Abstract
Purpose
Previous studies examining the association of body mass index (BMI) with risk of and survival from head and neck squamous cell carcinoma (HNSCC) have been inconsistent, although an inverse association has been noted for obesity and risk of HNSCC in several studies. Previous studies have not examined whether these associations differ by human papillomavirus (HPV) status.
Methods
We utilized the resources of a population-based case-control study of HNSCC from the greater Boston area (959 cases and 1208 controls were eligible for this analysis). Anthropometric history was collected through personal interviews and HPV status was assessed using serology. We analyzed the association between BMI (assessed 5 years prior to disease incidence) and disease risk and survival using logistic regression and Cox proportional hazards regression, respectively.
Results
After adjusting for known risk factors, the association between obesity and overall risk of HNSCC was not significant (OR=0.79, 95% CI: 0.60-1.04). However, obesity (BMI≥30 kg/m2) was inversely associated with HNSCC risk among HPV seronegative cases (OR=0.48, 95% CI: 0.32-0.70), but not among HPV seropositive cases (OR=0.91, 95% CI: 0.68-1.21). BMI was not associated with survival overall or by HPV status. However, being overweight (BMI:25-29.9 kg/m2) was associated with longer survival among HPV seropositive smokers (HR=0.48, 95%CI:0.31-0.74).
Conclusions
Our findings are consistent with previous observations that obesity is inversely associated with the risk of HNSCC; however this association appears to be confined to HPV seronegative cases. Overall obesity was not associated with HNSCC survival overall or by HPV status.
Impact
Obesity is associated with risk of non-HPV HNSCC, but not HPV HNSCC.
Keywords: Head and neck cancer, obesity, HPV serology
INTRODUCTION
In 2014, approximately 55,070 new cases of head and neck cancer, the majority of which are of a squamous histology (HNSCC), will be diagnosed in the United States and an estimated 12,000 individuals will die of these cancers [1]. Alcohol and tobacco use are the major risk factors for HNSCC. HPV is also strongly associated with some HNSCC, especially oropharynx cancers [2, 3].
HPV-negative HNSCC is more strongly associated with alcohol and tobacco consumption than HPV-positive HNSCC [4, 5]. Recent data indicate that patients with HPV-positive HNSCC have a better prognosis than those with HPV-negative tumors [6-8]. Thus, these two etiologically distinct types of HNSCC may each have a distinct pathogenesis.
Obesity has been associated with the occurrence of several types of cancer [9] and has also been associated with decreased survival from some cancers, including lung [10], breast [11], and pancreas [12]. The association between body mass index (BMI) and HNSCC risk is controversial; some case-control studies observed elevated risks of HNSCC among subjects who were underweight (BMI <18.5 kg/m2), relative to normal weight (BMI 18.5-24.9 kg/m2), and lower risks in overweight (BMI 25-29.9 kg/m2) and obese categories (BMI > 30 kg/m2) [13-17], while cohort studies reported no associations [18,19]. Three studies examined the association between prediagnostic BMI and HNSCC mortality: higher BMI was associated with lower risk of HNSCC mortality in two studies [16,20], while no association was observed in the third study [21].
No study has considered the potential role of HPV16 in the BMI-HNSCC relationship using HPV measurements. Therefore, we examined the association between BMI and HNSCC risk, as well as 5-year overall survival, stratified by high-risk HPV serology status in a large population-based case-control study.
MATERIALS AND METHODS
Study Subjects
The study population has been described in detail elsewhere [22-24]. Incident cases of HNSCC were identified from Departments of Oncology, Otolaryngology or Radiation Oncology at nine Boston-area medical facilities. Study phase I was conducted between December 1999 and December 2003 and phase II was conducted between October 2006 and June 2011. HNSCC cases were residents in the study area, all 18 years of age or older, with diagnosis codes 141, 143–146, 148, 149, and 161 according to International Classification of Disease, Ninth Revision (ICD-9). Recurrent cases and incident cases diagnosed more than six months before the time of patient contact were excluded. According to the Massachusetts Cancer Registry data, over 95% of the reported cases in the study area were identified and approached for study. The final data included 959 HNSCC: 153 laryngeal, 353 oral cavity and 440 pharyngeal. Controls (n=1208) with no prior history of HNSCC were selected from Massachusetts town books and frequency-matched to cases on age (+/− 3-years), sex, and town or neighborhood of residence. All-cause patient survival data were obtained from the social security death index and a search of public databases through September 1 of 2013. Study protocol and materials were approved by the Institutional Review Boards of the participating institutions and written informed consent was obtained from all cases and controls enrolled in the study.
Data Collection
Subjects completed a self-administered questionnaire that was subsequently reviewed in-person by research personnel. The questionnaire requested detailed information on sociodemographic characteristics and risk factors for HNSCC, including smoking and alcohol habits, medical history, and diet. Subjects were asked to report their height and weight 5 years prior to the interview date (or diagnosis, if a case) in order to minimize the likelihood that the preclinical disease process impacted these measures.
Laboratory Methods
Serologic high-risk HPV (HPV16, 18, 31, 33, 45, 52 and 58) testing for L1, E6 and E7 viral protein antibodies was conducted on cases and controls as a measure of exposure to HPV antibodies as previously described [23,25]. Serum from venous blood was separated within 24 hours of blood drawing and frozen at −80°C. Glutathione S-transferase capture multiplex serology assay was used for detection of HPV antibodies as previously described [26].
Statistical Analysis
Normality of continuous covariates was evaluated using the skewness-kurtosis test. In investigating the differences between case and controls, we used the two-sample T test for normally distributed continuous variables, two-sample spearman test for non-normally distributed continuous variables, and chi-square test for categorical variables.
Multivariable logistic regression was used to examine odds ratios (OR) and their confidence intervals (CI) of the association between BMI and HNSCC risk. The BMI of study participants at five years before diagnosis or interview was calculated as weight in kilograms divided by squared height in meters (kg/m2), and classified into: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), and obese (≥30 kg/m2) according to World Health Organization (WHO)/National Heart, Lung, and Blood Institute criteria. Underweight participants were excluded (n=20) as these subjects may have recorded their weight inaccurately. Normal weight participants were used as the reference group for the regression models. We defined HPV seropositive as being serologically positive L1, E6, and/or E7 for any of the high-risk HPV (HPV16, 18, 31, 33, 45, 52, 58). The model with all participants was adjusted for sex, age (continuous), race (Caucasian and non-Caucasian), education (high school or less or beyond high-school), alcohol consumption (average number of drinks per week), smoking status (never, ever), tobacco dose/duration (pack-years), and HPV serostatus. For the purpose of quantifying alcohol consumption, an alcoholic drink was defined as a 12 oz beer, 5 oz glass of wine, or 1.5 oz of liquor. Models were additionally stratified by HPV status (HPV seropositive/HPV seronegative). Subjects with missing HPV serology were excluded from the stratified analysis (138 cases and 170 controls). The excluded subjects were not different from the included ones with regard to BMI, tobacco and alcohol (p=0.25 for BMI, p=0.33 for tobacco, p=0.57 for alcohol). Interactions between high-risk HPV serostatus and BMI were tested by including a multiplicative term in the model and performing a likelihood ratio test. We further examined the association between BMI and HNSCC risk for non-smokers and smokers, according to HPV serostatus.
Survival analyses were conducted for all HNSCC cases, and separately by HPV serostatus, using the Kaplan-Meier method, where deaths from any cause were events and subjects lost to follow-up or who were alive on September, 1st, 2013, were right-censored. Survival time was measured in days from the date of diagnosis to censoring or death. Differences between BMI categories were assessed by the log-rank test. Cox proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CI) to evaluate the relation between the BMI categories and 5-year survival, controlling for sex, age at diagnosis (continuous), race (Caucasian and non-Caucasian), education (high school or less, college and professional schooling), alcohol (average intake per week, quartiles), smoking status (ever/never), tobacco dose and duration (pack-years, quartiles), tumor site (laryngeal, oral cavity and pharyngeal), stage (I, II, III and IV) and HPV serostatus. The Cox proportional hazards assumption was assessed by testing for an interaction by time in the model. The assumption was violated for stage in the models with all study patients and with HPV seronegative patients, and for sex and race in the model restricted to HPV seropositive patients. Thus, we stratified those variables in the Cox proportional hazard models. Interactions between HPV serostatus and BMI were tested by including a multiplicative term in the model. We further examined the association between BMI and HNSCC survival for never-smokers and ever-smokers, according to HPV serostatus. A subject was an ever-smoker if they reported having ever smoked 100 cigarettes or more (five packs) in their lifetime; if they had not, they were considered to be never smokers.
Statistical significance was based on 2-tailed tests and p-values ≤0.05. All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and R (http://www.r-project.org/).
RESULTS
Nine hundred and fifty nine cases and 1208 controls were available for this analysis. The majority of cases (73%) were male and study subjects were predominately Caucasian (91%; Table 1). Controls were slightly older than cases (60.7 vs. 60.2 years). There were 525 high-risk HPV seropositive cases, while only 127 controls were HPV seropositive. The few individuals with missing data with respect to sex, race, age, education, BMI, alcohol and tobacco consumption did not differ by serology status (data not shown). Significant differences in the distribution of education and BMI were observed between cases and controls, overall and among HPV seronegative participants, but not among the HPV seropositive participants. HPV seropositive cases were more likely to have pharyngeal tumors (50.8%), while laryngeal (28.3%), and oral cavity tumors (48.6%) were more common among HPV seronegative cases.
Table 1.
Distribution of selected characteristics among cases and controls, overall and by HPV serology
| All | High-risk HPVa seropositive | High-risk HPV seronegative | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Cases (n=959) | Controls (n=1208) | P value | Cases (n=525) | P valueb | Cases (n=296) | P value |
| Sex | |||||||
| Female | 258(26.9) | 323(26.7) | 0.93c | 121 (23.0) | 0.11 | 86 (29.0) | 0.42c |
| Male | 701(73.0) | 885(73.2) | 404 (76.9) | 210(70.9) | |||
| Race/Ethnicity | |||||||
| Caucasian | 871(90.8) | 1,090(90.2) | 0.59c | 487 (92.7) | 0.05 | 272(91.8) | 0.30c |
| Non-Caucasian | 87(9.0) | 118(9.7) | 38 (7.2) | 23 (7.7) | |||
| Age | |||||||
| 0-40 | 31(3.2) | 38(3.1) | 0.02b | 11 (2.0) | 0.001 | 16(5.4) | 0.45b |
| 40-50 | 131(13.6) | 133(11.0) | 78(14.8) | 38(12.8) | |||
| 50-60 | 324(33.7) | 384(31.7) | 192 (36.5) | 89(30.0) | |||
| 60-70 | 294(30.6) | 389(32.2) | 167 (31.8) | 87(29.3) | |||
| ≥70 | 179(18.6) | 264(21.8) | 77(14.6) | 66(22.2) | |||
| Education | |||||||
| High School | 401(41.8) | 340(28.1) | <.0001c | 194 (36.9) | 0.001 | 138(46.6) | <.0001c |
| College | 378(39.4) | 551(45.6) | 209 (39.8) | 114(38.5) | |||
| Professional School | 167(17.4) | 288(23.8) | 111 (21.1) | 43(14.5) | |||
| BMI | |||||||
| 18.5-25 | 325(33.8) | 360(29..8) | <0.01c | 154 (29.3) | 0.22 | 119 (40.2) | <0.001c |
| 25-30 | 421(43.8) | 504(41.7) | 240 (45.7) | 124 (41.8) | |||
| ≥30 | 213(22.2) | 344(28.4) | 131 (24.9) | 53(17.9) | |||
| Alcohol | |||||||
| First quartile | 173(18.0) | 302(25.0) | <.0001b | 98 (18.6) | <.0001 | 51 (17.2) | <.0001b |
| Second quartile | 152(15.8) | 300(24.8) | 86 (16.3) | 43 (14.5) | |||
| Third quartile | 189(19.7) | 302(25.0) | 121 (23.0) | 46 (15.5) | |||
| Forth quartile | 442(46.0) | 301(24.9) | 219 (41.7) | 154(52.0) | |||
| Smoking Statusd | |||||||
| Never | 236(24.6) | 486(40.2) | <.0001c | 158 (30.0) | <.0001 | 52 (17.5) | <.0001c |
| Former | 596(62.1) | 557(46.1) | 317 (60.3) | 190 (64.1) | |||
| Current | 116(12.0) | 152(12.5) | 42(8..0) | 52 (17.5) | |||
| Smoking | |||||||
| Never | 236(24.6) | 486(40.2) | <.0001b | 158 (30.0) | <0.001 | 52 (17.5) | <.0001b |
| Moderatee | 221(23.0) | 293(24.2) | 138(26.2) | 52 (17.5) | |||
| Heavyf | 502(52.3) | 429(35.5) | 229(43.6) | 192 (64.8) | |||
| Tumor Site | |||||||
| Laryngeal | 153(15.9) | 67 (12.7) | 64(21.6) | ||||
| Oral cavity | 353(36.8) | 149(28.3) | 144 (48.6) | ||||
| Pharyngeal | 440(45.8) | 305(58.0) | 84 (28.3) | ||||
| Tumor Stage | |||||||
| I | 131(13.6) | 49 (9.3) | 64 (21.6) | ||||
| II | 124(12.9) | 53(10.0) | 47(15.8) | ||||
| III | 153(15.9) | 86(16.3) | 44(14.8) | ||||
| IV | 485(50.5) | 319(60.7) | 115(38.8) | ||||
High-risk HPV include HPV16, 18, 31, 33, 45, 52, 58.
Spearman test for continuous variables and chi-square test for categorical variables.
Chi-square test for categorical variables.
Former (not smoking at time of interview) and current smokers (smoking at time of interview) reported having ever smoked 100 cigarettes or more (five packs) in their lifetime; if they had not, they were considered to be never smokers.
Moderate smoker was defined as those who smoke but less than 20 pack years.
Heavy smoker is defined as those who smoke more than 20 pack years.
HNSCC risk decreased with increasing BMI (Table 2), but associations were not statistically significant after adjusting for HPV serostatus, sex, race, education, age, smoking and alcohol intake (Table 2). BMI was not associated with HNSCC risk when restricting analysis to HPV seropositive cases (OR=1.15, 95% CI: 0.88-1.49, for overweight participants; OR=0.91, 95% CI: 0.68-1.21, for obese participants; Table 2). For the HPV seronegative cases, obesity was inversely associated with HNSCC risk (OR=0.48, 95% CI: 0.32-0.70) after adjusting for sex, race, education, age, smoking, and alcohol intake. Overweight participants had a non-significant 22% reduction in HNSCC risk as compared to normal weight participants (OR=0.78, 95% CI: 0.57-1.08; Table 2). Alternate definitions of smoking and alcohol intake did not alter the results for BMI among HPV seronegative participants (data not shown). Significant interactions were observed for HPV status and being overweight and obese (p = 0.008 and p = 0.01, respectively).
Table 2.
Body mass index, smoking and alcohol intake, and risk of head and neck squamous cell carcinoma overall and stratified by HPV serology.
| Total | High-risk HPV seropositive | High-risk HPV seronegative | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Cases | Control | OR (95%CI)a | P trend | Cases | OR (95%CI)b | P trend | Cases | OR (95%CI)b | P trend |
| BMI | ||||||||||
| 18.5-25 | 325 | 360 | 1[reference] | 0.08 | 154 | 1[reference] | 0.46 | 119 | 1[reference] | <0.0001 |
| 25-30 | 421 | 504 | 1.01 (0.79, 1.29) | 240 | 1.15(0.88,1.49) | 124 | 0.78(0.57,1.08) | |||
| ≥30 | 213 | 344 | 0.79 (0.60, 1.04) | 131 | 0.91(0.68,1.21) | 53 | 0.48(0.32,0.70) | |||
| Smoking Status | ||||||||||
| Never smoke | 236 | 486 | 1[reference] | 158 | 1[reference] | 52 | 1[reference] | |||
| Ever smoke | 723 | 722 | 1.32(1.01, 1.73) | 367 | 1.23(0.93,1.61) | 242 | 1.62(1.09,2.41) | |||
| Pack-years of smoking per 10 units | 959 | 1108 | 1.10(1.05, 1.16) | 525 | 1.05(1.00,1.10) | 294 | 1.15(1.09,1.21) | |||
| Alcohol | ||||||||||
| First quartile | 173 | 302 | 1[reference] | <0.0001 | 98 | 1[reference] | <0.0001 | 51 | 1[reference] | <0.0001 |
| Second quartile | 152 | 300 | 0.78(0.56, 1.07) | 86 | 0.82(0.58,1.17) | 43 | 0.84(0.53,1.32) | |||
| Third quartile | 189 | 302 | 1.03 (0.75, 1.42) | 121 | 1.16(0.83,1.61) | 46 | 0.87(0.55,1.39) | |||
| Forth quartile | 442 | 301 | 2.10 (1.54, 2.86) | 219 | 1.91(1.38,2.64) | 154 | 2.31(1.53,3.50) | |||
model includes HPV status, age, sex, race, education, smoking status, pack-years of smoking per 10 units, alcohol use
model includes age, BMI, sex, race, education, smoking status, pack-years of smoking, alcohol use
Associations between smoking and risk of HNSCC were stronger among HPV seronegative cases than HPVseropositive cases, but were similar for alcohol intake (Table 2). The association between BMI and HNSCC was also modified by smoking status; an inverse association was observed between obesity and risk of HNSCC among ever-smokers, relative to normal weight participants (OR = 0.71, 95% CI 0.50-0.99), while no association was found with obesityamong never-smokers (OR = 1.02, 95% CI =0.62-1.68; data not shown in table). We further stratified the data by both smoking status and HPV status (Table4). Similar inverse associations were observed between obesity and HNSCC risk among HPV seronegative smokers (OR=0.48, 95% CI: 0.31-0.74) and HPV seronegative never-smokers (OR=0.52 95%CI: 0.22-1.22), although only the former was statistically significant.
Table 4.
Body mass index and risk (OR) and survival (HR) of head and neck squamous cell carcinoma by HPV serology and smoking status
| High-risk HPV seropositive and ever smoker | High-risk HPV seropositive and never smoker | High-risk HPV seronegative and ever smoker | High-risk HPV seronegative and never smoker | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | Cases | OR (95%CI)a | HR (95%CI)c | Cases | OR (95%CI)b | HR (95%CI)d | Cases | OR (95%CI)a | HR (95%CI)c | Cases | OR (95%CI)b | HR (95%CI)d |
| 18.5-25 | 117 | 1 | 1 | 37 | 1 | 1 | 98 | 1 | 1 | 21 | 1 | 1 |
| 25-30 | 157 | 1.05 (0.76,1.44) | 0.58 (0.37,0.89) | 83 | 1.61 (0.55,4.77) | 1.51 (0.54,4.23) | 102 | 0.74 (0.51,1.05) | 1.09 (0.70,1.71) | 22 | 1.00 (0.50,2.00) | 3.04 (0.61,15.2) |
| ≥30 | 93 | 0.86 (0.61,1.23) | 0.71 (0.43,1.18) | 38 | 1.24 (0.40,3.87) | 1.67 (0.56,5.00) | 44 | 0.48 (0.31,0.74) | 1.51 (0.87,2.62) | 9 | 0.52 (0.22,1.22) | 2.72 (0.23,32.0) |
| P-trend | 0.43 | 0.14 | 0.82 | 0.37 | 0.96 | 0.17 | 0.15 | 0.27 | ||||
model includes age, sex, race, education, pack-years of smoking per 10 units, alcohol use.
model includes age, sex, race, education, alcohol intake.
model includes age, sex, race, education, tumor site, tumor stage, pack-years of smoking per 10 units, average alcohol intake per week
model includes age, sex, race, education, tumor site, tumor stage, average alcohol intake per week
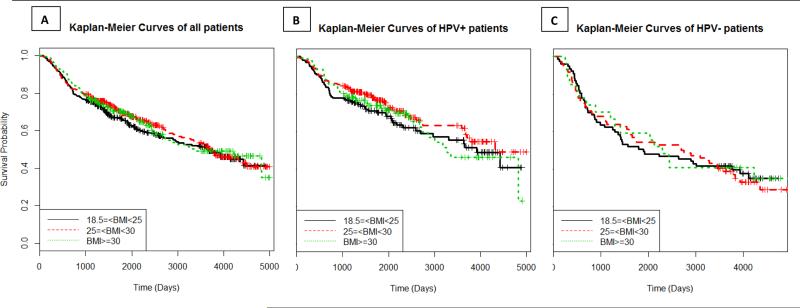
Obesity was not related to survival in the Kaplan-Meier analysis (p = 0.70; Figure 1A). Similiarly, the HRs did not vary across BMI categories (HR=0.87, 95% CI: 0.66-1.15 for overweight patients, and HR=1.01, 95% CI: 0.73-1.40 for obese patients) after adjusting for HPV status, age, tobacco, alcohol, education, race, sex, tumor site, and tumor stage (Table 3). Survival rates were not statistically associated with BMI when stratified by HPV serology (Figures 1 B and C); however, obesity was associated with a non-significant increase in risk of dying among those patients who were seronegative (HR = 1.43, 95% CI: 0.85, 2.42; Table 3). Smoking did not modify HNSCC survival (ever-smokers: HR = 0.97, 95% CI 0.68-1.39, for BMI≥30 compared with BMI≤25; never smokers: HR = 1.24, 95% CI 0.57-2.70, for BMI≥30 compared with BMI≤25, adjusting for age, race, education, HPV, alcohol, stage and site; data not shown in table). We further stratified the data according to smoking status and HPV status; among HPV seropositive smokers, overweight patients had improved survival (HR=0.58, 95%CI: 0.37-0.89), whereas no significant associations were observed in the other groups but suggestive positive associations were noted (Table 4).
Figure 1.
Kaplan-Meier curves and corresponding log-rank tests for 5-year survival rate, overall and by high-risk HPV serology.
Table 3.
Association of body mass index, smoking and alcohol intake with overall survival for HNSCC patients, overall and by HPV serology.
| Total | High-risk HPV seropositive | High-risk HPV seronegative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n) | Death(n) | HR (95%CI)a | P trend | Cases (n) | Death(n) | HR (95%CI)b | P trend | Cases (n) | Death(n) | HR (95%CI)b | P trend | |
| BMI | ||||||||||||
| 18.5-25 | 325 | 141 | 1[reference] | 0.96 | 154 | 59 | 1[reference] | 0.31 | 119 | 55 | 1[reference] | 0.24 |
| 25-30 | 421 | 167 | 0.87(0.66,1.15) | 240 | 71 | 0.68(0.46,1.01) | 124 | 70 | 1.09(0.72,1.66) | |||
| ≥30 | 213 | 86 | 1.01(0.73,1.40) | 131 | 46 | 0.83(0.53,1.29) | 53 | 30 | 1.43(0.85,2.42) | |||
| Smoking Status | ||||||||||||
| Never smoke | 236 | 63 | 1[reference] | 158 | 37 | 1[reference] | 52 | 19 | 1[reference] | |||
| Ever smoke | 723 | 331 | 1.05(0.74,1.50) | 367 | 139 | 1.12(0.72,1.73) | 242 | 136 | 0.95(0.48,1.87) | |||
| Pack-years of smoking per 10 units | 959 | 394 | 1.04(1.00,1.08) | 525 | 176 | 1.01(0.95,1.07) | 294 | 155 | 1.08(1.01,1.16) | |||
| Alcohol | ||||||||||||
| First quartile | 173 | 59 | 1[reference] | 0.04 | 98 | 27 | 1[reference] | 0.17 | 51 | 22 | 1[reference] | 0.23 |
| Second quartile | 152 | 37 | 0.73(0.44,1.19) | 86 | 16 | 0.70(0.36,1.35) | 43 | 12 | 0.75(0.34,1.67) | |||
| Third quartile | 189 | 72 | 1.17(0.77,1.78) | 121 | 39 | 1.01(0.59,1.73) | 46 | 24 | 1.51(0.75,3.03) | |||
| Forth quartile | 442 | 224 | 1.30(0.89,1.89) | 219 | 94 | 1.21(0.74,1.98) | 154 | 95 | 1.31(0.72,2.38) | |||
model includes HPV serostatus, age, sex, race, education, tumor site, tumor stage, BMI, smoking status, pack-years of smoking per 10 units, average alcohol intake per week
model includes age, sex, race, education, tumor site, tumor stage, BMI, smoking status, pack-years of smoking, average alcohol intake per week
DISCUSSION
The present study is the first to report the association between BMI and HNSCC risk, as well as survival, by measured HPV status. Our finding of effect modification by HPV status on the association between BMI and risk of HNSCC is consistent with the hypothesis that HPV seropositive and HPV seronegative HNSCC are pathologically distinct tumors and have different risk factors [27].
Our observation of an inverse association between BMI and HNSCC risk was consistent with a recent prospective analysis [18]. Moreover, the statistically significant inverse association with BMI among ever smokers was consistent with results from the International Head and Neck Cancer Epidemiology (INHANCE) consortium analysis of 17 case-control studies [14]. In the pooled analysis of 12,716 cases and 17,438 controls, obesity at 2–5 years before reference was not associated with overall risk of HNSCC (OR = 1.09, 95% CI 0.70- 1.71), but a strong inverse association was reported for obese individuals who were ever smokers (OR = 0.42, 95% CI 0.29-0.60) [14].
One of the major strengths of the current study is the inclusion of information on high-risk HPV status from serology. In this study, BMI was associated with a significantly lower risk of HNSCC among HPV seronegative subjects butBMI did not impact risk among those who were HPV seropositive. An inverse association between BMI and HNSCC has been consistently observed in case-control studies [15-17], as summarized in a recent pooled analysis [14]. The association of BMI and HNSCC in this sizable, collaborative study did not differ appreciably by tumor site [14].
To date only one study, the Caroline Head and Neck Cancer Epidemiology study, has examined the relation between BMI and HNSCC by presumed HPV status; in this study, sub-sites were used as a proxy for HPV status [28]. We and others have shown that HPV infection is not restricted to oropharyngeal cancer, although the risks are higher at those subsites [25]; thus, using site as a proxy for HPV infection may not accurately reflect true HPV status. In the Carolina Head and Neck Cancer Epidemiology Study, no associations were observed for BMI and risk of HNSCC among non-HPV associated sites among whites, but a positive association was noted for BMI in the HPV-related sites among whites (OR = 1.66, 95% CI = 1.10-2.51, BMI ≥30 vs BMI 18.5-24.9) [28]. In the same study, inverse associations were observed in HPV and non-HPV associated sites among African-Americans [28].
The inverse association between BMI and HNSCC risk found in the present study is similar to what has been reported in another tobacco-associated cancer that is not thought to be caused by HPV, namely lung cancer; a meta-analysis estimated that each unit increase in BMI is associated with a 3% decreased risk of lung cancer in men and a 4% decreased risk in women [9]. Two large studies with more than 400,000 participants reported that there was a significant inverse association between being overweight and lung cancer mortality (HR= 0.86, 95%CI: 0.77–0.96 and HR=0.78, 95%CI: 0.75–0.82 respectively) [21, 29]. The inverse association between BMI and HPV seronegative HNSCC risk may thus be due to an interaction with tobacco smoking and BMI, similar to that seen in lung cancer. The biological mechanisms for this interaction are not known.
BMI was not associated with overall survival, and HPV status did not modify the association between BMI and survival, although there was a suggestive increase in risk of dying among obese HPV-negative patients. We had limited power to stratify the analysis by both smoking and HPV serology, but did note differences and encourage other studies to examine how these factors impact survival.
The strengths of this study include the large number of patients and the information on HPV status. One weakness was the measure of obesity. BMI was self-reported, potentially allowing underreporting or over-reporting to occur. Even though previous studies have investigated the validity of self-reported past body weights and have found a high level of accuracy compared with measured weight [30], our study is still subject to the misclassification of BMI among cases who lost weight after diagnosis (as these patients may not recall their earlier weights accurately). Furthermore, we only collected weight information at a single time point (cases were asked to recall their weight 5-years prior to diagnosis) and we do not have BMI at earlier periods of life; if BMI fluctuated over time, this may have influenced the association between current BMI and HNSCC risk. If there is a true inverse association between BMI and HNSCC, misclassification of BMI due to weight gain secondary to quitting smoking could bias the result. Body fat distribution, such as waist circumference or waist to hip ratio, were not measured in this study, such that we could not evaluate the independent associations between distribution of body fat and cancers.
In conclusion, we observed that the association between BMI and HNSCC risk differs by high-risk HPV status; obesity is inversely associated with the risk of HNSCC among HPV seronegative subjects, but not among HPV seropositive subjects. While a higher BMI appeared to reduce survival among HPV seronegative patients, the association was not statistically significant.
Acknowledgments
Funding: NIH [CA078609, CA100679] and Flight Attendants Medical Research Institute
Footnotes
Authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Cancer Facts & Figures 2014. American Cancer Society, Inc.; Atlanta: 2014. [Google Scholar]
- 2.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–10. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 3.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–20. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 6.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 7.Furniss CS, McClean MD, Smith JF, et al. Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120:2386–92. doi: 10.1002/ijc.22633. [DOI] [PubMed] [Google Scholar]
- 8.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 10.Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1121–7. doi: 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast cancer research and treatment. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 12.Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. Journal of Clinical Oncology. 2013;31:4229–34. doi: 10.1200/JCO.2013.51.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubin JH, Gaudet MM, Olshan AF, et al. Body mass index, cigarette smoking, and alcohol consumption and cancers of the oral cavity, pharynx, and larynx: modeling odds ratios in pooled case-control data. Am J Epidemiol. 2010;171:1250–61. doi: 10.1093/aje/kwq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudet MM, Olshan AF, Chuang SC, et al. Body mass index and risk of head and neck cancer in a pooled analysis of case-control studies in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Int J Epidemiol. 2010;39:1091–102. doi: 10.1093/ije/dyp380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieto A, Sanchez MJ, Martinez C, et al. Lifetime body mass index and risk of oral cavity and oropharyngeal cancer by smoking and drinking habits. Br J Cancer. 2003;89:1667–71. doi: 10.1038/sj.bjc.6601347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreimer AR, Randi G, Herrero R, et al. Diet and body mass, and oral and oropharyngeal squamous cell carcinomas: analysis from the IARC multinational case-control study. Int J Cancer. 2006;118:2293–7. doi: 10.1002/ijc.21577. [DOI] [PubMed] [Google Scholar]
- 17.Garavello W, Randi G, Bosetti C, et al. Body size and laryngeal cancer risk. Ann Oncol. 2006;17:1459–63. doi: 10.1093/annonc/mdl166. [DOI] [PubMed] [Google Scholar]
- 18.Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee YC. obacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head & Neck. 2013;35:914–22. doi: 10.1002/hed.23052. [DOI] [PubMed] [Google Scholar]
- 19.Gaudet MM, Patel AV, Sun J, et al. Prospective studies of body mass index with head and neck cancer incidence and mortality. Cancer Epidemiol Biomarkers Prev. 2012;21:497–503. doi: 10.1158/1055-9965.EPI-11-0935. [DOI] [PubMed] [Google Scholar]
- 20.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. Journal of Clinical Oncology. 2006;24:5017–24. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 21.Parr CL, Batty GD, Lam TH, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11:741–52. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang C, Marsit CJ, Houseman EA, et al. Gene-environment interactions of novel variants associated with head and neck cancer. Head & Neck. 2012;34:1111–8. doi: 10.1002/hed.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang C, Marsit CJ, McClean MD, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72:5004–13. doi: 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langevin SM, McClean MD, Michaud DS, et al. Occupational dust exposure and head and neck squamous cell carcinoma risk in a population-based case-control study conducted in the greater Boston area. Cancer Medicine. 2013;2(6):978–986. doi: 10.1002/cam4.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaud DS, Langevin SM, Eliot M, et al. High-risk HPV types and head and neck cancer. Int J Cancer. 2014;135(7):1653–61. doi: 10.1002/ijc.28811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–53. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 27.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 28.Petrick JL, Gaudet MM, Weissler MC, et al. Body mass index and risk of head and neck cancer by race: the Carolina Head and Neck Cancer Epidemiology Study. Ann Epidemiol. 2014;24(2):160–164. doi: 10.1016/j.annepidem.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 30.Willett WC. Chapter 9. Anthropometric measures and body composition. In: Willett WC, editor. Nutritional Epidemiology,Third Edition. Oxford University Press; New York: 2013. [Google Scholar]