Abstract
Increased intracranial pressure (ICP) is associated with worse outcome after traumatic brain injury (TBI). The current guidelines and management strategies are aimed at maintaining adequate cerebral perfusion pressure and treating elevated ICP. Despite controversies, ICP monitoring is important particularly after severe TBI to guide treatment and in developed countries is accepted as a standard of care. We provide a narrative review of the recent evidence for the use of ICP monitoring and management of ICP in pediatric TBI.
Keywords: Craniectomy, intracranial pressure, traumatic brain injury
Introduction
Traumatic brain injury (TBI) and its complications are the leading cause of mortality and morbidity in children. In the US alone over 2,300 deaths, 42,000 hospitalizations, and 404,000 Emergency Department visits occur annually among children 0–14 years old related to TBI.[1,2] Mortality in children with severe TBI is often the result of a refractory increase in intracranial pressure (ICP). Therefore prevention and management of raised ICP is increasingly recognized as central to current pediatric neurocritical care. Increased ICP is an important cause of secondary brain injury in TBI and both degree and duration of high ICP is associated with poor outcomes. Sustained increases to >20 mm Hg for >5 min may need treatment.[3,4] Though the efficacy of treatment based on ICP monitoring has been questioned.[5] However there is enough evidence supporting the use of ICP monitoring in TBI. Therefore current protocols for treatment of children with severe TBI emphasize ICP monitoring and using either ICP and/or cerebral perfusion pressure (CPP) as the therapeutic target to minimize secondary brain injuries.[6]
Pathophysiology
The intracranial vault is comprised of three elements: Brain (80%), cerebrospinal fluid (CSF) (10%), and cerebral blood volume (CBV) (10%). It's essential when thinking about brain physiology to consider the Monro–Kellie doctrine which states that any individual component of the intra-cranial vault may undergo alterations but the total volume remains fixed as the space within the skull is fixed. Therefore any increase in one of these components will either cause a shift out of the box or increase in pressure within the box. The brain has certain normal compensatory mechanisms in place to maintain the normal ICP. Once these compensatory mechanisms are exhausted a small increase in volume will result in a large rise in ICP. This relationship between ICP and intracranial volume is best described by the volume-pressure curve[7] [Figure 1].
Figure 1.
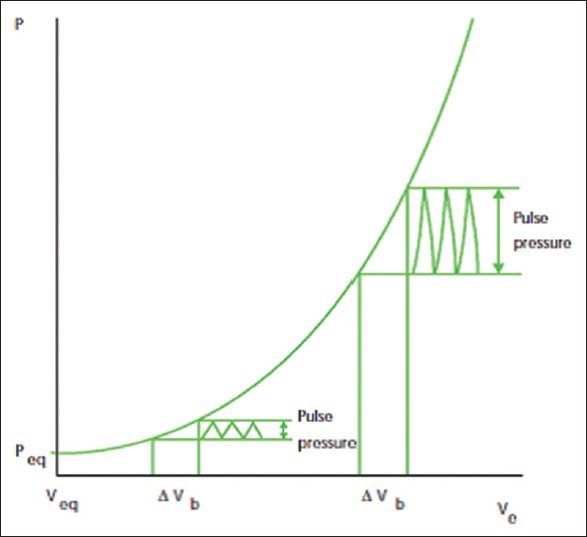
Volume-pressure curve showing relationship between intracranial pressure and intracranial volume. Reprinted with permission from Dunn[7]
The etiology of increase in ICP after TBI is multifactorial. It can be due to traumatic mass lesion, vascular engorgement and/or cerebral edema. Hyperemia occurs early after trauma and persists for few days. The increase in CBV is most likely caused by vasodilation which is a compensatory mechanism to maintain optimal cerebral blood flow (CBF). Studies have shown that it is the brain edema, and not increased CBV which is the major culprit for brain swelling after TBI.[8] Both vasogenic and cytotoxic cerebral edema occur in TBI, however incidence and onset depends on the nature of the injury. Vasogenic edema occurs due to transvascular leakage caused by failure of normally tight blood–brain barrier junction and was classically considered to be the prevalent edema after TBI[9,10] but recent work suggest that cytotoxic or cellular edema is the predominant form of edema after TBI.[10,11,12]
Cerebral Hemodynamics and the Effects of Blood Gases
In a normal brain, CBF is maintained at a constant level with a mean arterial blood pressure (MAP) of 60–150 mm Hg.[13,14] This is referred to as autoregulation. CPP is the pressure gradient across the brain and is defined as MAP - ICP. Autoregulation is maintained by ability of the cerebral vessels to change their diameter in response to changing physiological conditions[15] [Figure 2]. Arterial PaO2 and PaCO2 have effects on the CBF. Hypercapnia causes cerebral vasodilatation and increased CBF, whereas hypocapnia reduces CBF.[16] The physiological response of blood vessels to PaCO2 is the rationale for the use of brief hyperventilation as useful tool in the acute management of increased ICP. Arterial PaO2 also has its effects on cerebral vessels. As PaO2 declines it causes vasodilatation and increase in CBF which in presence of disrupted blood brain barrier promote the formation of vasogenic edema. Avoiding hypoxia is therefore important in the acute management of TBI.[17] If autoregulation is impaired, changes in blood pressure or ICP can have direct effects on CBF [Figure 2].
Figure 2.
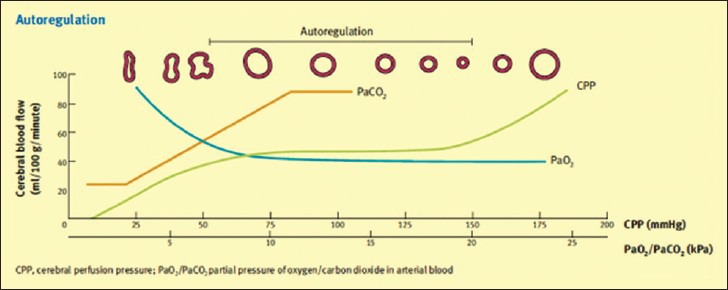
Cerebral autoregulation and the effect of the blood gases. Reproduced with permission from Tameem and Krovvidi[15]
Normal and Pathologic Intracranial Pressure
The normal range for ICP varies with age [Table 1].[7]
Table 1.
Normal ICP for age

The definition of intracranial hypertension depends on the specific pathology and age. In TBI literature therapeutic target for ICP treatment is based on adult studies, with an ICP treatment threshold of 20 mmHg.[18] The optimal ICP threshold for pediatric TBI is not yet defined. Various pediatric studies have suggested the the ICP threshold as low as 15 to as high as 35 for the initiation of treatment.[19,20,21] However there is sufficient evidence in pediatric literature that suggests an association between ICP of >20 mm Hg and poor outcome.[22,23,24] Treatment of ICP may therefore be considered at a threshold of 20 mm Hg.[6]
In the injured brain, there may be intra-parenchymal pressure gradients between the supra and infra-tentorial compartments[25] therefore assuming that there is one uniform ICP maybe questionable.[26]
Intracranial Pressure Monitoring
Indications
Intracranial pressure monitoring can be used as a guide to treatment. However there is no class I evidence to support standard treatment guidelines. Current guidelines suggest:[6]
ICP monitoring may be considered in infants and children with severe TBI (Glasgow Coma scale [GCS]: 3–8)
ICP monitoring is not routinely indicated in patients with mild or moderate head injury; the clinician may choose to monitor ICP in certain conscious patients with traumatic mass lesions who are at relative risk for neurologic deterioration or in whom serial neurologic examination is precluded by sedation, neuromuscular blockade, or anesthesia.
Techniques
Intracranial pressure cannot be accurately estimated clinically or by computed tomography findings and therefore must actually be measured. The lumbar CSF pressure measurement does not provide a reliable estimate of ICP and may be dangerous in the presence of elevated ICP. A variety of different techniques are available for the measurement and monitoring of ICP [Figure 3]. The “gold standard” technique for ICP monitoring is the intraventricular fluid filled catheter transducer system. The reference point for the transducer is the foramen of Monroe, although in practice the external auditory meatus is usually used. The advantages of ventricular catheters are the measurement of global ICP, ability to recalibrate the transducer, therapeutic drainage of CSF, and administration of drugs.[27,28] The disadvantages are difficulties with insertion into compressed or displaced ventricles, inaccuracies of pressure measurements because of obstruction, and infection.[29,30]
Figure 3.
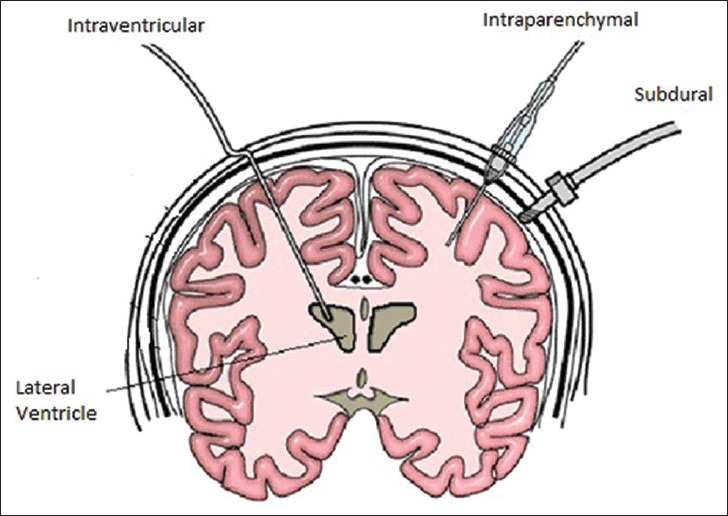
Various techniques of intracranial pressure monitoring
When the ventricles cannot be cannulated, alternatives can be used. Micotransducer-tipped ICP monitors can be inserted into the brain parenchyma or subdural space, either through a skull bolt, a small burr hole or during a neurosurgical procedure.[31] Microtransducer systems are reliable and easy to use, with recordings that are stable over time with minor zero drift.[32] They have low infection and other complication rates[33] however none of the transducer tipped catheters can be reset to zero after they are inserted into the skull and measured pressure may not be representative of true CSF pressure because of the intraparenchymal pressure gradients that may exist after TBI.[34]
Noninvasive methods of measuring ICP and methods using tympanic membrane displacement[35] and ultrasound measurement of optic nerve sheath diameter[36] have been described but currently they lack the accuracy of invasive techniques. Transcranial Doppler ultrasonography has also been used to provide a noninvasive estimate of ICP.[37]
Intracranial Pressure Waveform
The normal ICP waves are generated from a pressure wave transmitted through the cardiovascular system into the tissues within the intracranial cavity.[38] It has 3 peaked waves [Figure 4]. P1, the first and generally the tallest peak is also known as the percussion wave and corresponds to the transmitted systolic blood pressure; P2 (the dicrotic wave) has a more variable shape and reflects relative brain volume. The P2 component is often elevated in response to a rapidly expanding mass lesion such as a hematoma or an intracranial tumour[38,39] and P3 (the tidal wave) follows the dicrotic notch on the downslope of the individual ICP pulse waveform. As ICP increases, P2 and P3 rise and eventually surpass P1. Ultimately, with continued elevation of ICP, the waveform loses distinct peaks and assumes a triangular morphology.
Figure 4.
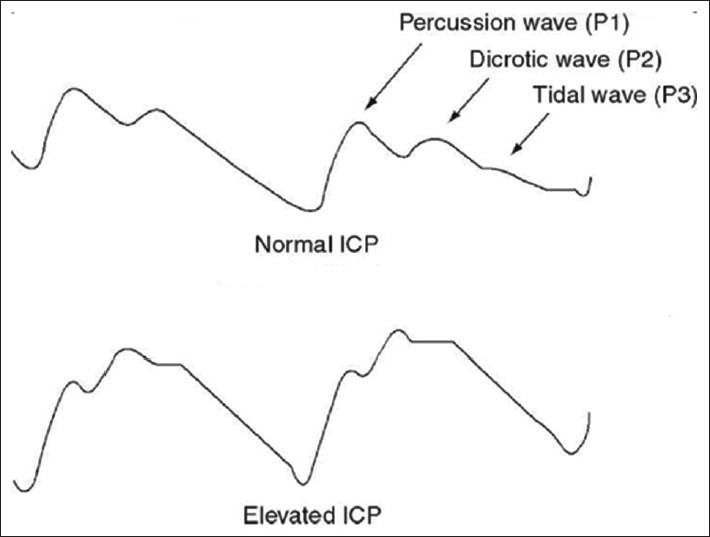
Waveform morphology with normal and elevated intracranial pressure
Intracranial pathology leading to sustained elevations of ICP may produce ICP slow waves, which were first classified in humans by Lundberg.[40]
A waves or “plateau” waves
They reflect a sudden dramatic rise in ICP to levels of 40–100 mm Hg, often lasting 5–20 min. These waves are always pathological and indicate greatly reduced compliance. They are frequently accompanied by neurological deterioration and occur in patients with intact autoregulation.[7,41]
B waves
These rhythmic oscillations occur every 1–2 min with peak ICP increasing to around 20–30 mm Hg above baseline. They are probably related to changes in cerebrovascular tone and CBV. B waves are also indicative of failing intracranial compensation.[7,41]
C waves
These oscillations occur with a frequency of 4–8/min and are of smaller amplitude than B waves. They occur with ABP and are of no pathological significance.[7,41]
Management
Initial stabilization
Treatment of raised ICP in TBI starts at the site of trauma. Circulation, airway and breathing should be taken care of. The most common cause of shock in trauma patients is hypovolemia. Isotonic solutions such as 0.9% normal saline (N.S) and/or packed red blood cells can be administered. Hypotonic solutions should be avoided. The criteria for intubation include hypoxemia, hypercarbia (PaCO2 >45 mm Hg), hypocapnia (hyperventilation causing PaCO2 <25 mm Hg), GCS <9, a drop in GCS >3 irrespective of initial GCS, anisocoria >1 mm, abnormal breathing due to cervical spine injury, chest wall dysfunction and loss of protective airway reflexes.[42] In prehospital settings, intubation should be performed only if there are skilled personnel available.[43] Because endotracheal intubation is a noxious stimuli and can increase ICP, appropriate medication should be used to help blunt the rise in ICP during intubation. Cervical spine precautions should be taken during intubation and nasotracheal intubation should be avoided because of the possibility of basal skull fracture.
General measures
After the initial resuscitation, management should be aimed at preventing secondary brain injury such as hypoxemia, hypercapnia, hyponatremia, hyperthermia, seizures and hypo/hyperglycemia and increase in ICP. First-tier Therapies for Raised Intracranial Pressure[44] are shown in Figure 5.
Figure 5.
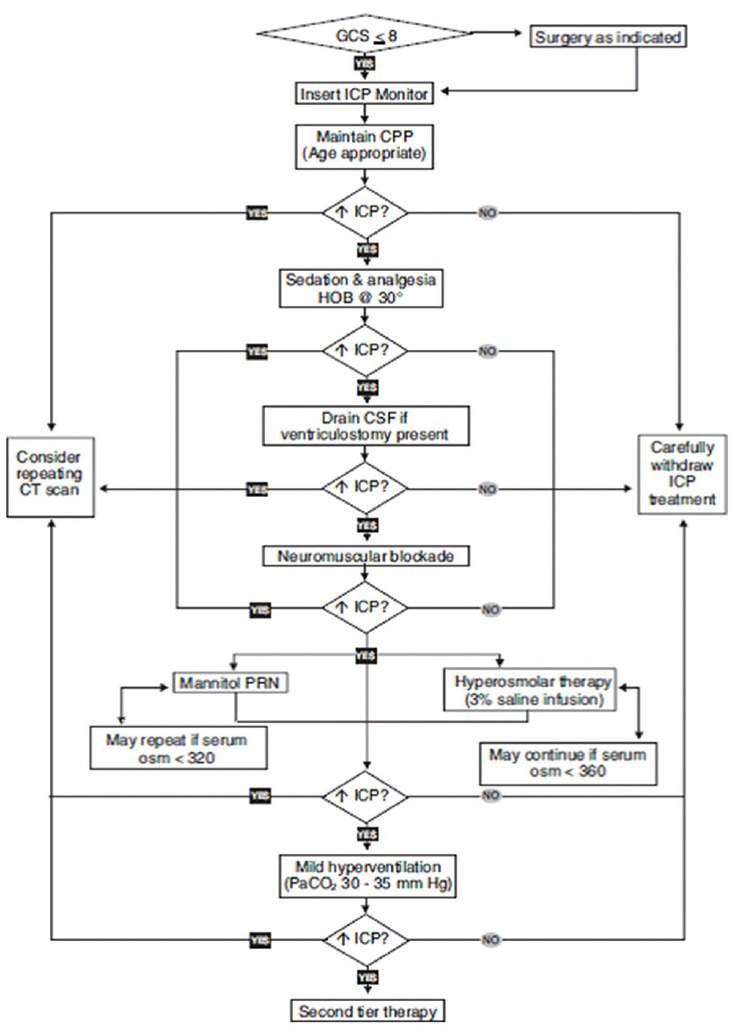
First Tier therapy. GCS, Glasgow Coma Scale; ICP, intracranial pressure; CPP, cerebral perfusion pressure; HOB, head of bed; CSF, cerebrospinal fluid; CT, computed tomography; PRN, as needed. Reproduced with permission from Adelson et al.[43]
Intracranial Pressure and Cerebral Perfusion Pressure
The basic principle in managing the pediatric patient with severe TBI after addressing the surgical evacuation of intracranial mass lesions is the insertion of an ICP monitor. The aim is to minimize ICP elevation and maintain normal cerebral homeostasis so as to prevent secondary ischemic injury. Treatment for intracranial hypertension should begin with ICP >20 mm Hg. In adults with raised ICP, CPP should be maintained around 60–70 mmHg.[45,46] The minimum acceptable level of CPP has not been clearly defined in children but is probably lower as compared to adults (e.g. 40–60 mmHg depending upon age).[6] CPP <40 mmHg is associated with poor outcome.[47,48]
Head position
Head elevation at 30° can lower ICP without adversely affecting MAP or CPP. However elevation >40° may decrease CPP.[49] Maintaining the head in a midline position to promote venous drainage is also recommended.
Sedation and analgesia
Pain and anxiety can increase the cerebral metabolic demand which can increase the CBF and ICP. Sedatives can be used. Etomidate may be considered, however there are risks of adrenal suppression. Barbiturates can also be tried in a sedative dose.[6] If ICP remains elevated despite adequate sedation, neuromuscular blockade may be required. Muscle relaxation also can prevent maneuvers that increase ICP such as coughing, straining and fighting against the ventilator. If paralysis is required, short-acting agents are preferred, and should be withheld periodically to permit neurologic examination.[50]
Ventricular cerebrospinal fluid drainage
Cerebrospinal fluid drainage decreases the intracranial volume and provides an immediate, but transient, decrease in ICP.[51] Depending on the clinical status, it can be done either continuously or intermittently. There is limited data on the effect of CSF drainage on ICP, CPP, CBF, and more importantly on outcome. One pediatric study showed that continuous CSF drainage was associated with lower concentration of markers of injury, lower mean ICP and increased volume of CSF drainage as compared to intermittent CSF drainage.[52]
Osmotherapy
Osmotic agents are used to reduce the brain tissue edema. Since its first use in 1962 to treat ICP, mannitol has been a cornerstone of intracranial hypertension management.[53] Mannitol reduces ICP by two mechanism:[1] It decreases blood viscosity thereby promoting reflex vasoconstriction by autoregulation and this mechanism decreases CBV and ICP. This rheologic effect occurs immediately and lasts about 75 min[54,55] Mannitol also produces an osmotic gradient, drawing fluid from the brain tissue into the vascular space.[56,57] Mannitol administration has the potential side effects of hypovolemia, electrolyte imbalance, and acute renal failure. There are also concerns that it may cross the injured blood-brain barrier and cause an exacerbation of cerebral edema.[58,59]
More recently hypertonic saline (HS) is gaining favour, its use has been shown to decrease ICP and improve outcome in pediatric TBI patients.[60,61] 3% N.S received a stronger level II recommendation over mannitol in the 2012 pediatric TBI guidelines.[6] Unlike mannitol it preserves intravascular status and can be administered in a hemodynamically unstable patient.[62] Additional benefits include restoration of normal cellular resting membrane potential and cell volume, stimulation of atrial natriuretic peptide release, inhibition of inflammation, and enhancement of cardiac output.[63,64,65]
The 2003 pediatric TBI guidelines recommended the upper safety threshold of 360 mOsm/L for serum osmolality[43] and caution should be taken if the serum osmolality approaches 320 mOsm/L as there may be an increased risk for renal insufficiency.[66] Other potential concerns with the use of HS include a rebound increase in ICP, central pontine myelinolysis, subarachnoid haemorrhage, natriuresis and hyperchloremic acidosis.[63]
Hyperventilation
Hyperventilation is one of the fastest methods to lower ICP in a child with impending herniation. However prophylactic hyperventilation without signs of impending herniation should be avoided. Hyperventilation decreases ICP by causing vasoconstriction which decreases CBF in a hyperemic brain. However recent studies have shown that hyperemia is uncommon and that children may actually have decreased CBF after TBI.[67,68] Hyperventilation therefore may dramatically decrease CBF which may cause further cerebral ischemia.[69] Despite the recommendations in 2003 guidelines that prophylactic hyperventilation (PaCO2 <35 mm hg) should be avoided, it still remains a commonly used therapy in pediatric TBI.[70,71] The 2012 guidelines has a stronger level III recommendation against prophylactic hyperventilation.
Hyperventilation
If hyperventilation is to be used for refractory intracranial hypertension then advanced neuromonitoring should be considered for evaluation of cerebral ischemia. Second-Tier Therapies for Raised Intracranial Pressure[43] are shown in Figure 6.
Figure 6.
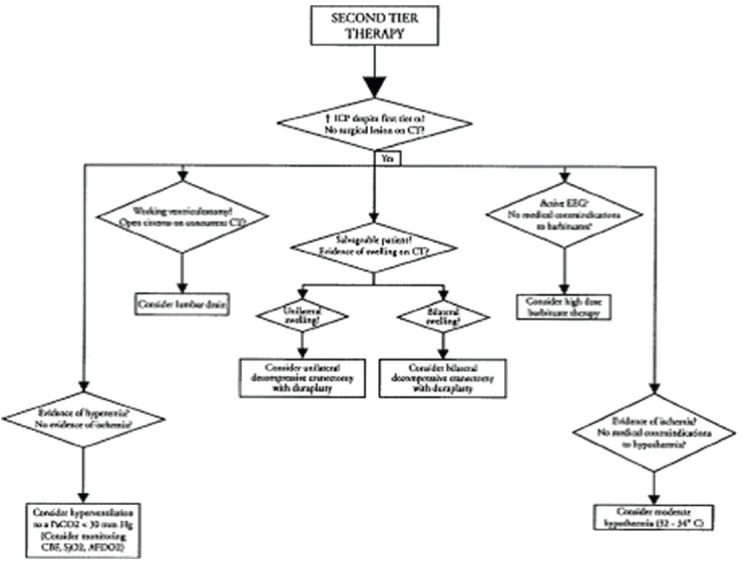
Second Tier therapy. ICP, intracranial pressure; CT, computed tomography; EEG, electroencephalogram; CBF, cerebral blood flow; SjO2, jugular venous oxygen saturation; AJDO2, arterial-jugular venous difference in oxygen content. Reproduced with permission from Adelson et al.[43]
Barbiturates
Barbiturates are used to treat intracranial hypertension that is refractory to other therapies. Barbiturates decrease ICP by decreasing cerebral metabolic rate but may cause significant hemodynamic instability.[72,73] Pentobarbitone is the most commonly used barbiturate. Additionally these patients require EEG monitoring to maintain a burst suppression pattern.
There are limited pediatric studies to show the effectiveness of barbiturate and its role in outcome, some studies have shown improved ICP with good outcome.[74] Similarly, adult studies have also reported pentobarbital as an effective treatment to decrease ICP.[75] However some studies have shown no benefit in outcome[76] or even a worse neurological outcome.[77] Barbiturates produce cardiac suppression, which may result in hypotension - therefore appropriate hemodynamic monitoring and inotropic support must be provided during barbiturate therapy.
Decompressive craniectomy
For a decompressive craniectomy (DECRA), part of the skull is removed to allow a swelling brain to expand in order to reduce increased ICP. This can be done at the time of mass lesion evacuation procedure (secondary craniectomy) or as a primary treatment for refractory ICP (primary craniectomy). Use of this technique is controversial and this option may be considered in patients with clinical deterioration, herniation or refractory intracranial hypertension.[6] The only randomized clinical trial (RCT) comparing early DECRA in children with TBI to medical management of intracranial hypertension revealed that 54% of children in decompression group had favourable outcome as compared to the 14% in the medical group.[78] Despite being a RCT, this study was not included in the guidelines as a evidence because of its inclusion of patients with GCS >8. Other potential flaws of this study were that the statistical analysis was based on the outcome data and additionally, the surgical procedure performed in this study was different from the other comparative studies. Other small class III case series also suggest improved outcome with decompressive craniotomy in pediatric patients.[2,79,80]
The recent DECRA Trial with severe TBI in adults showed that DECRA decreased the ICP, total duration of mechanical ventilation and Intensive Care Unit stay however the patients had unfavourable outcomes as compared to the standard care group.[81] Little inference can be made in terms of the applicability of the adult RCT to the pediatric population because pediatric patients have different outcomes as compared to their adult counterparts.[82,83]
Potential complications of surgery include herniation through the skull defect, spinal fluid leak, wound infection, epidural and subdural hematoma and post traumatic hydrocephalus.[84]
Hypothermia
Hypothermia has been found to be neuroprotective by decreasing cerebral metabolism, excitotoxicity, production of free radicals, and nitric oxide synthesis.[85]
For adult patients with refractory intracranial hypertension due to TBI controlled hypothermia has been shown to provide a modest improvement in morbidity and mortality in some conditions but is not recommended as standard therapy.[86]
Two trials of hypothermia therapy in children with TBI have shown no improvement in neurologic or other outcomes; one showed a nonsignificant increase in mortality.[87,88] Given the controversies associated with hypothermia, moderate hypothermia (core temperature 32–33°C) for ICP not controlled by conventional measures, beginning within 8 h of injury, and maintained for 48 h may be applied. If hypothermia is employed the speed of rewarming should not exceed more than 0.5°C/h.[6]
Lumbar cerebrospinal fluid drainage
For refractory intracranial hypertension, addition of lumbar drainage may be considered if the ventricular catheter is patent, the basal cisterns are open, and there is no evidence of midline shift or a significant mass lesion on neuroimaging.[6,89]
Seizure prevention
In children with TBI, seizures most commonly occur within the first 24 h after injury[90] and may increase the ICP, and therefore should be treated aggressively. Prophylactic anticonvulsants are only effective in the 1st week after TBI to decrease the incidence of early onset seizures.[91,92]
Steroids
The use of corticosteroids is not recommended in the management of increased ICP after TBI.
Summary
Elevated ICP in pediatric TBI causes a secondary brain injury. ICP monitoring is the important and established method in management after TBI. Supporting circulation, airway and breathing are the mainstay of therapy. Head elevation, sedation, analgesia, osmotherapy and hyperventilation can rapidly lower ICP. In refractory cases barbiturate coma, moderate hypothermia and surgical decompression may be helpful.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Rutigliano D, Egnor MR, Priebe CJ, McCormack JE, Strong N, Scriven RJ, et al. Decompressive craniectomy in pediatric patients with traumatic brain injury with intractable elevated intracranial pressure. J Pediatr Surg. 2006;41:83–7. doi: 10.1016/j.jpedsurg.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin MR, Marion DW. Cerebral blood flow and vasoresponsivity within and around cerebral contusions. J Neurosurg. 1996;85:871–6. doi: 10.3171/jns.1996.85.5.0871. [DOI] [PubMed] [Google Scholar]
- 4.Marmarou A. Increased intracranial pressure in head injury and influence of blood volume. J Neurotrauma. 1992;9(Suppl 1):S327–32. [PubMed] [Google Scholar]
- 5.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–81. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents – Second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 7.Dunn LT. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;3:i23–7. doi: 10.1136/jnnp.73.suppl_1.i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marmarou A, Fatouros PP, Barzó P, Portella G, Yoshihara M, Tsuji O, et al. Contribution of edema and cerebral blood volume to traumatic brain swelling in head-injured patients. J Neurosurg. 2000;93:183–93. doi: 10.3171/jns.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 9.Marmarou A. Pathophysiology of traumatic brain edema: Current concepts. Acta Neurochir Suppl. 2003;86:7–10. doi: 10.1007/978-3-7091-0651-8_2. [DOI] [PubMed] [Google Scholar]
- 10.Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–9. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Marmarou A, Signoretti S, Aygok G, Fatouros P, Portella G. Traumatic brain edema in diffuse and focal injury: Cellular or vasogenic? Acta Neurochir Suppl. 2006;96:24–9. doi: 10.1007/3-211-30714-1_6. [DOI] [PubMed] [Google Scholar]
- 12.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg. 2006;104:720–30. doi: 10.3171/jns.2006.104.5.720. [DOI] [PubMed] [Google Scholar]
- 13.Bruzzone P, Dionigi R, Bellinzona G, Imberti R, Stocchetti N. Effects of cerebral perfusion pressure on brain tissue PO2 in patients with severe head injury. Acta Neurochir Suppl. 1998;71:111–3. doi: 10.1007/978-3-7091-6475-4_33. [DOI] [PubMed] [Google Scholar]
- 14.Bouma GJ, Muizelaar JP, Fatouros P. Pathogenesis of traumatic brain swelling: Role of cerebral blood volume. Acta Neurochir Suppl. 1998;71:272–5. doi: 10.1007/978-3-7091-6475-4_79. [DOI] [PubMed] [Google Scholar]
- 15.Tameem AB, Krovvidi HR. Cerebral physiology. Contin Educ Anaesth Crit Care Pain. 2013;13:113–8. [Google Scholar]
- 16.Reivich M. Arterial pco2 and cerebral hemodynamics. Am J Physiol. 1964;206:25–35. doi: 10.1152/ajplegacy.1964.206.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Biros MH, Heegaard W. Prehospital and resuscitative care of the head-injured patient. Curr Opin Crit Care. 2001;7:444–9. doi: 10.1097/00075198-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24(Suppl 1):S55–8. doi: 10.1089/neu.2007.9988. [DOI] [PubMed] [Google Scholar]
- 19.Cruz J, Nakayama P, Imamura JH, Rosenfeld KG, de Souza HS, Giorgetti GV. Cerebral extraction of oxygen and intracranial hypertension in severe, acute, pediatric brain trauma: Preliminary novel management strategies. Neurosurgery. 2002;50:774–9. doi: 10.1097/00006123-200204000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–54. doi: 10.1227/01.neu.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- 21.Chambers IR, Treadwell L, Mendelow AD. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver-operating characteristic curves: An observational study in 291 patients. J Neurosurg. 2001;94:412–6. doi: 10.3171/jns.2001.94.3.0412. [DOI] [PubMed] [Google Scholar]
- 22.Jagannathan J, Okonkwo DO, Yeoh HK, Dumont AS, Saulle D, Haizlip J, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2:240–9. doi: 10.3171/PED.2008.2.10.240. [DOI] [PubMed] [Google Scholar]
- 23.Michaud LJ, Rivara FP, Grady MS, Reay DT. Predictors of survival and severity of disability after severe brain injury in children. Neurosurgery. 1992;31:254–64. doi: 10.1227/00006123-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Esparza J, M-Portillo J, Sarabia M, Yuste JA, Roger R, Lamas E. Outcome in children with severe head injuries. Childs Nerv Syst. 1985;1:109–14. doi: 10.1007/BF00706691. [DOI] [PubMed] [Google Scholar]
- 25.Rosenwasser RH, Kleiner LI, Krzeminski JP, Buchheit WA. Intracranial pressure monitoring in the posterior fossa: A preliminary report. J Neurosurg. 1989;71:503–5. doi: 10.3171/jns.1989.71.4.0503. [DOI] [PubMed] [Google Scholar]
- 26.Wolfla CE, Luerssen TG, Bowman RM. Regional brain tissue pressure gradients created by expanding extradural temporal mass lesion. J Neurosurg. 1997;86:505–10. doi: 10.3171/jns.1997.86.3.0505. [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Dujovny M, Park HK, Perez E, Perlin AR, Diaz FG. Advances in ICP monitoring techniques. Neurol Res. 2003;25:339–50. doi: 10.1179/016164103101201661. [DOI] [PubMed] [Google Scholar]
- 28.Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. Br J Anaesth. 2006;97:26–38. doi: 10.1093/bja/ael110. [DOI] [PubMed] [Google Scholar]
- 29.Birch AA, Eynon CA, Schley D. Erroneous intracranial pressure measurements from simultaneous pressure monitoring and ventricular drainage catheters. Neurocrit Care. 2006;5:51–4. doi: 10.1385/NCC:5:1:51. [DOI] [PubMed] [Google Scholar]
- 30.Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984;310:553–9. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 31.Gopinath SP, Robertson CS, Contant CF, Narayan RK, Grossman RG. Clinical evaluation of a miniature strain-gauge transducer for monitoring intracranial pressure. Neurosurgery. 1995;36:1137–40. doi: 10.1227/00006123-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Koskinen LO, Olivecrona M. Clinical experience with the intraparenchymal intracranial pressure monitoring Codman MicroSensor system. Neurosurgery. 2005;56:693–8. doi: 10.1227/01.neu.0000156609.95596.24. [DOI] [PubMed] [Google Scholar]
- 33.Martínez-Mañas RM, Santamarta D, de Campos JM, Ferrer E. Camino intracranial pressure monitor: Prospective study of accuracy and complications. J Neurol Neurosurg Psychiatry. 2000;69:82–6. doi: 10.1136/jnnp.69.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahuquillo J, Poca MA, Arribas M, Garnacho A, Rubio E. Interhemispheric supratentorial intracranial pressure gradients in head-injured patients: Are they clinically important? J Neurosurg. 1999;90:16–26. doi: 10.3171/jns.1999.90.1.0016. [DOI] [PubMed] [Google Scholar]
- 35.Gwer S, Sheward V, Birch A, Marchbanks R, Idro R, Newton CR, et al. The tympanic membrane displacement analyser for monitoring intracranial pressure in children. Childs Nerv Syst. 2013;29:927–33. doi: 10.1007/s00381-013-2036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soldatos T, Chatzimichail K, Papathanasiou M, Gouliamos A. Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg Med J. 2009;26:630–4. doi: 10.1136/emj.2008.058453. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt B, Czosnyka M, Raabe A, Yahya H, Schwarze JJ, Sackerer D, et al. Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003;34:84–9. doi: 10.1161/01.str.0000047849.01376.ae. [DOI] [PubMed] [Google Scholar]
- 38.Kirkness CJ, Mitchell PH, Burr RL, March KS, Newell DW. Intracranial pressure waveform analysis: Clinical and research implications. J Neurosci Nurs. 2000;32:271–7. doi: 10.1097/01376517-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Morgalla MH, Stumm F, Hesse G. A computer-based method for continuous single pulse analysis of intracranial pressure waves. J Neurol Sci. 1999;168:90–5. doi: 10.1016/s0022-510x(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg N, Troupp H, Lorin H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury. A preliminary report. J Neurosurg. 1965;22:581–90. doi: 10.3171/jns.1965.22.6.0581. [DOI] [PubMed] [Google Scholar]
- 41.Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg. 2008;106:240–8. doi: 10.1213/01.ane.0000297296.52006.8e. [DOI] [PubMed] [Google Scholar]
- 42.Tasker RC. Head and spinal cord trauma. In: Nichols DG, editor. Rogers’ Textbook of Pediatric Intensive Care. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2008. pp. 887–911. [Google Scholar]
- 43.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Prehospital airway management. Pediatr Crit Care Med. 2003;4(Chapter 3):S9–11. [PubMed] [Google Scholar]
- 44.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(Chapter 17):S65–7. [PubMed] [Google Scholar]
- 45.Unterberg AW, Kiening KL, Härtl R, Bardt T, Sarrafzadeh AS, Lanksch WR. Multimodal monitoring in patients with head injury: Evaluation of the effects of treatment on cerebral oxygenation. J Trauma. 1997;42:S32–7. doi: 10.1097/00005373-199705001-00006. [DOI] [PubMed] [Google Scholar]
- 46.Juul N, Morris GF, Marshall SB, Marshall LF. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- 47.Downard C, Hulka F, Mullins RJ, Piatt J, Chesnut R, Quint P, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654–8. doi: 10.1097/00005373-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Elias-Jones AC, Punt JA, Turnbull AE, Jaspan T. Management and outcome of severe head injuries in the Trent region 1985-90. Arch Dis Child. 1992;67:1430–5. doi: 10.1136/adc.67.12.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg. 1992;76:207–11. doi: 10.3171/jns.1992.76.2.0207. [DOI] [PubMed] [Google Scholar]
- 50.Chesnut RM. Medical management of severe head injury: Present and future. New Horiz. 1995;3:581–93. [PubMed] [Google Scholar]
- 51.Kerr EM, Marion D, Sereika MS, Weber BB, Orndoff AP, Henker R, et al. The effect of cerebrospinal fluid drainage on cerebral perfusion in traumatic brain injured adults. J Neurosurg Anesthesiol. 2000;12:324–33. doi: 10.1097/00008506-200010000-00005. [DOI] [PubMed] [Google Scholar]
- 52.Shore PM, Thomas NJ, Clark RS, Adelson PD, Wisniewski SR, Janesko KL, et al. Continuous versus intermittent cerebrospinal fluid drainage after severe traumatic brain injury in children: Effect on biochemical markers. J Neurotrauma. 2004;21:1113–22. doi: 10.1089/neu.2004.21.1113. [DOI] [PubMed] [Google Scholar]
- 53.Wise BL, Chater N. The value of hypertonic mannitol solution in decreasing brain mass and lowering cerebro-spinal-fluid pressure. J Neurosurg. 1962;19:1038–43. doi: 10.3171/jns.1962.19.12.1038. [DOI] [PubMed] [Google Scholar]
- 54.Muizelaar JP, Wei EP, Kontos HA, Becker DP. Mannitol causes compensatory cerebral vasoconstriction and vasodilation in response to blood viscosity changes. J Neurosurg. 1983;59:822–8. doi: 10.3171/jns.1983.59.5.0822. [DOI] [PubMed] [Google Scholar]
- 55.Muizelaar JP, Lutz HA, 3rd, Becker DP. Effect of mannitol on ICP and CBF and correlation with pressure autoregulation in severely head-injured patients. J Neurosurg. 1984;61:700–6. doi: 10.3171/jns.1984.61.4.0700. [DOI] [PubMed] [Google Scholar]
- 56.Paczynski RP. Osmotherapy. Basic concepts and controversies. Crit Care Clin. 1997;13:105–29. doi: 10.1016/s0749-0704(05)70298-0. [DOI] [PubMed] [Google Scholar]
- 57.Nath F, Galbraith S. The effect of mannitol on cerebral white matter water content. J Neurosurg. 1986;65:41–3. doi: 10.3171/jns.1986.65.1.0041. [DOI] [PubMed] [Google Scholar]
- 58.Allen CH, Ward JD. An evidence-based approach to management of increased intracranial pressure. Crit Care Clin. 1998;14:485–95. doi: 10.1016/s0749-0704(05)70012-9. [DOI] [PubMed] [Google Scholar]
- 59.Kaufmann AM, Cardoso ER. Aggravation of vasogenic cerebral edema by multiple-dose mannitol. J Neurosurg. 1992;77:584–9. doi: 10.3171/jns.1992.77.4.0584. [DOI] [PubMed] [Google Scholar]
- 60.Khanna S, Davis D, Peterson B, Fisher B, Tung H, O’Quigley J, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144–51. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 61.Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136–43. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 62.Walsh JC, Zhuang J, Shackford SR. A comparison of hypertonic to isotonic fluid in the resuscitation of brain injury and hemorrhagic shock. J Surg Res. 1991;50:284–92. doi: 10.1016/0022-4804(91)90192-o. [DOI] [PubMed] [Google Scholar]
- 63.Qureshi AI, Suarez JI. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28:3301–13. doi: 10.1097/00003246-200009000-00032. [DOI] [PubMed] [Google Scholar]
- 64.Nakayama S, Kramer GC, Carlsen RC, Holcroft JW. Infusion of very hypertonic saline to bled rats: Membrane potentials and fluid shifts. J Surg Res. 1985;38:180–6. doi: 10.1016/0022-4804(85)90025-3. [DOI] [PubMed] [Google Scholar]
- 65.Arjamaa O, Karlqvist K, Kanervo A, Vainionpää V, Vuolteenaho O, Leppäluoto J. Plasma ANP during hypertonic NaCl infusion in man. Acta Physiol Scand. 1992;144:113–9. doi: 10.1111/j.1748-1716.1992.tb09275.x. [DOI] [PubMed] [Google Scholar]
- 66.Dominguez TE, Priestley MA, Huh JW. Caution should be exercised when maintaining a serum sodium level>160 meq/L. Crit Care Med. 2004;32:1438–9. doi: 10.1097/01.ccm.0000124860.91886.c2. [DOI] [PubMed] [Google Scholar]
- 67.Muizelaar JP, Marmarou A, DeSalles AA, Ward JD, Zimmerman RS, Li Z, et al. Cerebral blood flow and metabolism in severely head-injured children. Part 1: Relationship with GCS score, outcome, ICP, and PVI. J Neurosurg. 1989;71:63–71. doi: 10.3171/jns.1989.71.1.0063. [DOI] [PubMed] [Google Scholar]
- 68.Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: A preliminary report. Pediatr Neurosurg. 1997;26:200–7. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 69.Skippen P, Seear M, Poskitt K, Kestle J, Cochrane D, Annich G, et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402–9. doi: 10.1097/00003246-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 70.Curry R, Hollingworth W, Ellenbogen RG, Vavilala MS. Incidence of hypo- and hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines. Pediatr Crit Care Med. 2008;9:141–6. doi: 10.1097/PCC.0B013e318166870e. [DOI] [PubMed] [Google Scholar]
- 71.Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA, et al. UK Paediatric Traumatic Brain Injury Study Group. Intracranial pressure complicating severe traumatic brain injury in children: Monitoring and management. Intensive Care Med. 2006;32:1606–12. doi: 10.1007/s00134-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 72.Kassell NF, Hitchon PW, Gerk MK, Sokoll MD, Hill TR. Alterations in cerebral blood flow, oxygen metabolism, and electrical activity produced by high dose sodium thiopental. Neurosurgery. 1980;7:598–603. doi: 10.1227/00006123-198012000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Cormio M, Gopinath SP, Valadka A, Robertson CS. Cerebral hemodynamic effects of pentobarbital coma in head-injured patients. J Neurotrauma. 1999;16:927–36. doi: 10.1089/neu.1999.16.927. [DOI] [PubMed] [Google Scholar]
- 74.Pittman T, Bucholz R, Williams D. Efficacy of barbiturates in the treatment of resistant intracranial hypertension in severely head-injured children. Pediatr Neurosci. 1989;15:13–7. doi: 10.1159/000120433. [DOI] [PubMed] [Google Scholar]
- 75.Goodman JC, Valadka AB, Gopinath SP, Cormio M, Robertson CS. Lactate and excitatory amino acids measured by microdialysis are decreased by pentobarbital coma in head-injured patients. J Neurotrauma. 1996;13:549–56. doi: 10.1089/neu.1996.13.549. [DOI] [PubMed] [Google Scholar]
- 76.Ward JD, Becker DP, Miller JD, Choi SC, Marmarou A, Wood C, et al. Failure of prophylactic barbiturate coma in the treatment of severe head injury. J Neurosurg. 1985;62:383–8. doi: 10.3171/jns.1985.62.3.0383. [DOI] [PubMed] [Google Scholar]
- 77.Cruz J. Adverse effects of pentobarbital on cerebral venous oxygenation of comatose patients with acute traumatic brain swelling: Relationship to outcome. J Neurosurg. 1996;85:758–61. doi: 10.3171/jns.1996.85.5.0758. [DOI] [PubMed] [Google Scholar]
- 78.Taylor A, Butt W, Rosenfeld J, Shann F, Ditchfield M, Lewis E, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154–62. doi: 10.1007/s003810000410. [DOI] [PubMed] [Google Scholar]
- 79.Figaji AA, Fieggen AG, Peter JC. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666–73. doi: 10.1007/s00381-003-0804-3. [DOI] [PubMed] [Google Scholar]
- 80.Jagannathan J, Okonkwo DO, Dumont AS, Ahmed H, Bahari A, Prevedello DM, et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: A 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106:268–75. doi: 10.3171/ped.2007.106.4.268. [DOI] [PubMed] [Google Scholar]
- 81.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 82.Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–92. doi: 10.1097/00006123-199707000-00018. [DOI] [PubMed] [Google Scholar]
- 83.Weintraub D, Williams BJ, Jane J., Jr Decompressive craniectomy in pediatric traumatic brain injury: A review of the literature. NeuroRehabilitation. 2012;30:219–23. doi: 10.3233/NRE-2012-0748. [DOI] [PubMed] [Google Scholar]
- 84.Kan P, Amini A, Hansen K, White GL, Jr, Brockmeyer DL, Walker ML, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105(5 Suppl):337–42. doi: 10.3171/ped.2006.105.5.337. [DOI] [PubMed] [Google Scholar]
- 85.Tokutomi T, Morimoto K, Miyagi T, Yamaguchi S, Ishikawa K, Shigemori M. Optimal temperature for the management of severe traumatic brain injury: Effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery. 2003;52:102–11. [PubMed] [Google Scholar]
- 86.Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: A systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71. doi: 10.1089/neu.2007.0424. [DOI] [PubMed] [Google Scholar]
- 87.Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): A phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–53. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 88.Hutchison JS, Ward RE, Lacroix J, Hébert PC, Barnes MA, Bohn DJ, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 89.Levy DI, Rekate HL, Cherny WB, Manwaring K, Moss SD, Baldwin HZ. Controlled lumbar drainage in pediatric head injury. J Neurosurg. 1995;83:453–60. doi: 10.3171/jns.1995.83.3.0453. [DOI] [PubMed] [Google Scholar]
- 90.Mazzola CA, Adelson PD. Critical care management of head trauma in children. Crit Care Med. 2002;30:S393–401. doi: 10.1097/00003246-200211001-00003. [DOI] [PubMed] [Google Scholar]
- 91.Young B, Rapp RP, Norton JA, Haack D, Tibbs PA, Bean JR. Failure of prophylactically administered phenytoin to prevent late posttraumatic seizures. J Neurosurg. 1983;58:236–41. doi: 10.3171/jns.1983.58.2.0236. [DOI] [PubMed] [Google Scholar]
- 92.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]


