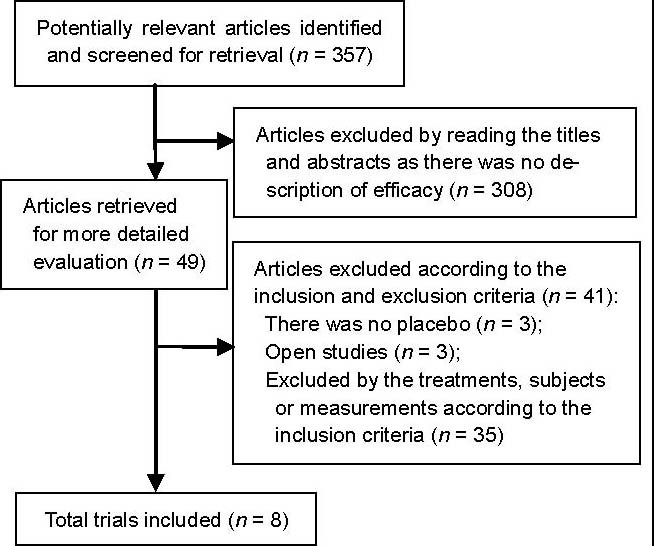
Trial flow for selection of study reports.
Baseline analysis and quality estimation
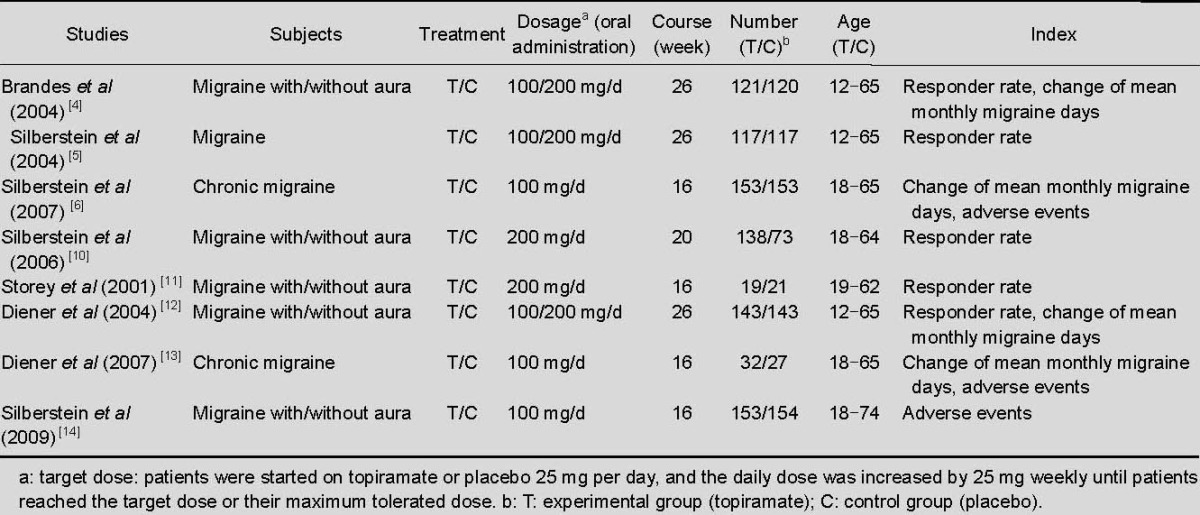
All eight included trials were randomized double-blind controlled trials. On the improved Jadad scale, two studies achieved seven points[4,5], two studies achieved six points[6,13], and the remaining four studies achieved four points[10,11,12,14]. Characteristics of the included trials are shown in Table 1.
Table 1.
Characteristics of included trials
Meta-analysis
Comparison of effects of topiramate 200 mg/d and placebo on responder rate
Regarding the comparison of topiramate 200 mg/d with placebo, five studies[4,5,10,11,12] reported outcomes for responder rate. There was no significant heterogeneity among the studies (I2 = 47%), and the data were calculated using a fixed effects model. Topiramate 200 mg/d was clearly superior to placebo with OR for a responder rate of 2.35 (95% CI: 1.77–3.12, P < 0.01; Figure 2).
Figure 2.
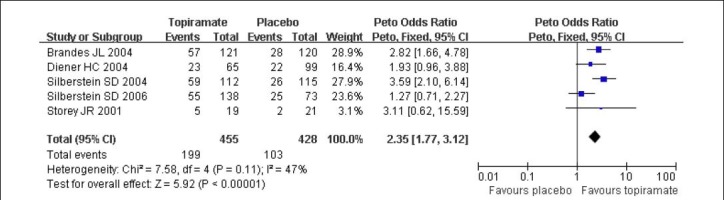
Forest plot of topiramate 200 mg/d vs. placebo for responder rate.
Studies shown in the figure (from up to down) are citations [4,12,5,10,11]. CI: Confidence interval; df: degrees of freedom.
Comparison of topiramate 100 mg/d with placebo
Three studies[4,5,12] reported outcomes for responder rate. There was no significant heterogeneity among the studies (I2 = 10%, P = 0.33), and the data were calculated using the fixed effects model. Topiramate 100 mg/d was significantly superior to placebo with OR for a responder rate of 2.97 (95% CI: 2.17–4.08, P < 0.01; Figure 3).
Figure 3.
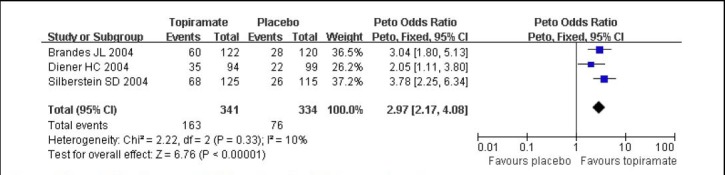
Forest plot of topiramate 100 mg/d vs. placebo for responder rate.
Studies shown in the figure (from up to down) are citations [4,12,5]. CI: Confidence interval; df: degrees of freedom.
Mean monthly migraine days after topiramate prophylaxis
Regarding comparison of topiramate 100 mg/d with placebo, four studies[4,6,12,13] reported outcomes for the change in mean monthly migraine days. The data were calculated using a random effects model because of significant heterogeneity among the studies (I2 = 98%, P < 0.01). Topiramate 100 mg/d was significantly superior to placebo with MD for reduction of mean monthly migraine days (P < 0.01; Figure 4).
Figure 4.
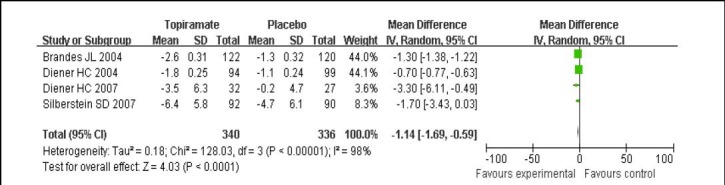
Forest plot of topiramate 100 mg/d vs. placebo for change of mean monthly migraine days.
Studies shown in the figure (from up to down) are citations [4,12,13,6]. CI: Confidence interval; df: degrees of freedom.
Adverse events
Regarding the comparison of topiramate (100 mg/d) with placebo, three studies[6,13,14] reported the total incidence rate of adverse events. There was no significant heterogeneity among the studies (I2 = 14%, P = 0.31), and the data were calculated using a fixed effects model. The total incidence rate of adverse events of topiramate 100 mg/d was higher than that of placebo (P < 0.01; Figure 5). The most common adverse events in the topiramate group were paresthesia, nausea, anorexia, weight loss, upper respiratory tract infection, and fatigue.
Figure 5.
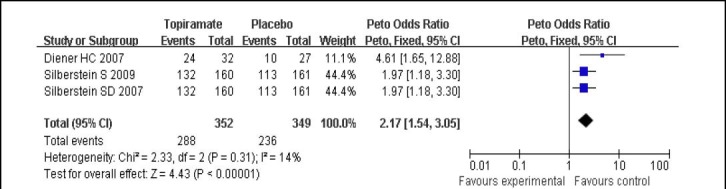
Forest plot of topiramate 100 mg/d vs. placebo for adverse events.
Studies shown in the figure (from up to down) are citations [13,14,6]. CI: Confidence interval; df: degrees of freedom.
Sensitivity analysis
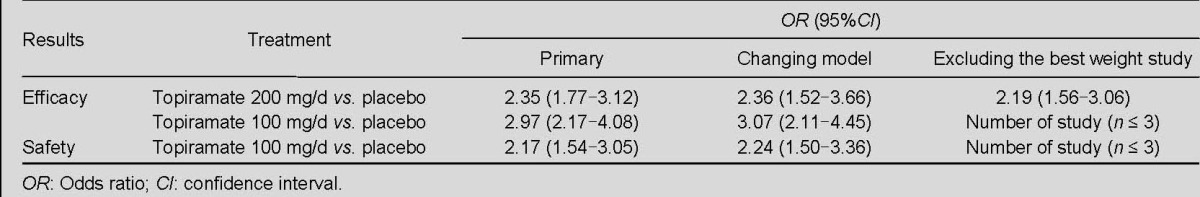
Regarding comparisons of topiramate (200 mg/d) with placebo for responder rate, and topiramate (100 mg/d) with placebo for change of mean monthly migraine days, sensitivity analyses determined by calculating model and weight did not yield different results. Regarding comparisons of topiramate (100 mg/d) with placebo for responder rate and topiramate (100 mg/d) with placebo for adverse events, sensitivity analyses by weight were not possible (n ≤ 3), sensitivity analyses conducted by changing the model (comparing the fixed effects and random effects models) did not yield different results. The results of the sensitivity analysis are shown in Table 2.
Table 2.
Results of sensitivity analysis
Bias analysis
We assessed the possibility of publication bias by evaluating a funnel plot, in the comparison of topiramate 200 mg/d with placebo for responder rate; no evidence of publication bias was found (Figure 6). Regarding the comparisons of topiramate 100 mg/d with placebo for responder rate, change of mean monthly migraine days and adverse events, there were less than five studies included in each comparison; it was not possible to conduct bias analysis by evaluating funnel plot.
Figure 6.
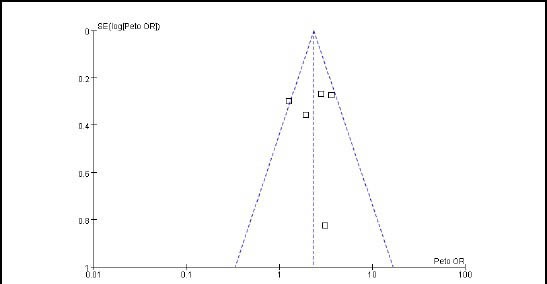
Funnel plot of topiramate 200 mg/d vs. placebo for responder rate.
X-axis: Log odds ratios (ORs); Y-axis: 1/standard error (log ORs).
DISCUSSION
All eight studies identified in this meta-analysis reported the efficacy of topiramate in migraine prophylaxis[4,5,6,10,11,12,13,14], and the results revealed a significant difference between topiramate and placebo in the responder rate and change of mean monthly migraine days for migraine prophylaxis. All eight studies reported adverse events, but only three of them reported the total incidence rate of adverse events for topiramate and placebo. The most common adverse events in the topiramate group were paresthesia, nausea, anorexia, weight loss, upper respiratory tract infection, and fatigue. Most adverse events were mild to moderate, and severe adverse events were rarely reported[11,13,14] (no evidence to confirm that they were relative to the treatment). Data from randomized controlled trials revealed that topiramate was significantly superior to placebo in migraine prophylaxis, and was generally safe.
In the present study, the responder rate was defined as at least a 50% reduction in average monthly migraine frequency. The mean monthly headache days, categorical responses (based on mean monthly migraine/migrainous days, mean monthly (28-day) migraine days, and mean monthly total headache days), using acute drugs days, headache severity and headache duration have all been used to represent efficacy in previous studies. Two studies compared the reduction of mean monthly headache days in the topiramate and placebo groups, and found a significant improvement in the topiramate group[4,12]. Two studies reported categorical responses[13,14], and six studies reported a change in the mean monthly number of migraine days before and after treatment[4,5,6,12,13,14], suggesting that topiramate is superior to placebo in reducing migraine attacks. Three studies have confirmed a lack of significant differences in the change of headache intensity between topiramate (50–200 mg/d) and placebo[11,14,15]. Two studies reported the duration of headache[6,15], and no significant difference was found between the topiramate (100 mg/d) group and the placebo group, but in one study a significant reduction of headache duration was reported in the topiramate (200 mg/d) group[4]. Three studies reported a significant reduction of the number of days in which acute drugs were used in the topiramate (100 mg/d) group compared with placebo[4,5,12]. As the number of cases was limited, no unified criteria were used to describe the results in these studies, and more trials are needed to confirm these conclusions.
All eight trials in this study were high quality studies, achieving more than four points on the improved Jadad scale. Only two studies (which achieved seven points on the improved Jadad scale) described the blinding and randomization precisely. In contrast, the remaining six studies may have been affected by shortcomings in blinding and randomizaton[4,5]. The trials may have been affected by selection bias, execution bias, detection bias and other potential biases, which may have influenced the quality of the results. To exclude the influence of publication bias, we included as many smaller high quality trials as possible, producing a symmetrical funnel plot, suggesting that potential publication bias exerted no substantive effect on results. The age of subjects, drug dosage and course of treatment differed between trials, and there was clinical heterogeneity among the studies. These factors may have influenced the validity of our conclusion. In future studies, methods of randomization, allocation concealment, and blinding should be described in detail. In addition, it is important for future studies to implement uniform duration and dosage, to reduce potential bias.
In conclusion, the results revealed that topiramate is effective in migraine prophylaxis. The responder rate and reduction in mean monthly number of migraine days were found to be better in the treatment than in the placebo group, and the drug was found to be generally safe. As the number of included studies was small, and there was heterogeneity among studies, the conclusion requires confirmation in future. More high-quality randomized controlled clinical trials are needed to provide more robust evidence for the efficacy of topiramate in migraine prophylaxis.
Footnotes
Conflicts of interest: None declared.
(Edited by Wang HL, Qi JP/Qiu Y/Song LP)
REFERENCES
- [1].Cephalalgia. 2nd ed. suppl 1. Vol. 24. The International Classification of Headache Disorders; 2004. Headache Classification Subcommittee of the International Headache Society; pp. 9–160. [DOI] [PubMed] [Google Scholar]
- [2].Mulleners WM, Chronicle EP. Anticonvulsants in migraine prophylaxis: a Cochrane review. Cephalalgia. 2008;28(6):585–697. doi: 10.1111/j.1468-2982.2008.01571.x. [DOI] [PubMed] [Google Scholar]
- [3].Winner P, Gendolla A, Stayer C, et al. Topiramate for migraine prevention in adolescents: a pooled analysis of efficacy and safety. Headache. 2006;46(10):1503–1510. doi: 10.1111/j.1526-4610.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- [4].Brandes JL, Saper JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA. 2004;291(8):965–973. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- [5].Silberstein SD, Neto W, Schmitt J, et al. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol. 2004;61(4):490–495. doi: 10.1001/archneur.61.4.490. [DOI] [PubMed] [Google Scholar]
- [6].Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. Headache. 2007;47(2):170–180. doi: 10.1111/j.1526-4610.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- [7].Hu YM, Lv Y, Zhang XQ, et al. Topiramate for migraine prevention-Meta analysis. Guoji Binglixue yu Linchuang Zazhi. 2009;29(5):374–377. [Google Scholar]
- [8].Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- [9].Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary. Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- [10].Silberstein SD, Hulihan J, Karim MR, et al. Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: a randomized, placebo-controlled, double-blind, 12-week pilot study. Clin Ther. 2006;28(7):1002–1011. doi: 10.1016/j.clinthera.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [11].Storey JR, Calder CS, Hart DE, et al. Topiramate in migraine prevention: a double-blind, placebo-controlled study. Headache. 2001;41(10):968–975. doi: 10.1046/j.1526-4610.2001.01190.x. [DOI] [PubMed] [Google Scholar]
- [12].Diener HC, Tfelt-Hansen P, Dahlöf C, et al. Topiramate in migraine prophylaxis--results from a placebo-controlled trial with propranolol as an active control. J Neurol. 2004;251(8):943–950. doi: 10.1007/s00415-004-0464-6. [DOI] [PubMed] [Google Scholar]
- [13].Diener HC, Bussone G, Van Oene JC, et al. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27(7):814–823. doi: 10.1111/j.1468-2982.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- [14].Silberstein S, Lipton R, Dodick D, et al. Topiramate treatment of chronic migraine: a randomized, placebo-controlled trial of quality of life and other efficacy measures. Headache. 2009;49(8):1153–1162. doi: 10.1111/j.1526-4610.2009.01508.x. [DOI] [PubMed] [Google Scholar]
- [15].Lakshmi CV, Singhi P, Malhi P, et al. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J Child Neurol. 2007;22(7):829–835. doi: 10.1177/0883073807304201. [DOI] [PubMed] [Google Scholar]


