Abstract
The lack of curative therapies for neurodegenerative diseases has high economic impact and places huge burden on the society. The contribution of stem cells to cure neurodegenerative diseases has been unraveled and explored extensively over the past few years. Beyond substitution of the lost neurons, stem cells act as immunomodulators and neuroprotectors. A large number of preclinical and a small number of clinical studies have shown beneficial outcomes in this context. In this review, we have summarized the current concepts of stem cell therapy in neurodegenerative diseases and the recent advances in this field, particularly between 2010 and 2012. Further studies should be encouraged to resolve the clinical issues and vague translational findings for maximum optimization of the efficacy of stem cell therapy in neurodegenerative diseases.
Keywords: stem cells, neurodegenerative diseases, Parkinson's disease, Huntington's disease, Alzheimer's disease, amyotrophic lateral sclerosis, embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells, neural stem cells
Research Highlights
-
(1)
Stem cell therapy is probably the only potential treatment modality which offers ‘cure’ for neurodegenerative diseases.
-
(2)
The structural and functional improvements seen in animals need further evaluation prior to extrapolation to humans. Hence, the clinical outcome and long term safety of stem cell therapy in humans with neurodegenerative diseases are still questionable.
-
(3)
Of the 4 types of neurodegenerative diseases described in the literature, there is relatively more evidence for stem cell therapy in Parkinson's disease and amyotrophic lateral sclerosis compared to Huntington's disease and Alzheimer's disease.
Abbreviations
PD, Parkinson's disease; HD, Huntington's disease; ALS, amyotrophic lateral sclerosis; ESCs, embryonic stem cells; IPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells or mesenchymal stromal cells; SVZ, subventricular zone; SGZ, subgranular zone; GDNF, glial cell line-derived neurotrophic factor; AD, Alzheimer's disease
INTRODUCTION
Stem cell therapy has revolutionized the field of medicine over the past few decades. Stem cells have been proven to be invaluable in the treatment of numerous diseases affecting various organs in the human body. The list of therapeutic applications of stem cells in clinical practice continues to expand steadily over the years. Neurodegenerative diseases became a landmark in the history of stem cell therapy back in the 1980s when patients suffering from Parkinson's disease (PD) in Mexico were treated with this form of therapy with variable outcome[1]. Today, stem cell therapy offers promising hope for almost all forms of neurodegenerative diseases including PD, Huntington's disease (HD), Alzheimer's and amyotrophic lateral sclerosis (ALS). The fundamental mechanism underlying all forms of neurodegenerative diseases is progressive loss of structure, function or number of neurons, including death of neurons[2]. At a molecular level, there are many parallels among the different forms of neurodegenerative diseases. Unfortunately, the current available treatment options neither pharmacological nor neurosurgical are efficient in arresting the progression of the neurodegenerative processes. Stem cell therapy, on the other hand, enables regeneration of neural tissue which ameliorates neurodegeneration occurring at different levels of the neuronal circuitry. We searched the electronic databases of the PubMed and the Cochrane Library for related studies encompassing basic sciences, translational, clinical and epidemiological research. In this review, we have summarized the principles, clinical applications and the recent progress of stem cell therapy in neurodegenerative diseases.
TYPES OF STEM CELLS
Embryonic stem cells (ESCs)
ESCs are pluripotent and retain the ability to self-renew indefinitely with the capacity to differentiate into almost all cell types of the central nervous system. These cells are currently being used as an inexhaustible source of neurons to perform experiments involving new candidate drugs in neurodegenerative diseases[3]. The first clinical application of ESCs-derived tissue in the central nervous system was oligodendrocytes in the treatment of spinal cord injury. The initial approval for this clinical application by the Food and Drug Administration (FDA) in January 2009 (http://www.geron.com/products/productinformation/spinalcordinjury.aspx) was put on hold after preclinical studies showed a higher frequency of epithelial cysts within the grafted spinal cords compared to the rodent studies. The subsequent applications for clinical trials using ESCs-derived tissues are still awaiting approval[4,5].
In 2011, Nistor et al[6], for the first time described a method for producing human neuronal progenitors in large quantity and high purity from ESCs. The human neuronal progenitor population produced displayed characteristic neuronal-specific markers and spontaneously differentiated into neuronal subtypes in vitro, i.e. cholinergic, serotonergic, dopaminergic, noradrenergic, and medium spiny striatal neurons. In this recent study, the ESCs derived human neuronal progenitors, when transplanted into the injured spinal cord and integrated into host tissue, matured into a variety of neurons.
Translating ESCs into novel therapies in neurodegenerative diseases needs careful consideration as it is associated with the risk of tumour formation. This threat is due to the persistence of nondifferentiated cells that undergo malignant transformation and genetic instability following prolonged time in culture[7,8].
Induced pluripotent stem cells (IPSCs)
The main breakthrough in regenerative medicine is the reprogramming of adult somatic cells to acquire similar characteristics as ESCs. These cells are referred to as IPSCs. Although the initial reprogramming required retroviral transfer into fibroblasts of four transcription factors, subsequent research demonstrated that fewer factors might suffice[9,10,11].
Such reprogrammed cells now offer the promising avenue to generate autologous dopaminergic neurons for transplantation in PD patients. Of note, the IPSCs platform has a distinct advantage over ESCs in the sense that IPSCs are autologous and therefore the transplantation does not require immunosuppressive agents[12]. However, similar to ESCs, an important risk of IPSCs is tumour formation. The differentiation of IPSCs into mature neurons is more difficult than ESCs. Hence, the clinical application of IPSC in neurodegenerative diseases is still not feasible in the near future owing to the lack of in-depth research evaluating its therapeutic safety among human subjects[8].
Vierbuchen et al[13] in 2010 through their elegant work demonstrated that it may be possible to transform fibroblasts from adult mice into mature neurons without the intermediate step of IPSCs. This strategy which is not only ethically acceptable, may also overcome the risk of tumour formation.
Mesenchymal stem cells or mesenchymal stromal cells (MSCs)
MSCs, with pluripotent differentiation capacity, are an ideal source for cell transplantation in neurodegenerative diseases. The International Society for Cellular Therapy proposed a panel of markers for the identification of MSCs which include CD44, CD73, CD90, and CD105[14]. The exact regulating mechanism underlying MSC proliferation and differentiation remains vague. This critical issue has to be addressed for safe translational clinical application. Increasing depth of MSCs knowledge has led to a few successful FDA-approved phase I–III clinical trials for bone and cartilage diseases. MSC-derived functional neurons appear more promising in neurodegenerative diseases compared to ESCs given the less related ethical and immunorejection problems[15].
In preclinical studies of neurodegenerative diseases, MSCs were delivered via either intracerebral or intrathecal injection. Following transplantation, MSCs promote neuronal growth, decrease apoptosis, reduce release of free radicals, and suppress inflammation. At present, there are ongoing clinical trials evaluating MSCs in the treatment of amyotrophic lateral sclerosis, traumatic brain injury, and stroke[16,17].
In a recent study by Gong et al[18] published in 2011, immortalized mesenchymal stem cells were generated by using SSR#69 retrovirus expressing simian virus 40 large T (SV40T) antigen. Immortalized MSCs outperformed primary MSCs by demonstrating higher proliferation capacity and anti-senescence ability in addition to basic features of primary MSCs. Senescence was a serious drawback in MSCs application prospects for cell-based regenerative medicine. This study highlighted that immortalized MSCs were a superior alternative to MSCs in neuronal differentiation and neuroregeneration.
Neural stem cells (NSCs)
NSCs can be produced from fetal or adult central nervous tissue via the dissection of specific brain regions. Several growth media facilitate the proliferation of such cells when supplemented with mitogens such as epidermal growth factor and fibroblast growth factor 2. NSCs have the capacity to differentiate into oligodendrocytes, neurons, and astrocytes[19].
A study by Huang et al in 2012, demonstrated for the first time that NSCs can synthesize d-serine[20]. d-Serine, the co-agonist of N-methyl-D-aspartate receptors, has been recognized as an important gliotransmitter in the central nervous system. d-serine has been shown to regulate neurogenesis by promoting NSC differentiation into neurons. Degradation of endogenous d-serine in their study, on the other hand, significantly inhibited the proliferation and neuronal differentiation of NSCs[20].
Low oxygen conditions and hypoxia-inducible factor 1 alpha were identified to be critical for NSC development. These conditions were successfully used to expand epidermal growth factor/fibroblast growth factor 2 responding cells for a period of more than a year[21,22,23,24,25]. A recent study by Wei et al[26] indicated that Wnt/beta-catenin signaling is critical in the control of proliferation and differentiation of NSCs in the hippocampus. In the study, the effects of low-dose radiation in stimulating Wnt/beta-catenin signaling and neurogenesis in the hippocampus were identified in both in vitro and in vivo animal studies. Low-dose radiation (0.3 Gy) induced significant increase of Wnt1, Wnt3a, Wnt5a, and beta-catenin expression in NSCs. Besides, it promoted cell survival and reduced apoptosis of NSCs by flow cytometry analysis.
Midbrain-derived cells could differentiate into dopaminergic neurons at a low rate[27,28,29,30]. Human NSCs which have the potential for stable expansion and in vitro differentiation into neurons, are an attractive cell source for regenerative strategies in many brain diseases such as neurodegenerative diseases, stroke, and spinal cord injury. At present, only fetal tissue-derived NSCs have made it into the clinical arena. Preclinical murine studies revealed that transplantation in the brain of mice with a disorder similar to infantile neuronal ceroid lipofuscinosis (Batten's disease) integrate into the host cells, release the defective enzyme, and also offer neuroprotection[29]. The favorable outcome of this study is likely to foster further clinical applications of human NSCs in central nervous system diseases. Unlike ESCs, NSCs are considered safe and less tumorigenic.
NSCs have been modified genetically by researchers via ectopic expression of the oncogene c-myc to produce immortalized NSCs with increased proliferative potential. Clinical application of such cells was approved among patients post-stroke[30,31].
EVOLVING CONCEPTS OF NEUROGENESIS
It has become convincingly apparent that neurogenesis in the central nervous system of primates, including humans occurs throughout life. About 9 000 to 10 000 new cells are generated daily in the rodent hippocampus of which up to 90% differentiate into neurons[32]. This capability has been clearly demonstrated particularly at two locations: the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus. Neurons born in the SVZ, migrate through the rostral migratory stream to the olfactory bulb and become granule neurons and periglomerular neurons. As the precursors migrate towards their destination, three well-defined processes take place i.e. proliferation, cell death/survival, and differentiation. Neurons born in the SGZ, on the other hand, migrate and become dentate granule cells in the granule cell layer of the dentate gyrus[33,34]. Recent studies have proven that newborn neurons in the brains integrate into the existing neuronal circuitry and receive functional input.
Adult neurogenesis is regulated by various physiological and pathological factors at all levels from the proliferation of NSCs to maturation of the neurons. Apart from aging, the known down-regulating factors of neurogenesis are stress, glucocorticoids and inflammation[35,36,37]. Conversely, the up-regulating factors are estrogen, antidepressant drugs and growth factors such as brain-derived neurotrophic factor (BDNF) and insulin growth factor 1[38,39,40,41,42,43]. There are still controversies on whether neurogenesis occurs at sites other than the SVZ and SGZ[44].
Over the past decade, the field of regenerative medicine has witnessed remarkable progress in the concepts and understanding of neurogenesis. There are a few basic principles derived from recent studies which were summarized by Zhao et al in 2008[44]. (1) Neurogenesis is conserved in all mammalian species. (2) The process of neurogenesis is readily influenced by numerous external factors. (3) Adult neurogenesis shares similar mechanisms with embryonic neurogenesis. (4) The mechanisms of SVZ and SGZ neurogenesis are quite different, despite sharing certain common regulators. (5) The activities of newborn neurons from both SVZ and SGZ are critical for effective neurogenesis.
Although the processes described above occur throughout the life span of humans, there is a sharp decline from youth to the aged brain. In 2012, Encinas et al[45] pointed out that NSC deforestation is the culprit leading to age-related decline in hippocampal neurogenesis. Deforestation through differentiation of NSCs into astrocytes contributed to the diminishing pool of quiescent neural progenitors in the dentate gyrus[46,47]. The “NSC deforestation model” explained that in terms of frequency, astrogliogenesis occurred parallel to neurogenesis.
Recently, Cunningham et al[48] discovered that hypoxia-inducible factor-1alpha played a central role in the survival of NSCs through Notch and Wnt/beta-catenin signaling pathways. Hypoxia-inducible factor-1alpha is a mediator of adaptive cellular responses to hypoxia through regulation of cellular metabolism and angiogenesis. These molecules are involved in the maintenance of the ideal vascular environment of the NSCs niche enhancing the repair of the brains.
The fundamental mechanism for the observed improvements after stem cell therapy in the central nervous system is believed to be neuroprotection. Neuroprotection is achieved through the secretion of growth factors i.e. brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor, and nerve growth factor. Genetically engineered stem cells in recent studies have the tendency to overexpress growth factors which in turn enhance their neuroprotective capacity[49].
The outcome of stem cell therapy can be improved through the combination with other adjunct therapies. For example, stem cell therapy with erythropoietin demonstrated synergistic effects on neurogenesis in a rat model[50]. Exploration of other strategies in this regard has been sparse. Post stem cell therapy, the survival of stem cells can be jeopardised by poor vascularisation, inflammation or rejection mediated by the immune response of the host[49].
Despite the remarkable advance in our scientific knowledge and understanding of stem cell biology, there are still many hurdles to cross and multiple challenges to overcome. Unfortunately, the vast majority of studies are based on animal models. Researchers have not answered the compelling question of whether human stem cell populations will exhibit similar functional properties to their murine and rodent counterparts.
Translating stem cell therapy to the neurology clinics is not possible without the identification of a suitable source of stem cells for therapeutic applications. Different neurological conditions are better suited to a certain cell type than others. For example, NSCs are a good option for spinal cord injury whereas MSCs are better for multiple sclerosis. The cost, time and labor intensive nature of stem cell therapy limit its use especially in developing and third world countries. A potential solution for this concern is the use of broad spectrum stem cell therapy with multipotent adult precursor cells (MAPC cells). These cells are currently being tested in neural pathologies[51].
EXPERIMENTS AND EXPERIENCES OF STEM CELL THERAPY IN NEURODEGENERATIVE DISEASES
PD
PD is typically a disease of the basal ganglia characterized by progressive degeneration of dopaminergic neurons in the substantia nigra. The depletion of dopamine in the nigrostriatal pathway leads to motor dysfunction. The current available therapies for PD address symptoms but do not cure this illness.
Over the past two decades, preclinical and clinical trials in PD patients have demonstrated that stem cell therapy of human embryonic mesencephalic tissue has the capacity to reinnervate the striatum. PD, in fact, has emerged as the best-suited neurodegenerative diseases for stem cell therapy[52,53]. The essence of stem cell therapy in PD is the ability of stem cells to differentiate into dopaminergic neurons. Through genetic engineering, dopaminergic neurons were obtained from rat NSCs, mouse fibroblasts and human ESCs[1,54].
Soldner and colleagues’ finding that fibroblasts from PD patients can be reprogrammed to differentiate into dopaminergic neurons was a turning point in the clinical area of PD[52]. ESCs, NSCs and bone marrow stem cells (BMSCs) all successfully survived when grafted in animal models of PD[53,54]. Of note, not only were ESCs found to release significant amounts of dopamine but both ESCs and NSCs derived from the embryonic ventral mesencephalon produced promising functional outcome[54,55].
There were a few double-blinded trials presented with negative results[56,57]. In those trials, patients who were transplanted with fetal tissues did not improve significantly especially the older patients[56,57]. These inconsistent results could be due mainly to variation in the collection of stem cells[56,58,59,60]. Several factors have been proposed to influence the development of dopaminergic neurons such as orthodenticle homeobox 2 (OTX2), orphan nuclear receptor-related factor 1 (Nurr1), tyrosine hydroxylase, fibroblast growth factor 8 (FGF8), engrailed genes (En1, En2), Wingless (Wnt), (Fox) A2 and neurogenin (Ngn). Figure 1 summarizes the sources of stem cells and factors regulating stem cells differentiation in PD.
Figure 1.
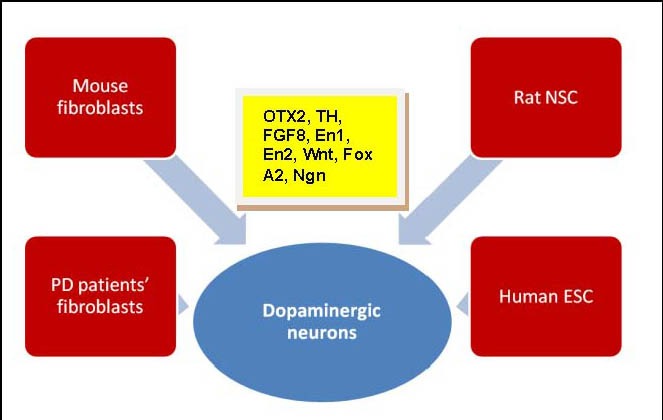
Sources of stem cells in Parkinson's disease (PD).
Factors regulating stem cells differentiation into dopaminergic neurons are orthodenticle homeobox 2 (OTX2), orphan nuclear receptor-related factor1 (Nurr1), tyrosine hydroxylase (TH), fibroblast growth factor 8 (FGF8), engrailed genes (En1, En2), Wingless (Wnt), (Fox) A2 and neurogenin (Ngn).
NSC: Neural stem cells; ESC: embryonic stem cells.
A recent study demonstrated that multipassaged neural precursor cells from human ESCs had reduced life span[61]. Forced expression of Bcl-XL and SHH is believed to be able to tackle this problem[61]. A few studies have looked into generating dopaminergic neurons by overexpression of Nurr1 in stem cells[62,63,64,65].
Introducing Nurr 1 into stem cells resulted in motor function improvement with ESCs but not with NSCs[65,66]. Intriguingly, in the presence of stromal cells, dopaminergic neurons had improved survival and were better incorporated in the striatum[67,68].
A more recent study published in 2012, stated that stem cell therapy with human amniotic fluid stem cells and MSCs ameliorated bladder dysfunction in a PD rat model. In this study, cystometric parameters improved as early as 14 days after stem cell therapy[69]. Besides, glial cell line-derived neurotrophic factor (GDNF) has been implicated in the survival of ventral midbrain dopaminergic neurons. Treatment of midbrain NSCs with GDNF increased the sphere diameter of the stem cells, and reduced the expression of caspase 3 while enhancing the expression of Bcl-2[70].
Deleidi et al[71] in 2011 reported for the first time that Oct-4 induced pluripotency caused successful differentiation of adult NSC into functional midbrain dopaminergic neurons in the SVZ of the brains. Dopaminergic neurons produced from Oct4- reprogrammed NSCs improved motor deficits in a rat model of PD.
Despite the impressive potential of stem cell therapy in PD, it carries the serious risk of graft-induced dyskinesias. Politis et al[72] in their study found that positron emission tomography after 14 years posttransplantation revealed an elevated serotonin to dopamine transporter ratio in the grafted striatum. This was compatible with serotonergic hyperinnervation which was the underlying mechanism for graft-induced dyskinesias.
HD
HD is a fatal progressive neurodegenerative diseases of autosomal dominant inheritance due to an expansion of cytosine-adenine-guanine repeats in the Huntingtin gene (htt). Mutated htt induces a preferential loss of medium spiny neurons of the striatum giving rise to motor, cognitive and emotional deficits. Experiments utilizing stem cells in HD is barely a decade old. Research on neural regeneration has been less intense in HD compared to PD with fewer preclinical and clinical trials. Nevertheless, there is convincing evidence that NSCs confer behavioral benefits in phenotypic models of HD[73,74].
Dey et al[75] in 2010 reported that MSCs, harvested from mouse femurs which were genetically engineered to over-express BDNF or nerve growth factor reduced behavioral deficits in the YAC 128 mouse model of HD. The transplanted mice showed less neuronal loss in the striatum on immunohistological examination. This study highlighted that intrastriatal transplantation of MSCs that over-express BDNF may create a conducive environment within the striatum that retards neurodegenerative processes.
Ebert et al[76], on the other hand, identified mouse NSC act as growth factor (GDNF) delivery vehicles to the brains of murine models of HD. GDNF has shown promising results in HD by reducing neuronal death and the resultant motor impairment.
Snyder et al[77] in their study discovered that the implantation of human multipotent stromal cells from bone marrow (hMSCs) into the dentate gyrus of the hippocampus of mice models of HD was shown to enhance proliferation and neural differentiation of endogenous NSCs for up to 30 days post stem cell therapy. Interestingly, these effects were seen despite the rapid disappearance of the implanted hMSCs over 3 to 15 days.
In HD monkeys, dental pulp stem cells were a potential source of personal stem cells for therapeutic purposes. dental pulp stem cells had multipotent differentiation capabilities. The advantage of this source of stem cell is they are true personal stem cells and can be readily isolated from individuals at any age[78]. It is rational to speculate that stem cell therapy with personal stem cells would require less intensive immunosuppressive therapy post transplant in humans.
Lin et al[79] concluded from their study that human bone marrow-derived MSCs based stem cell therapy in HD offered neuroprotection and neurorestoration through neural differentiation, neurotrophic support capability and anti-apoptotic effects. Mice models showed significant improvement in motor dysfunction as early as 10 weeks after stem cell therapy. Post transplantation, there were increased levels of laminin, Von Willebrand factor, stromal cell-derived factor-1, and its receptor Cxcr4.
Almost all studies on stem cell therapy in HD are animal based (Figure 2) and hence this area is still at its infancy. The applicability of the findings of the studies described earlier in humans is still a gray area. For the time being, stem cell therapy with regard to HD, is still far from being practiced in the neurology clinics worldwide.
Figure 2.
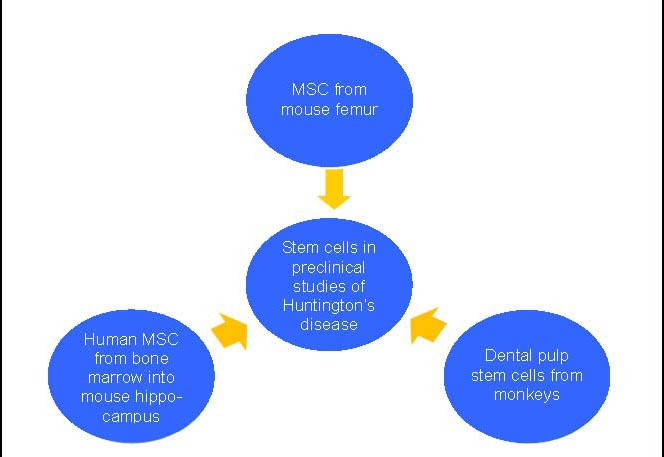
Stem cells evaluated in the recent preclinical studies of Huntington's disease.
MSC: Mesenchymal stem cells or mesenchymal stromal cells.
ALS
ALS is an adult-onset disease which is usually sporadic. ALS is characterized by the death of upper and lower motor neurons with subsequent muscular paralysis and atrophy. Unfortunately, despite the advent of modern medicinal chemistry, there is only one approved treatment i.e. riluzole, which has modest therapeutic effects[80,81].
Compared to other neurodegenerative diseases, there are certain features of ALS that makes it more challenging to experiment stem cell therapy. The most important aspect is the unknown pathogenesis followed by the lack of knowledge on how the disease spreads in the human body. Choosing the ideal site to implant stem cell is difficult without answers to the above[80,82]. Theoretically, the objective of stem cell therapy in ALS is substitution of motor neurons. The fundamental strategies of stem cell therapy in ALS consist of the regulation of inflammation and the expression of neurotrophic factors. Stem cells modified to release GDNF improved motor function in transgenic rats[83,84].
In 2010, the FDA approved a clinical trial with NSCs in patients suffering from ALS. The phase I of the trial, at Emory University, assessed the safety of implanting NSCs into the spinal cord of 18 affected patients. A year later, FDA approved the continuation of the trial[80,85].
More recently, Martinez et al described the safety of stem cell transplantation into the frontal motor cortex of 67 patients with ALS[86]. This was probably one of the largest human studies in the history of stem cell therapy in neurodegenerative diseases. Autologous stem cell therapy into the frontal motor cortex was found to be safe with encouraging results of 90% survival at 1 year[86]. Similarly, surgical implantation of MSC into the dorsal spinal cord was well tolerated with no immediate or long term complications during a follow up period of 9 years[87]. Both these human studies, however, focused on the safety rather than the clinical benefits.
Hence, stem cell therapy in ALS is still in a preliminary stage. The ideal cellular type and optimal anatomical site for implantation which would yield favorable clinical outcome are yet to be determined.
Alzheimer's disease (AD)
AD is the most common form of dementia accounting for more than half of all dementias. It is characterized by the insidious onset of dementia and, histologically, by senile plaques and neurofibrillary tangles[88]. There is accumulating evidence that the pathogenesis of AD involves oxidative stress and inflammation[89,90]. Most patients only seek treatment at an advanced stage of the disease with neuritic plaques, neurofibrillary tangles, and neurodegeneration. To arrest the disease progression at this stage, clinicians are expected to adopt a multifaceted approach aiming to promote cell survival and substitute the lost neurons[55].
Using the mouse models of AD, Blurton-Jones et al[91] in 2009 reported that NSCs transplanted in the hippocampus improved memory deficits via BDNF mediated response. The role of BDNF in ameliorating cognitive function was further emphasized by Nagahara et al[92] in the same year. Furthermore, observations from animal studies have pointed out that transplanted SC migrate and differentiate into cholinergic neurons, astrocytes, and oligodendrocytes. Apart from replacement of the lost neurons, stem cells stimulate endogenous neural precursors, promote structural neuroplasticity, inhibit proinflammatory cytokines, suppress neuronal apoptosis and express growth factors[93].
Kern et al[94] in 2011 discovered that implantation of NSCs into the hippocampus of aged Down syndrome mice resulted in a significant decrease in the tau/reelin-positive granules. The authors of the study suggested that changes in granule density could be used to assess the effectiveness of novel therapies such as stem cell therapy. Generation of patient-specific iPSCs associated with AD is in progress. Researchers are working on creating stem cell lines from patients with amyloid precursor protein duplications, tau mutations and preselenin-1. In this regard, cholinergic cells of the nucleus basalis are a reasonable initial target[95].
FUTURE DIRECTIONS AND RESEARCH AGENDA
Neurorestoration is a concept which is evolving at an accelerated pace over the past decade. A very recent study identified up to 106 of either ongoing or planned neurorestorative clinical trials. The vast majority of the trials were targeting one of the following diseases: multiple sclerosis, stroke, PD and ALS[96].
stem cell therapy has set off both interests and alarms in the scientific community. Despite all the elegant studies in this area, there are still more questions than answers. In future, researchers should attempt to identify the ideal SC type and route of administration for each neurodegenerative diseases. Clearly, a tailored approach is required for each neurodegenerative diseases to effectively salvage the neuronal networks. Besides, less invasive methods of stem cell implantation across the blood brain barrier are being explored.
CONCLUSION
neurodegenerative diseases have devastating sequelae with conventional pharmacological therapies and to date stem cell therapy is probably the only potential treatment modality which offers ‘cure’ for neurodegenerative diseases. The vast majority of studies are in animal models. Thus, there is still paucity of data on clinical outcome and long term safety of stem cell therapy in humans with neurodegenerative diseases. The structural and functional improvements seen in animals need further evaluation prior to extrapolation to humans. Of the four types of neurodegenerative diseases described above, there are relatively more studies on PD and ALS compared to HD and AD. Prior to the clinical applications of stem cell therapy in neurodegenerative diseases, several issues need to be addressed. The cost, safety, manpower requirement and post-transplant monitoring are some of the many concerns.
Footnotes
Conflicts of interest: None declared.
(Edited by Rodella LF/Fernandez M/Song LP)
REFERENCES
- [1].Hedlund E, Perlmann T. Neuronal cell replacement in Parkinson's disease. J Intern Med. 2009;266(4):358–371. doi: 10.1111/j.1365-2796.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- [2].Hung CW, Chen YC, Hsieh WL, et al. Ageing and neurodegenerative diseases. Ageing Res Rev. 2010;9(Suppl 1):S36–46. doi: 10.1016/j.arr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- [3].Malgrange B, Borgs L, Grobarczyk B, et al. Using human pluripotent stem cells to untangle neurodegenerative disease mechanisms. Cell Mol Life Sci. 2011;68(4):635–649. doi: 10.1007/s00018-010-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nistor GI, Totoiu MO, Haque N, et al. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49(3):385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- [6].Nistor G, Siegenthaler MM, Poirier SN, et al. Derivation of high purity neuronal progenitors from human embryonic stem cells. PLoS One. 2011;6(6):e20692. doi: 10.1371/journal.pone.0020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lefort N, Feyeux M, Bas C, et al. Human embryonic stem cells reveal recurrent genomic instability at 20q11.21. Nat Biotechnol. 2008;26:1364–1366. doi: 10.1038/nbt.1509. [DOI] [PubMed] [Google Scholar]
- [8].Schwarz SC, Schwarz J. Translation of stem cell therapy for neurological diseases. Transl Res. 2010;156(3):155–160. doi: 10.1016/j.trsl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- [9].Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [10].Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454(7204):646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- [12].Verma A, Verma N. Induced pluripotent stem cells and promises of neuroregenerative medicine. Neurol India. 2011;59(4):555–557. doi: 10.4103/0028-3886.84337. [DOI] [PubMed] [Google Scholar]
- [13].Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1031–1032. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hilfiker A, Kasper C, Hass R, et al. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396(4):489–497. doi: 10.1007/s00423-011-0762-2. [DOI] [PubMed] [Google Scholar]
- [15].Chen BY, Wang X, Chen LW, et al. Molecular targeting regulation of proliferation and differentiation of the bone marrow-derived mesenchymal stem cells or mesenchymal stromal cells. Curr Drug Targets. doi: 10.2174/138945012799499749. in press. [DOI] [PubMed] [Google Scholar]
- [16].Olson SD, Pollock K, Kambal A, et al. Genetically engineered mesenchymal stem cells as a proposed therapeutic for Huntington's disease. Mol Neurobiol. 2012;45(1):87–98. doi: 10.1007/s12035-011-8219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Delcroix GJ, Schiller PC, Benoit JP, et al. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials. 2010;31(8):2105–2120. doi: 10.1016/j.biomaterials.2009.11.084. [DOI] [PubMed] [Google Scholar]
- [18].Gritti A, Bonfanti L, Doetsch F, et al. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22:437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johe KK, Hazel TG, Muller T, et al. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10(24):3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- [20].Huang X, Kong H, Tang M, et al. d-serine regulates proliferation and neuronal differentiation of neural stem cells from postnatal mouse forebrain. CNS Neurosci Ther. 2012;18(1):4–13. doi: 10.1111/j.1755-5949.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Milosevic J, Adler I, Manaenko A, et al. Non-hypoxic stabilization of hypoxia-inducible factor alpha (HIF-alpha): relevance in neural progenitor/stem cells. Neurotox Res. 2009;15(4):367–380. doi: 10.1007/s12640-009-9043-z. [DOI] [PubMed] [Google Scholar]
- [22].Milosevic J, Maisel M, Wegner F, et al. Lack of hypoxia-inducible factor-1 alpha impairs midbrain neural precursor cells involving vascular endothelial growth factor signaling. J Neurosci. 2007;27(2):412–421. doi: 10.1523/JNEUROSCI.2482-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Milosevic J, Schwarz SC, Krohn K, et al. Low atmospheric oxygen avoids maturation, senescence and cell death of murine mesencephalic neural precursors. J Neurochem. 2005;92(4):718–729. doi: 10.1111/j.1471-4159.2004.02893.x. [DOI] [PubMed] [Google Scholar]
- [24].Milosevic J, Brandt A, Roemuss U, et al. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J Neurochem. 2006;99(3):913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- [25].Sabolek M, Baumann B, Heinrich M, et al. Initiation of dopaminergic differentiation of Nurr1(-) mesencephalic precursor cells depends on activation of multiple mitogen-activated protein kinase pathways. Stem Cells. 2009;27(8):2009–2021. doi: 10.1002/stem.122. [DOI] [PubMed] [Google Scholar]
- [26].Wei LC, Ding YX, Liu YH, et al. Low-dose radiation stimulates Wnt/beta-catenin signaling, neural stem cell proliferation and neurogenesis of the mouse hippocampus in vitro and in vivo. Curr Alzheimer Res. 2012 doi: 10.2174/156720512800107627. [DOI] [PubMed] [Google Scholar]
- [27].Schaarschmidt G, Schewtschik S, Kraft R, et al. A new culturing strategy improves functional neuronal development of human neural progenitor cells. J Neurochem. 2009;109(1):238–247. doi: 10.1111/j.1471-4159.2009.05954.x. [DOI] [PubMed] [Google Scholar]
- [28].Wegner F, Kraft R, Busse K, et al. Glutamate receptor properties of human mesencephalic neural progenitor cells: NMDA enhances dopaminergic neurogenesis in vitro. J Neurochem. 2009;111(1):204–216. doi: 10.1111/j.1471-4159.2009.06315.x. [DOI] [PubMed] [Google Scholar]
- [29].Tamaki SJ, Jacobs Y, Dohse M, et al. Neuroprotection of host cells by human central nervous system stem cells in a mouse model of infantile neuronal ceroid lipofuscinosis. Cell Stem Cell. 2009;5(3):310–319. doi: 10.1016/j.stem.2009.05.022. [DOI] [PubMed] [Google Scholar]
- [30].Amariglio N, Hirshberg A, Scheithauer BW, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miljan EA, Sinden JD. Stem cell treatment of ischemic brain injury. Curr Opin Mol Ther. 2009;11(4):394–403. [PubMed] [Google Scholar]
- [32].Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- [33].Rakic P. Neurogenesis in adult primates. Prog Brain Res. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- [34].Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8(6):481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- [35].Gould E, Daniels DC, Cameron HA, et al. Expression of adrenal steroid receptors by newly born cells and pyknotic cells in the dentate gyrus of the postnatal rat. Mol Cell Neurosci. 1992;3(1):44–48. doi: 10.1016/1044-7431(92)90007-o. [DOI] [PubMed] [Google Scholar]
- [36].Gould E, Tanapat P, McEwen BS, et al. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor- dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21(3):512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- [39].Malberg JE, Eisch AJ, Nestler EJ, et al. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Manev H, Uz T, Smalheiser NR, et al. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411(1-2):67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- [41].Perez-Martin M, Azcoitia I, Trejo JL, et al. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur J Neurosci. 2003;18(4):923–930. doi: 10.1046/j.1460-9568.2003.02830.x. [DOI] [PubMed] [Google Scholar]
- [42].Zigova T, Pencea V, Wiegand SJ, et al. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11(4):234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- [43].Gil-Mohapel J, Simpson JM, Ghilan M, et al. Neurogenesis in Huntington's disease: can studying adult neurogenesis lead to the development of new therapeutic strategies? Brain Res. 2011;1406:84–105. doi: 10.1016/j.brainres.2011.06.040. [DOI] [PubMed] [Google Scholar]
- [44].Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- [45].Encinas JM, Sierra A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res. 2012;227(2):433–439. doi: 10.1016/j.bbr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- [46].Lugert S, Basak O, Knuckles P, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- [47].Aizawa K, Ageyama N, Terao K, et al. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging. 2011;32(1):140–150. doi: 10.1016/j.neurobiolaging.2008.12.011. [DOI] [PubMed] [Google Scholar]
- [48].Cunningham LA, Candelario K, Li L. Roles for HIF-1 alpha in neural stem cell function and the regenerative response to stroke. Behav Brain Res. 2012;227(2):410–417. doi: 10.1016/j.bbr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ritfeld GJ, Roos RA, Oudega M. Stem cells for central nervous system repair and rehabilitation. PM R. 2011;3(6 Suppl 1):S117–122. doi: 10.1016/j.pmrj.2011.02.011. [DOI] [PubMed] [Google Scholar]
- [50].Esneault E, Pacary E, Eddi D, et al. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2008;28(9):1552–1563. doi: 10.1038/jcbfm.2008.40. [DOI] [PubMed] [Google Scholar]
- [51].Miller RH, Bai L. Translating stem cell therapies to the clinic. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.01.043. [DOI] [PubMed] [Google Scholar]
- [52].Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol. 2007;20(6):688–692. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- [53].Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87(10):2183–2200. doi: 10.1002/jnr.22054. [DOI] [PubMed] [Google Scholar]
- [54].Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson's disease. Trends Pharmacol Sci. 2009;30(5):260–267. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- [55].Shihabuddin LS, Aubert I. Stem cell transplantation for neurometabolic and neurodegenerative diseases. Neuropharmacology. 2010;58(6):845–854. doi: 10.1016/j.neuropharm.2009.12.015. [DOI] [PubMed] [Google Scholar]
- [56].Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- [57].Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- [58].Piccini P, Brooks DJ, Bjorklund A, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2(12):1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- [59].Olanow CW, Freeman T, Kordower J. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;345(2):146. [PubMed] [Google Scholar]
- [60].Xi J, Zhang SC. Stem cells in development of therapeutics for Parkinson's disease: a perspective. J Cell Biochem. 2008;105(5):1153–1160. doi: 10.1002/jcb.21916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kim HJ, Sugimori M, Nakafuku M, et al. Control of neurogenesis and tyrosine hydroxylase expression in neural progenitor cells through bHLH proteins and Nurr1. Exp Neurol. 2007;203(2):394–405. doi: 10.1016/j.expneurol.2006.08.029. [DOI] [PubMed] [Google Scholar]
- [62].Sakurada K, Ohshima-Sakurada M, Palmer TD, et al. Nurr1, an orphan nuclear receptor, is a transcriptional activator of endogenous tyrosine hydroxylase in neural progenitor cells derived from the adult braint. Developmen. 1999;126(18):4017–4026. doi: 10.1242/dev.126.18.4017. [DOI] [PubMed] [Google Scholar]
- [63].Lee HS, Bae EJ, Yi SH, et al. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells. 2010;28(3):501–512. doi: 10.1002/stem.294. [DOI] [PubMed] [Google Scholar]
- [64].Ko JY, Lee HS, Park CH, et al. Conditions for tumor-free and dopamine neuron-enriched grafts after transplanting human ES cell-derived neural precursor cells. Mol Ther. 2009;17(10):1761–1770. doi: 10.1038/mt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wagner J, Akerud P, Castro DS, et al. Induction of a midbrain dopaminergic phenotype in Nurr1- overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17(7):653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- [66].Kim JH, Auerbach JM, Rodriguez-Gomez JA, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418(6893):50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- [67].Takagi Y, Takahashi J, Saiki H, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115(1):102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim HJ. Stem cell potential in Parkinson's disease and molecular factors for the generation of dopamine neurons. Biochim Biophys Acta. 2011;1812(1):1–11. doi: 10.1016/j.bbadis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- [69].Soler R, Fullhase C, Hanson A, et al. Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J Urol. 2012 doi: 10.1016/j.juro.2011.11.079. [DOI] [PubMed] [Google Scholar]
- [70].Lei Z, Jiang Y, Li T, Zhu J, et al. Signaling of glial cell line-derived neurotrophic factor and its receptor GFRalpha1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol. 2011;70(9):736–747. doi: 10.1097/NEN.0b013e31822830e5. [DOI] [PubMed] [Google Scholar]
- [71].Deleidi M, Cooper O, Hargus G, et al. Oct4-induced reprogramming is required for adult brain neural stem cell differentiation into midbrain dopaminergic neurons. PLoS One. 2011;6(5):e19926. doi: 10.1371/journal.pone.0019926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Politis M, Oertel WH, Wu K, et al. Graft-induced dyskinesias in Parkinson's disease: High striatal serotonin/dopamine transporter ratio. Mov Disord. 2011;26(11):1997–2003. doi: 10.1002/mds.23743. [DOI] [PubMed] [Google Scholar]
- [73].Bosch M, Pineda JR, Sunol C, et al. Induction of GABAergic phenotype in a neural stem cell line for transplantation in an excitotoxic model of Huntington's disease. Exp Neurol. 2004;190(1):42–58. doi: 10.1016/j.expneurol.2004.06.027. [DOI] [PubMed] [Google Scholar]
- [74].McBride JL, Behrstock SP, Chen EY, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J Comp Neurol. 2004;475(2):211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- [75].Dey ND, Bombard MC, Roland BP, et al. Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington's disease. Behav Brain Res. 2010;214(2):193–200. doi: 10.1016/j.bbr.2010.05.023. [DOI] [PubMed] [Google Scholar]
- [76].Ebert AD, Barber AE, Heins BM, et al. Ex vivo delivery of GDNF maintains motor function and prevents neuronal loss in a transgenic mouse model of Huntington's disease. Exp Neurol. 2010;224(1):155–162. doi: 10.1016/j.expneurol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- [77].Snyder BR, Chiu AM, Prockop DJ, et al. Human multipotent stromal cells (MSCs) increase neurogenesis and decrease atrophy of the striatum in a transgenic mouse model for Huntington's disease. PLoS One. 2010;5(2):e9347. doi: 10.1371/journal.pone.0009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Snyder BR, Cheng PH, Yang J, et al. Characterization of dental pulp stem/stromal cells of Huntington monkey tooth germs. BMC Cell Biol. 2011;12:39. doi: 10.1186/1471-2121-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lin YT, Chern Y, Shen CK, et al. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington's disease mouse models. PLoS One. 2011;6(8):e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Galan L, Guerrero-Sola A, Gomez-Pinedo U, et al. Cell therapy in amyotrophic lateral sclerosis: science and controversy. Neurologia. 2010;25(8):467–469. [PubMed] [Google Scholar]
- [81].Mattis VB, Svendsen CN. Induced pluripotent stem cells: a new revolution for clinical neurology? Lancet Neurol. 2011;10(4):383–394. doi: 10.1016/S1474-4422(11)70022-9. [DOI] [PubMed] [Google Scholar]
- [82].Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–2041. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- [83].Suzuki M, McHugh J, Tork C, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Suzuki M, McHugh J, Tork C, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16(12):2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lepore AC, Rauck B, Dejea C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Martinez HR, Molina-Lopez JF, Alez-Garza MT, et al. Stem cell transplantation in amyotrophic lateral sclerosis patients. Methodological approach, safety, and feasibility. Cell Transplant. 2012 doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- [87].Mazzini L, Mareschi K, Ferrero I, et al. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy. 2012;14(1):56–60. doi: 10.3109/14653249.2011.613929. [DOI] [PubMed] [Google Scholar]
- [88].Zhu X, Raina AK, Perry G, et al. Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res. 2006;3(4):393–396. doi: 10.2174/156720506778249470. [DOI] [PubMed] [Google Scholar]
- [89].Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35(3):419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- [90].Onyango IG, Khan SM. Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2006;3(4):339–349. doi: 10.2174/156720506778249489. [DOI] [PubMed] [Google Scholar]
- [91].Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–23599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Abdel-Salam OM. Stem cell therapy for Alzheimer's disease. CNS Neurol Disord Drug Targets. 2011;10(4):459–485. doi: 10.2174/187152711795563976. [DOI] [PubMed] [Google Scholar]
- [94].Kern DS, Maclean KN, Jiang H, et al. Neural stem cells reduce hippocampal tau and reelin accumulation in aged Ts65Dn Down syndrome mice. Cell Transplant. 2011;20(3):371–379. doi: 10.3727/096368910X528085. [DOI] [PubMed] [Google Scholar]
- [95].Cundiff PE, Anderson SA. Impact of induced pluripotent stem cells on the study of central nervous system disease. Curr Opin Genet Dev. 2011;21(3):354–361. doi: 10.1016/j.gde.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bednar MM, Perry A. Neurorestoration therapeutics for neurodegenerative and psychiatric disease. Neurol Res. 2012 doi: 10.1179/1743132811Y.0000000069. [DOI] [PubMed] [Google Scholar]


