Abstract
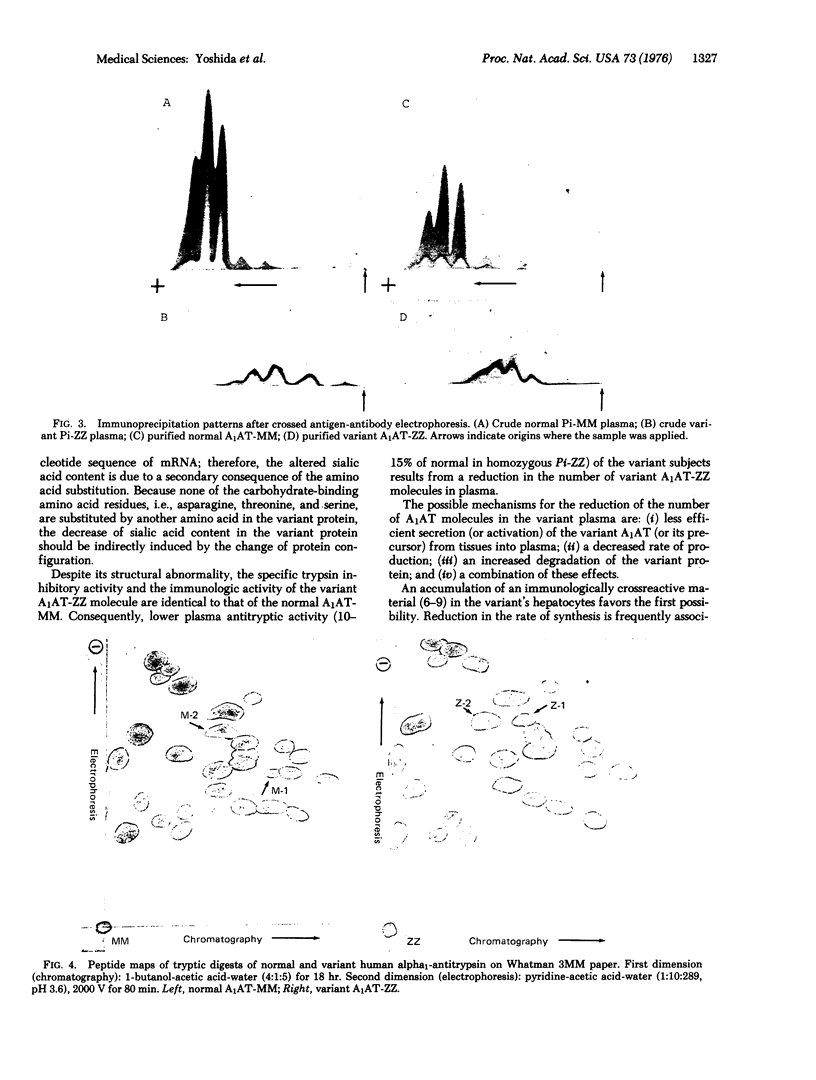
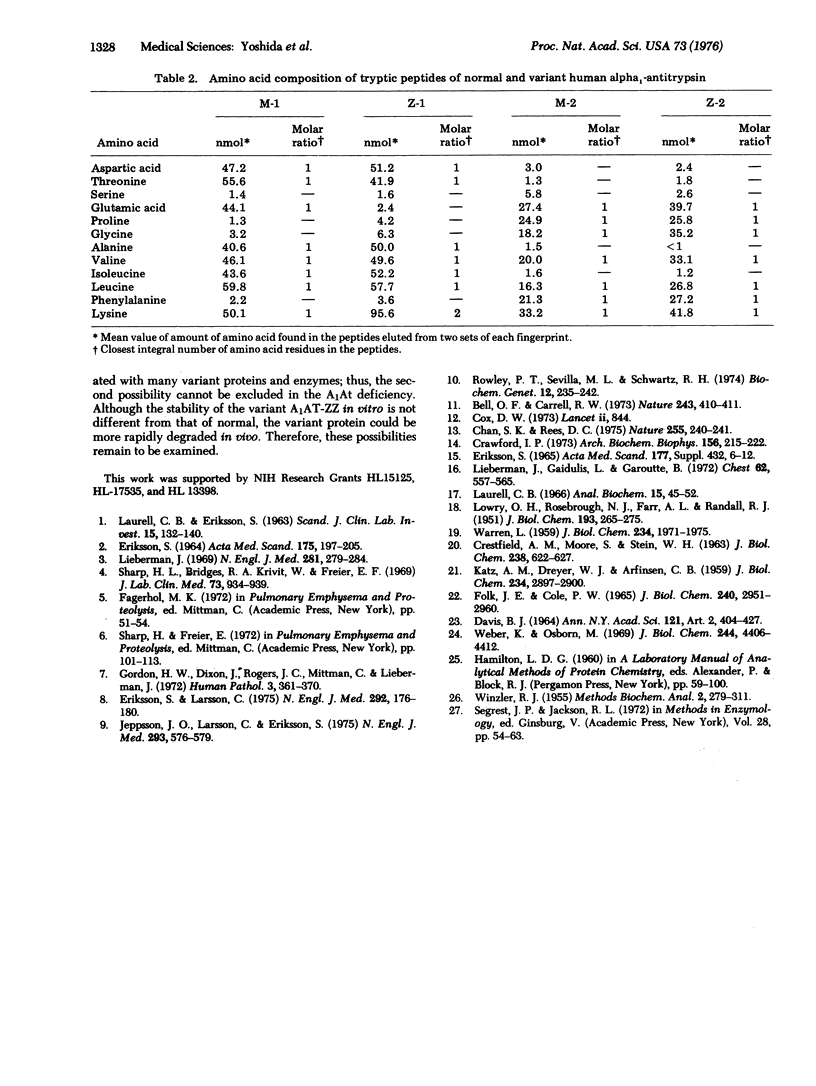
A human alpha1-antitrypsin variant protein was purified to homogeneity from homozygous variant subjects (Pi-ZZ) who had a deficiency of plasma trypsin inhibitory capacity. Molecular weight, specific trypsin inhibitory capacity, and immunologic activity of the variant protein were identical to those of normal. Amino acids, N-acetylglucosamine, and hexose contents were closely similar in the normal and variant proteins, but the sialic acid content in the variant protein was significantly lower than normal. The structural difference between the normal and the variant alpha1-antitrypsin was elucidated by fingerprinting of their tryptic peptides. Two amino acid substitutions, i.e., glutamic acid in the normal protein to lysine in the variant protein, and glutamic acid in the normal protein to glutamine in the variant protein, were found.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell O. F., Carrell R. W. Basis of the defect in alpha-1-antitrypsin deficiency. Nature. 1973 Jun 15;243(5407):410–411. doi: 10.1038/243410a0. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chan S. K., Rees D. C. Molecular basis for the alpha1-protease inhibitor deficiency. Nature. 1975 May 15;255(5505):240–241. doi: 10.1038/255240a0. [DOI] [PubMed] [Google Scholar]
- Cox D. W. Letter: Defect in alpha1-antitrypsin deficiency. Lancet. 1973 Oct 13;2(7833):844–845. doi: 10.1016/s0140-6736(73)90884-2. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Purification and properties of normal human alpha 1-antitrypsin. Arch Biochem Biophys. 1973 May;156(1):215–222. doi: 10.1016/0003-9861(73)90359-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ERIKSSON S. PULMONARY EMPHYSEMA AND ALPHA1-ANTITRYPSIN DEFICIENCY. Acta Med Scand. 1964 Feb;175:197–205. doi: 10.1111/j.0954-6820.1964.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Larsson C. Purification and partial characterization of pas-positive inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Jan 23;292(4):176–180. doi: 10.1056/NEJM197501232920403. [DOI] [PubMed] [Google Scholar]
- FOLK J. E., COLE P. W. STRUCTURAL REQUIREMENTS OF SPECIFIC SUBSTRATES FOR GUINEA PIG LIVER TRANSGLUTAMINASE. J Biol Chem. 1965 Jul;240:2951–2960. [PubMed] [Google Scholar]
- Gordon H. W., Dixon J., Rogers J. C., Mittman C., Lieberman J. Alpha 1 -antitrypsin (A1AT) accumulation in livers of emphysematous patients with A1AT deficiency. Hum Pathol. 1972 Sep;3(3):361–370. doi: 10.1016/s0046-8177(72)80037-6. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Larsson C., Eriksson S. Characterization of alpha1-antitrypsin in the inclusion bodies from the liver in alpha 1-antitrypsin deficiency. N Engl J Med. 1975 Sep 18;293(12):576–579. doi: 10.1056/NEJM197509182931203. [DOI] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Gaidulis L., Garoutte B., Mittman C. Identification and characteristics of the common alpha 1 -antitrypsin phenotypes. Chest. 1972 Nov;62(5):557–564. doi: 10.1378/chest.62.5.557. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Heterozygous and homozygous alpha-antitrypsin deficiency in patients with pulmonary emphysema. N Engl J Med. 1969 Aug 7;281(6):279–284. doi: 10.1056/NEJM196908072810601. [DOI] [PubMed] [Google Scholar]
- Rowley P. T., Sevilla M. L., Schwartz R. H. Pathogenesis of deficient serum alpha1-antitrypsin in the type ZZ homozygote. Biochem Genet. 1974 Sep;12(3):235–242. doi: 10.1007/BF00486092. [DOI] [PubMed] [Google Scholar]
- Sharp H. L., Bridges R. A., Krivit W., Freier E. F. Cirrhosis associated with alpha-1-antitrypsin deficiency: a previously unrecognized inherited disorder. J Lab Clin Med. 1969 Jun;73(6):934–939. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WINZLER R. J. Determination of serum glycoproteins. Methods Biochem Anal. 1955;2:279–311. doi: 10.1002/9780470110188.ch10. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]