Abstract
Aim:
To determine the association between Vitamin D and autism, and the difference in level of Vitamin D in autism children and control.
Design:
Case–control study conducted between June 2011 and May 2013, among autism at the Hamad Medical Corporation and controls at the School Health Clinics and Primary Health Care Clinics
Subjects and Methods:
A total of 254 cases and 254 controls. The Autism Diagnostic Observation Schedule-Generic is a semi-structured, standardized assessment of social interaction, communication, play and imaginative use of materials for individuals suspected of having autism spectrum disorders. Data on clinical manifestations and laboratory, family history, body mass index (BMI) and clinical biochemistry variables including serum 25-hydroxy Vitamin D, calcium, phosphorus and magnesium were obtained. Univariate and multivariate statistical analyzes were performed.
Results:
Of the total number of 508 children surveyed, 254 of autism and 254 of healthy children were contacted. The mean age (± standard deviation, in years) for autism versus control children was 5.51 ± 1.58 versus 5.76 ± 1.56. There were statistically significant differences between autism and healthy children control subjects with respect to educational level of mother (P = 0.016); occupation of mother (P = 0.005); BMI (P < 0.001); consanguinity (P = 0.015); exposure to sun (P = 0.002) and walking time per day <60 min (P < 0.001). The mean value of Vitamin D in autism children was much lower than the normal value, and there was a significant difference found in the mean values of Vitamin D between autism (18.39 ± 8.2 with median 18) and versus control children (21.59 ± 8.4) (P < 0.0001) and with median 21 (P = 0.004). Besides mean values of calcium, phosphorous, magnesium, glucose, potassium and alkaline phosphate were statistically significant higher in control healthy children compared to autism children (P < 0.001). Multivariate logistic regression analysis revealed that the mean serum Vitamin D level, calcium, consanguinity, BMI, physical activity, child order, and ferritin, were considered as the main factors associated with autism. Of total 254 of autism children, 14.2% had severe Vitamin D deficiency (<10 ng/ml), 43.7% had moderate insufficient levels (between 10 and 20 ng/ml), 28.3% had mild insufficient levels (between 20 and 30 ng/ml), and only 13.8% of autism had sufficient levels (>30 ng/ml). Similarly, of the total 254 of healthy children 8.3% had severe Vitamin D deficiency (<10 ng/ml), 37% had moderate insufficient levels (between 10 and 20 ng/ml), 37.4% had mild insufficient levels (between 20 and 30 ng/ml), and only 17.3% had sufficient levels (>30 ng/ml). Furthermore, there was statistically significant differences between autism and control subjects with respect to the serum level of Vitamin D (P = 0.023).
Conclusion:
The present study revealed that Vitamin D deficiency was higher in autism children compared to healthy children and supplementing infants with Vitamin D might be a safe and more effective strategy for reducing the risk of autism.
Keywords: Association, autism, autism spectrum disorders, epidemiology, risk factors, Vitamin D
Introduction
Autism may affect all aspects of a child's life, which is a common disorder among 3–8 aged children.[1,2] Genetic, nutrition, and environmental factors have each been implicated as sources of risk for autism.[3,4,5] Oxidative stress, including low plasma levels of the antioxidant glutathione, has been reported by numerous autism studies, which can disrupt methylation-dependent epigenetic regulation of gene expression with neurodevelopmental consequences.[6] The risk of autism is generally acknowledged to reflect both genetic and environmental factors.[3,5,6] In fact, autism spectrum disorder (ASD) is a complex neuro developmental disorder, with multiple genetic and environmental risk factors; therefore, the interplay between genetic and environmental factors has become the subject of intensified research in the last several years.[3,6,7] Vitamin D deficiency has recently been proposed as a possible environmental risk factor for ASD.[7] Vitamin D has a unique role in brain homeostasis, embryogenesis and neurodevelopment, immunological modulation (including the Brain's Immune System), antioxidation, anti-apoptosis, neural differentiation and gene regulation.[7,8,9,10] Children with ASD had significantly lower serum levels of 25-hydroxy Vitamin D (25(OH) D) than healthy children. Therefore, Vitamin D deficiency during pregnancy and early childhood may be an environmental trigger for ASD.[11,12]
Vitamin D deficiency is a major health problem noticed in many parts of the world.[10,11,12,13,14,15,16,17] It is not restricted to sunshine-limited regions of the globe. It is still commonly seen in sunshine-rich areas such as Asia-Pacific,[13] Africa and Middle East regions.[10,11,12,13,14,15,18] There are several studies indicating Vitamin D deficiency is quite common among mental health children,[9,10] pregnant women,[11] asthmatic children[15] and diabetic children.[19,20] Any theory of autism's etiology must take into account its strong genetic basis while explaining its striking epidemiology. The apparent increase in the prevalence of autism over the last 20 years corresponds with increasing medical advice to avoid the sun, advice that has probably lowered Vitamin D levels and would theoretically greatly lower activated Vitamin D (calcitriol) levels in developing brains.[17]
Although autism has a significant genetic component, it is primarily diagnosed through behavioral characteristics.[3,4,5] Diagnosing autism has been formalized with instruments carefully designed to measure impairments indicative of autism in three developmental areas: language and communication, reciprocal social interactions and restricted or stereotypical interests and activities. One of the most widely used instruments is the Autism Diagnostic Observation Schedule-Generic (ADOS).[6] The ADOS consists of a variety of semi-structured activities designed to measure social interaction, communication, play and imaginative use of materials. The exam is divided into four modules, each geared toward a specific group of individuals based on their language and developmental level, ensuring coverage for a wide variety of behavioral manifestations.
The aim of this study was to find an association between Vitamin D and autism, and study the difference in level of Vitamin D in autism and control. Additionally to determine predictor risk factors.
Subjects and Methods
This is a case–control study that was designed to determine the relationship between Vitamin D and autism in subjects younger than 8 years of age in Qatar. The survey was conducted over a period from June 2011 to May 2013. This current study is based on 254 autism cases and 254 control subjects.
The study was approved by the Hamad General Hospital, Hamad Medical Corporation. All human studies have been approved by the Research Ethics Committee and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki. All the persons who agreed to participate in this study gave their informed consent prior to their inclusion in the study.
Data collection
Study measurement-clinical evaluation of autistic patients
This was based on clinical history taken from caregivers, clinical examination, and neuropsychiatric assessment. In addition, the degree of the disease severity was assessed using the ADOS; is an instrument for diagnosing and assessing autism based on specific coded behaviors that are included in a scoring algorithm using the Diagnostic and Statistical Manual of Mental Disorders-IV diagnostic criteria, resulting in a communication score, a reciprocal social interaction score, and a total score.[6] The ADOS is an observation measure designed to assess reciprocal social interaction and communication, play, and use of imagination. The ADOS attempts to set a “social world” in which behaviors associated with ASD can be observed via play, tasks, and conversation. The ADOS can be used to assist with educational planning.[6,21] The ADOS was originally developed to be used in conjunction with the autism diagnostic interview (ADI).[22] This combination of instruments has been deemed the “gold standard” for the assessment of ASD.[3,7,17] Categorized observations are subsequently combined to produce quantitative scores for analysis. Research-determined cut-offs identify the potential diagnosis of autism or related ASDs, allowing a standardized assessment of autistic symptoms. The ADI-revised, a companion instrument, is a structured interview conducted with the parents of the referred individual and covers the subject's full developmental history.[22] The ADOS has been widely used in research, and academic centers for approximately 15 years to classify children with an ASD diagnosis for research studies and to assist in making clinical diagnoses. Published validity studies also suggest good predictive validity, with sensitivities ranging from 90% to 97%, and specificities ranging from 87% to 94% for autism/ASD versus other clinical diagnoses.[6,21,22] The ADOS observation is run by a certified professional in a clinical environment, and its duration can range from 30 to 60 min. Following the observation period, the administrator will then score the individual to determine their ADOS-based diagnosis, increasing the total time from observation through scoring to between 60 and 90 min in length.[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]
Autism subjects aged below 9 years were identified from Pediatrics Clinics and School Health as a part of cohort study a random sample of 336 autism children approached and 254 gave consent and participated in study with a response rate (75.5%). The study excluded the subjects with following characteristics: Calcium supplements or Vitamin D intake during the last 6 months before the study; history of epilepsy or antiepileptic drugs since they affect Vitamin D; any history of sun block use and the pubertal age, since we know that behavioral problems and 25(OH) D2 are affected by puberty and use of sun block.
Selection of controls
Control subjects aged below 9 years were identified from healthy subjects if not ever been diagnosed as attention deficit hyperactivity disorder (ADHD). This group involved a random sample of 348 healthy subjects who visited the Primary Health Care centers for any reason other than acute or chronic disease, and only 254 subjects were included due to either refusal of the mother or difficulty in drawing blood from very uncooperative subjects; with a response rate (72.9%). The healthy subjects were selected in a way matching to the age, gender and ethnicity of cases to give a good representative sample of the studied population.
Laboratory investigation
Blood collection and serum measurements of Vitamin D
Trained phlebotomist collected venous blood sample, and serum separated and stored at −70°C until analysis. Serum 25(OH) D, a Vitamin D metabolite, was measured using a commercially available kit (DiaSorin Corporate Headquarter, Saluggia, Italy). Vitamin D deficiency which is defined as serum 25(OH) D is lower than 30 ng/ml and optimum levels are between 30 and 80 ng/ml.[10,11,16,23,24] The treated samples were then assayed using competitive binding radioimmunoassay technique. Subjects were classified into four categories: (1) severe Vitamin D deficiency, 25(OH) D <10 ng/ml; (2) moderate deficiency, 25(OH) D 10–19 ng/ml; (3) mild deficiency, 25(OH) D 20–29 ng/ml; and normal/optimal level is between 30 and 80 ng/ml.[12] According to the recommendations of other studies,[23,24] we categorized Vitamin D levels as deficient if 25(OH) D is <20 ng/ml, insufficient if it is between 20 and 29 ng/ml and sufficient if >30 ng/ml. Other baseline biochemical parameters measured from the serum included Vitamin D, calcium, phosphorus, magnesium, urea, parathyroid hormone, bilirubin, albumin, cholesterol, and triglycerides on the basis of previous recommendations.[10,11,15,16] Serum levels of these biochemical parameters were determined according to standard laboratory procedures. Furthermore, during the screening period, each patient provided a complete history, and a comprehensive examination was performed.
The questionnaire was designed to meet the objective of this study. The survey was conducted by physicians and based on standardized interviews performed by trained health professionals and nurses. The participants were interviewed by health professionals and nurses concerning their socio-demographic information such as age, gender, place of residence (urban and semi-urban), and monthly income. Height and weight were measured using standardized methods and all the participants wore light clothes and no shoes for this part of the examination. Body mass index (BMI) was calculated as the weight in kilograms (with 1 kg subtracted to allow for clothing) divided by height in meters squared. BMI <85th percentile was considered normal weight, 85th–95th percentile as overweight and >95th percentile as obese.
Data are expressed as median, the arithmetic mean and standard deviation (SD) unless otherwise stated. Student's t-test was used to ascertain the significance of differences between mean values of two continuous variables and nonparametric Mann–Whitney test was used. The Fisher's exact test (two-tailed) and Chi-square tests were performed to test for differences in proportions of categorical variables between two or more groups. Multivariate logistic regression analysis using the forward inclusion and backward deletion method was used to assess the relationship between dependent and independent variables and to adjust for potential confounders and orders the importance of risk factors (determinants) for autism. The level P < 0.05 was considered as the cut-off value for significance.
Results
Table 1 shows socio-demographic characteristics of the studied children according to autism and healthy children control subjects. Of the total number of 508 children surveyed, 254 of autism and 254 of healthy children were contacted. The mean age (± SD, in years) for autism versus control children was 5.51 ± 1.58 versus 5.76 ± 1.56. There were statistically significant differences between autism and healthy children control subjects with respect to educational level of mother (P = 0.016); occupation of mother (P = 0.005); BMI (P < 0.001); consanguinity (P = 0.015); exposure to sun (P = 0.002) and walking time per day <60 min (P < 0.001).
Table 1.
Socio-demographic characteristics of studied autism and control subject
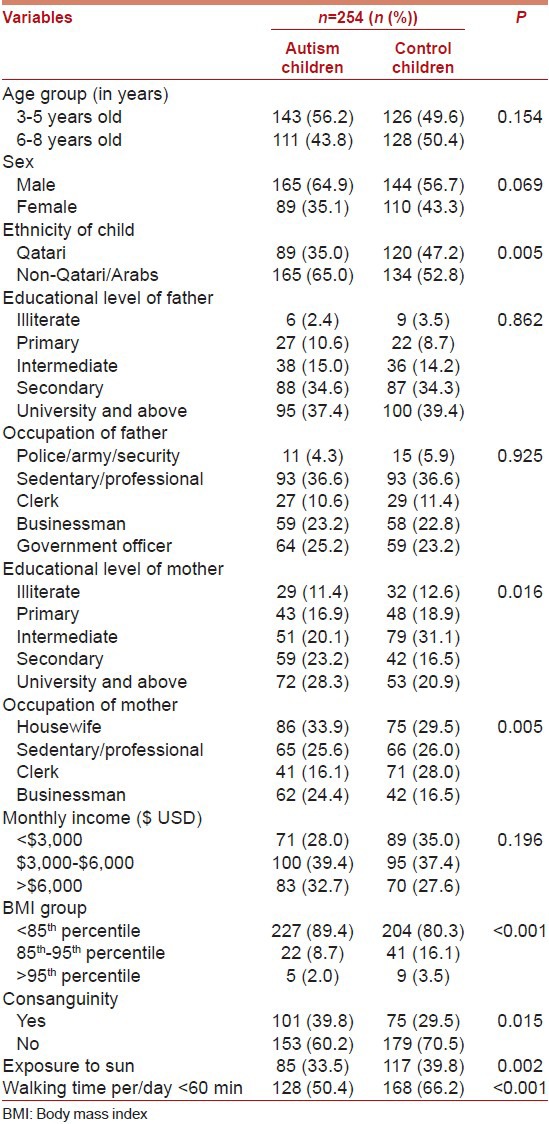
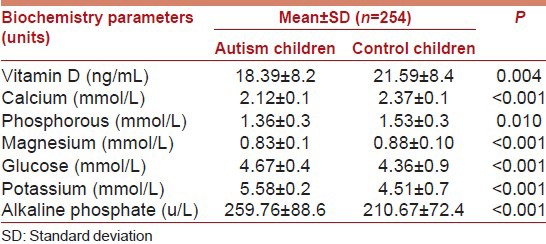
Table 2 presents baseline chemistry biomarker values among autism and control children. The study revealed that Vitamin D deficiency was considerably more common among autism children compared to healthy children. The mean value of Vitamin D in autism children was much lower than the normal value and there was a significant difference found in the mean values of Vitamin D between autism (18.39 ± 8.2 with median 18) and versus control children (21.59 ± 8.4) (P < 0.0001) and with median 21 (P = 0.004). Besides mean values of calcium; phosphorous; magnesium; glucose; potassium and alkaline phosphate were statistically significantly higher in control healthy children compared to autism children (P < 0.001).
Table 2.
Biochemistry baseline value among autism and control subjects
The predictors for autism in children using multivariate logistic regression analysis are shown in Table 3 the mean serum Vitamin D level, calcium, consanguinity, BMI, physical activity, child order, and ferritin, were considered as the main factors associated with autism after adjusting for age and gender.
Table 3.
Multivariate logistic regression analysis as predictors for autism children
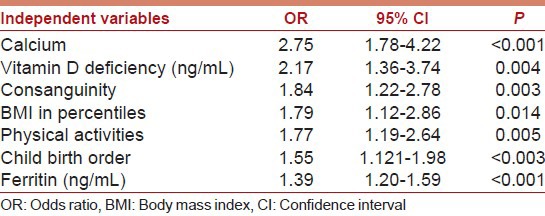
Figure 1 reveals the distribution of serum Vitamin D in children with autism and healthy controls. Of total 254 autism children 14.2% had severe Vitamin D deficiency (<10 ng/ml), 43.7% had moderate insufficient levels (between 10 and 20 ng/ml), 28.3% had mild insufficient levels (between 20 and 30 ng/ml), and only 13.8% of autism had sufficient levels (>30 ng/ml). Similarly, of the total 254 of healthy children 8.3% had severe Vitamin D deficiency (<10 ng/ml), 37% had moderate insufficient levels (between 10 and 20 ng/ml), 37.4% had mild insufficient levels (between 20 and 30 ng/ml), and only 17.3% had sufficient levels (>30 ng/ml). As can be seen from Figure 1, there was statistically significant differences between autism and control subjects with respect to the serum level of Vitamin D (P = 0.023).
Figure 1.
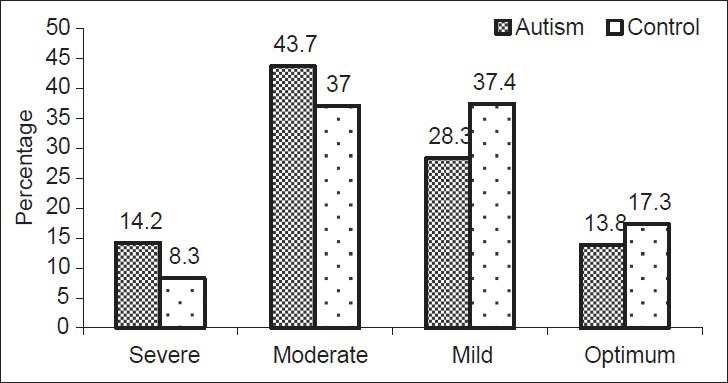
Serum level of Vitamin D among autism and control children (P = 0.023)
Discussion
This case–control study presents, to the best of our knowledge, the first report on the association of Vitamin D in children with autism. The exact etiopathological changes leading to autism are not well defined. Still with all these studies, the epigenetics of Vitamin D deficiency is still not well understood.[9,10]
Children with Vitamin D deficient rickets have several autistic markers that apparently disappear with high-dose Vitamin D treatment.[17,24] Estrogen and testosterone have very different effects on calcitriol's metabolism, differences that may explain the striking male/female sex ratios in autism.[17] Calcitriol down-regulates production of inflammatory cytokines in the brain, cytokines that have been associated with autism. Consumption of Vitamin D containing fish during pregnancy reduces autistic symptoms in offspring.[11,12] Autism and ADHD are more common in areas of impaired ultraviolet B (UVB) penetration such as poleward latitudes, urban areas, areas with high air pollution, and areas of high precipitation.[9,10] Autism is more common in dark-skinned persons, and severe maternal Vitamin D deficiency is exceptionally common in the dark-skinned. More recently a study reported[17] that simple Gaussian distributions of the enzyme that activates neural calcitriol combined with widespread gestational and/or early childhood Vitamin D deficiency may explain both the genetics and epidemiology of autism. The effects of Vitamin D on brain development and function, as a neuro-immunomodulatory agent, leading to behavioral, and neuro-psychiatric diseases have recently been reviewed.[25] Vitamin D deficiency is being associated with a number of psychiatric conditions with a developmental basis, such as autism and schizophrenia. Vitamin D deficiency in early life affects neuronal differentiation, axonal connectivity, dopamine ontogeny, and brain structure and function.[25,26] Some investigators reported reduced serum 25(OH) D in autistic children. These studies could classify them as being “Vitamin D inadequate”, which lends support to the hypothesis that autism is a Vitamin D deficiency disorder.[27,28] Although, over the last decade, the association of Vitamin D with neuro-psychiatric diseases conditions has been the focus of interest of multiple studies including those mental health disorders.[17,18,19,20,21,22,23,24,25,26,27,28]
Worldwide, the rate of autism has been steadily rising. In the present work, serum 25(OH) D levels had significant negative correlations with ADOS (P < 0.001) which signifies the possible link between the extent of Vitamin D deficiency and the degree of the severity of autism. Vitamin D receptors and Vitamin D metabolizing enzymes are present in central nervous system. Calcitriol, the active Vitamin D, affects numerous neurotransmitters and neurotrophic factors, relevant for mental disorders.[29] Furthermore, studies revealed that developmental Vitamin D deficiency leads to abnormalities in the brain, large lateral ventricles, poor tissue differentiation, and reduced expression of neurotropic factors. This study presents an association, in children, between autism with its disruptive behavior and hypovitaminosis D.
Further, the average age of diagnosis in the United States is 5.7 years and an estimated 27% remain undiagnosed at 8 years of age,[21] this is consistent with the average age of diagnosis in Qatar 5.51. At these late stages in the development, many of the opportunities to intervene with therapy have evaporated.
Finally, basic, genetic, and epidemiological studies indicate a potential role of Vitamin D in the prevention of autoimmune diseases and autism.[17,24,25,26,27,28,29,30] Three treatment modalities exist for Vitamin D deficiency which include; sunlight, Vitamin D3 supplementation, and artificial UVB radiation.[9,10,15] Treatment of Vitamin D deficiency in patients with 2000–7000 IU Vitamin D per day should be sufficient to maintain year-round levels between 40 and 70 ng/mL.[31] Children with chronic illnesses such as autism, diabetes, and/or frequent infections should be supplemented with higher doses of sunshine or Vitamin D3.[32] Furthermore, low maternal Vitamin D level is a risk factor for premature delivery. The risk of autism increases with each week a baby is born early. In addition, maternal seafood consumption during pregnancy may lower the baby's risk of autism. Perhaps, cold water ocean fish are a good source of Vitamin D and omega-3 fatty acids. Both are important for brain health.
Limitations of study
Although our study included a large sample of participants, and it is case-controlled, it has some limitations. Our study was limited by the content of existing repositories that, for reasons related to the recruitment processes of those studies, contain very few individuals who did not meet the criteria for an autism classification. Data on the mother Vitamin D deficiency before and after delivery are lacking. As confounding factors, we cannot exclude the possibility that they have contributed, to some extent, in determining the association of Vitamin D and autism. Another limitation is the Vitamin D source. It is known that vitamin is readily available either by oral intake or skin biosynthesis through UVB. The study did not include data on children kept on avoidance/restriction diet. It is known that avoidance or restriction diet is one of the modalities of therapy in some case of autism and for a relatively short period. Data on duration of outdoor activity is lacking, another limitation in our study. In Vitamin D deficient children with autism, Vitamin D treatment should be initiated. However, whether Vitamin D supplementation should be considered as an additional mode of therapy of these children needs further investigation.
Conclusion
The present study revealed that Vitamin D deficiency was higher in autism children compared to healthy children. Supplementing infants with Vitamin D might prove to be a safe and effective strategy for reducing the risk of autism, but, further genomic and some other relevant tests need to be done.
What's known on this subject
Studies suggest convincing biological or behavioral evidence linking Vitamin D deficiency to brain dysfunction. However, data are lacking regarding the association between Vitamin D and autism in human beings.
What this study adds
The association between Vitamin D deficiency and autism in young children and associated risk factors has never been reported in the literature. Perhaps, this is the first study to investigate the association between circulating levels of Vitamin D and autism among highly endogamous population. The present study revealed that Vitamin D deficiency was higher in autism children compared to healthy children. Supplementing infants with Vitamin D might be a safe and effective strategy for reducing the risk of autism.
Contributors’ statement page
AB designed and supervised the study and was involved in data collection, data analysis, involved in the interpretation of data; writing manuscript and approved submission. AOK and MMA were involved in data collection, interpretation of data; writing the manuscript and approved the final manuscript as submitted.
Acknowledgments
We are very grateful to Dr. Madeeha Kamal for partially to help and support in data collection. This work was generously supported and funded by the Qatar Foundation Grant No. Grant NPRP08-760-3-153. The authors would like to thank the Hamad Medical Corporation for their support and ethical approval (RC HMC IRB # RC/0760/2009 an Research Protocol # 8226/08 and HMC IRB # 10226/10).
Footnotes
Source of Support: This research was supported by the by the Qatar National Research Fund- QNRF NPRP08-760-3-153. The sponsor of the study had no role in study design; in the collection, analysis and interpretation of data; in the writing of this report; and in the decision to submit the paper for publication.
Conflict of Interest: None declared.
References
- 1.Mefford HC, Batshaw ML, Hoffman EP. Genomics, intellectual disability, and autism. N Engl J Med. 2012;366:733–43. doi: 10.1056/NEJMra1114194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120:A258–60. doi: 10.1289/ehp.1104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostafa GA, Al-Ayadhi LY. Reduced serum concentrations of 25-hydroxy vitamin D in children with autism: Relation to autoimmunity. J Neuroinflammation. 2012;9:201. doi: 10.1186/1742-2094-9-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Grabrucker AM. Environmental factors in autism. Front Psychiatry. 2012;3:118. doi: 10.3389/fpsyt.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- 7.Duan XY, Jia FY, Jiang HY. Relationship between vitamin D and autism spectrum disorder. Zhongguo Dang Dai Er Ke Za Zhi. 2013;15:698–702. [PubMed] [Google Scholar]
- 8.Grant WB, Cannell JJ. Autism prevalence in the United States with respect to solar UV-B doses: An ecological study. Dermatoendocrinol. 2013;5:159–64. doi: 10.4161/derm.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bener A, Kamal M. Predict attention deficit hyperactivity disorder? Evidence -based medicine. Glob J Health Sci. 2013;6:47–57. doi: 10.5539/gjhs.v6n2p47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamal M, Bener A, Ehlayel MS. Is high prevalence of vitamin D deficiency a correlate for attention deficit hyperactivity disorder? Atten Defic Hyperact Disord. 2014;6:73–8. doi: 10.1007/s12402-014-0130-5. [DOI] [PubMed] [Google Scholar]
- 11.Bener A, Al-Hamaq AO, Saleh NM. Association between vitamin D insufficiency and adverse pregnancy outcome: Global comparisons. Int J Womens Health. 2013;5:523–31. doi: 10.2147/IJWH.S51403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012;4:208–30. doi: 10.3390/nu4030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prentice A. Vitamin D deficiency: A global perspective. Nutr Rev. 2008;66:S153–64. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- 14.Whiting SJ, Green TJ, Calvo MS. Vitamin D intakes in North America and Asia-Pacific countries are not sufficient to prevent vitamin D insufficiency. J Steroid Biochem Mol Biol. 2007;103:626–30. doi: 10.1016/j.jsbmb.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 15.Bener A, Ehlayel MS, Tulic MK, Hamid Q. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol. 2012;157:168–75. doi: 10.1159/000323941. [DOI] [PubMed] [Google Scholar]
- 16.Bener A, Al-Ali M, Hoffmann GF. High prevalence of vitamin D deficiency in young children in a highly sunny humid country: A global health problem. Minerva Pediatr. 2009;61:15–22. [PubMed] [Google Scholar]
- 17.Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70:750–9. doi: 10.1016/j.mehy.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 18.El-Rassi R, Baliki G, Fulheihan GE. Vitamin D status in Middle East and Africa. 2009. [Lst accessed on 2014 May 15]. Available from: http://www.iofbonehealth.org/download/osteofound/filemanager/health_professionals/pdf/Vitamin-D-reports/Vitamin_D-MEast_Africa.pdf .
- 19.Bener A, Alsaied A, Al-Ali M, Al-Kubaisi A, Basha B, Abraham A, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009;46:183–9. doi: 10.1007/s00592-008-0071-6. [DOI] [PubMed] [Google Scholar]
- 20.Bener A, Hoffmann GF. Nutritional Rickets among Children in a Sun Rich Country. Int J Pediatr Endocrinol 2010. 2010:410502. doi: 10.1155/2010/410502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry. 2009;48:474–83. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, et al. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. J Am Acad Child Adolesc Psychiatry. 2008;47:642–51. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 25.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, et al. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology. 2009;34(Suppl 1):S247–57. doi: 10.1016/j.psyneuen.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Kocovská E, Fernell E, Billstedt E, Minnis H, Gillberg C. Vitamin D and autism: Clinical review. Res Dev Disabil. 2012;33:1541–50. doi: 10.1016/j.ridd.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Meguid NA, Hashish AF, Anwar M, Sidhom G. Reduced serum levels of 25-hydroxy and 1,25-dihydroxy vitamin D in Egyptian children with autism. J Altern Complement Med. 2010;16:641–5. doi: 10.1089/acm.2009.0349. [DOI] [PubMed] [Google Scholar]
- 29.Humble MB. Vitamin D, light and mental health. J Photochem Photobiol B. 2010;101:142–9. doi: 10.1016/j.jphotobiol.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Kinney DK, Barch DH, Chayka B, Napoleon S, Munir KM. Environmental risk factors for autism: Do they help cause de novo genetic mutations that contribute to the disorder? Med Hypotheses. 2010;74:102–6. doi: 10.1016/j.mehy.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]
- 32.Cannell JJ. On the aetiology of autism. Acta Paediatr. 2010;99:1128–30. doi: 10.1111/j.1651-2227.2010.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


