Abstract
The anorectal and urogenital systems arise from a common embryonic structure termed cloaca. Subsequent development leads to the division/septation of the cloaca into the urethra, urinary bladder, vagina, anal canal, and rectum. Defective cloacal development and the resulting anorectal and urogenital malformations are some of the most severe congenital anomalies encountered in children. In the most severe form in females, the rectum, vagina, and urethra fail to develop separately and drain via a single common channel known as a cloaca into the perineum.
In this review, we summarize our current knowledge of embryonic cloaca development and malformation, and compare them to what has already been described in literature. We describe the use of mouse models of cloaca malformation to understand which signaling pathways and cellular mechanisms are involved in the process of normal cloaca development. We also discuss the embryological correlation of the epithelial and stromal histology found in step sections of the common channel in fourteen human cloaca malformations. Finally, we highlight the significance of these findings, compare them to prior studies, and discuss their implications for the pediatric surgeons. Understanding and identifying the molecular basis for cloaca malformation could provide foundation for tissue engineering efforts that in the future would reflect better surgical reconstruction and improved quality of life for patients.
Keywords: anorectal malformation, cloaca, common channel
Mammals develop separate anorectal and urogenital canals by division (also called septation) of a common transient embryonic structure called cloaca which develops by the fourth week of intrauterine development in humans1;2 (Fig. 1A) and between days 10.5-12.5 post-fertilization in mice3. Cloaca is also defined as a small cavity at the posterior end of the gut tube lined by endoderm which is surrounded by mesenchyme derived from the splanchnopleuric mesoderm. By the sixth week in humans, the embryonic cloaca is divided into a ventral urogenital sinus and a separate dorsal hindgut (Fig. 1B). At the end of 6 weeks, the urorectal septum derived from the mesoderm completely separates the urogenital sinus from the anorectum. By the twelfth week, the anal canal, vaginal and urethral openings are established (Fig. 1C).
Fig. 1. Cloacal septation in human.
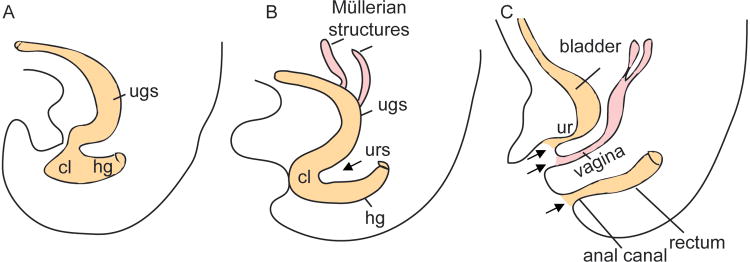
(A) At 5 weeks gestation the cloaca is not separated. (B) At 6 week, the cloaca is divided into the urogenital sinus and the hindgut. (C) By 12 weeks, the 3 openings (anus, vagina and urethra), denoted by the black arrows, are formed. cl: cloaca; ugs: urogenital sinus; urs: urorectal septum; hg: hindgut; ur: urethra.
The simple columnar epithelium of the majority of the urethra becomes stratified at the seventh week of gestation. At gestational term, this epithelium is mature and composed of transitional epithelium as it exits the bladder (proximal end), pseudostratified and stratified columnar epithelium (midportion), and stratified squamous cells near the external urethral orifice (distal end). Müllerian columnar epithelium of the vaginal canal is replaced by stratified squamous vaginal mucosa by 18 to 20th week of gestation4;5. By 32 weeks, the muscularis propria has an outer and inner muscle layer, and a discrete lamina propria layer is recognized by histology6. Lastly, at term, the anorectal mucosa is lined with pseudostratified columnar epithelium (proximal end), transitional epithelium, and squamous epithelium (distal end).
Although the urethra, vagina, and anorectum all arise from a common structure, they are morphologically and functionally different, and the molecular and morphogenetic mechanisms that give rise to these different epithelial surfaces are unknown. The cloaca mesenchymal cells have been considered to be essential in the differentiation process1;7;8. These mesenchymal cells express intrinsic regulators crucial during genitourinary tract formation, including cloacal separation9. A proper balance of epithelial cell death called apoptosis, cell growth, and maturation is needed in the process of cloaca separation.
Cloaca malformation: the most complex and severe form of anorectal malformation
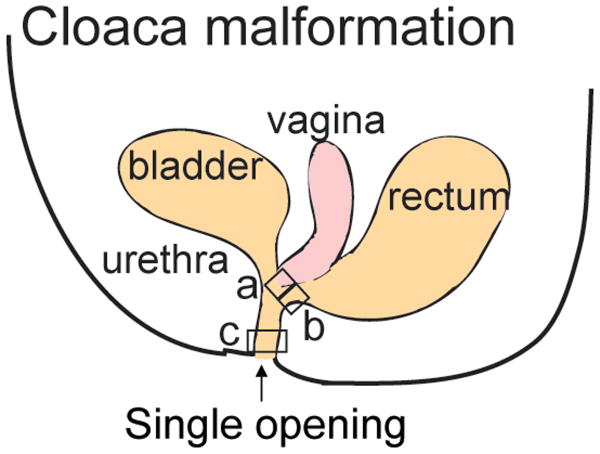
Anorectal malformations (ARM) represent a common pathology affecting children and occur in approximately 1 in 5000 live births. They are part of a spectrum of disease that goes from the most benign type called recto-perineal fistula with excellent functional prognosis to very complex malformations such as cloaca and cloacal exstrophy. In females with cloaca, the rectum, vagina and urethra fail to develop separately and instead drain via a common channel that opens into the perineum as a single orifice7 (Fig. 2).
Fig. 2. Analysis of different regions of the common channel found in cloaca malformation.
In cloaca malformation, the urethra, vagina and rectum fail to separate, and drain via a single common channel. Three areas shown by the squares labelled “a”, “b” and “c” have been analyzed histologically and molecularly55. Area “a” is closest to the vagina, area “b” is closest to the rectum and area “c” is the most distal part of the common channel.
The spectrum of anomalies associated with cloaca malformation likely results from the timing of embryological developmental arrest11. Although the precise etiology of the developmental arrest is unknown, some studies implicate the pathogenesis lies within the homeobox and sonic hedgehog signaling pathways rather than teratogenic or hereditary causes12;13. The defects are categorized by the length of the common channel that can be measured endoscopically. The length of the common channel can vary from 1 to 10 cm. The longer the common channel (>3 cm), the higher the chance for poor bowel control, neurogenic bladder, and reproductive abnormalities. When the common channel is shorter than 3 cm, about 20% of the patients will require intermittent catheterization in order to be able to empty their bladders. On the other hand when the common channel is more than 3 cm, 80% of the patients require intermittent catheterization14.
Patients with cloaca malformation have more urological risk of severe renal impairment with the ultimate need of renal transplant3;15. Moreover, 30% of these patients suffer from hydrocolpos a condition that must be suspected, diagnosed and treated during the newborn period as it can aggravate an already existing urological condition by compressing the trigone of the bladder causing megaureters and hydronephrosis16. Upon observation of uterine and vaginal duplication, Manzella et al, speculated that cloaca malformations or urogenital sinus interfered with müllerian duct fusion17. When a series of 22 patients born with cloaca reached puberty, a concern arose as it was found that 60% these patients were found to have duplicated Müllerian systems that might become obstructed and manifest as an acute abdomen during menarche18. This subsequently led to a change in practice resulting in early evaluation of the patency of the Müllerian structures at the time of the main repair or at time of colostomy closure19.
Prior to sexual activity, patients with cloaca should be evaluated to determine if the introitus and vagina are adequate for intercourse19;20. Fertility and obstetric outcomes in patients with cloaca are still a subject of investigation with few reports in the literature of successful pregnancies21-25.
The genetic cause of ARM has not yet been established, and it deserves special analysis and further investigation. For example, 95% of patients born with Down syndrome and ARM have a specific type of ARM called imperforate anus without fistula that is otherwise very uncommon (less than 5%) in patients without Down syndrome26. Certain types of ARM such as recto-perineal fistula and recto-vestibular fistula are also more common to run in families when compared to more complex malformations27. Recently a genome-wide copy number variation study indicates that ARM are genetically a heterogeneous disease but this study did not include cloaca anomalies28.
While some of the pioneering surgical techniques used to correct ARM have been developed by the faculty at our institution29, there is often a profound impact on the function of the digestive and urogenital system including poor bowel control, neurogenic bladder, chronic urinary tract infections, and renal failure associated with mortality due to inherited prognostic factors such as quality of the sacrum and presence of tethered cord that can not be altered with surgical reconstruction. Moreover, 25% of cloaca patients also require a type of vaginal replacement, most commonly with bowel. Disadvantages of having a bowel neovagina includes the possibility of future complications such as ulcerative colitis in the neovagina30 and malignant potential31;32 where the neovagina epithelium created with rectum or colonic type of tissue creates a transition zone in which the graft is suddenly subjected to new contacts or stresses33. We and others have shown that these transition zones are susceptible to tumor formation in human and mice34;35. Therefore, a critical need to investigate the molecular reasons leading to the failed separation of the embryonic cloaca remains.
Signaling pathways involved in cloaca malformations
What are the signals that specify the portion of the endoderm to become the cloaca? What are the signaling pathways involved in the division of the cloaca which give rise to the various organ lineages including anorectum, vagina, and urogenital system? Even today, summarized below, little is known about cloaca patterning, polarity, and the correlation between the embryology/histology of cloaca malformation which may provide information on cloaca septation.
Patterning of the cloaca endoderm and cloaca mesenchyme
Using human embryos, Li et al, found dorso-ventral patterning of the cloaca may involve a noncanonical Wnt ligand, WNT5a. Its expression was predominately found on the dorsal side of the cloaca that became the anorectum, and almost absent on the ventral side which give rise to the urogenital sinus36.
Paracrine factors that mediate cross-talk between the mesenchyme and epithelium are crucial into the development of cloaca derivatives. For example, the transcription factors Six1 and Six2, which are asymmetrically expressed in the mesenchyme surrounding the embryonic cloaca, are required for the genitourinary tract formation including cloaca septation37;38. This asymmetrical expression has been suggested to create an unbalanced growth of the mesenchyme which turns out to be the driving force that separates the cloaca38. This morphogenetic movement has also been described in the urorectal septum where BMP7 promote cell survival and proliferation of cloaca endoderm39.
Cell-cell adhesion is also an important event in the movement of the cells as they migrate during the process of cloaca septation. The Eph family of receptor tyrosine kinase and their membrane anchored ephrin ligands play major roles cell adhesion. Mouse mutants for those signaling genes40 develop hypospadias and incomplete cloacal septation, indicating that bidirectional signaling mediated by these proteins plays an important role in the formation of the anorectal and urogenital organs.
Sonic hedgehog (Shh) is an endoderm-derived secreted signaling molecule implicated in the first phase of signaling from the endoderm to the mesoderm. Shh acts to specify positional identities, and to promote cell proliferation and survival in a wide range of organ systems41-43. Shh is expressed in the cloaca endoderm and has both early and late functions during anorectal and urogenital development44. Mice with mutations in Shh signaling pathways recapitulate the whole spectrum of ARM that are seen in humans44-48.
Oriented cell division and cell polarity
Asymmetric cell divisions, in which the mitotic spindle orients perpendicularly to the basement membrane49, in some epithelia such as the skin has been shown to be required for proper columnar stratification, differentiation, and tissue organization50. Disruption of oriented cell divisions has been reported in a model of ARM in which BMP7 knockout mice have an arrest in cloaca septation39;51 as a result of dysfunction of the polarity pathway c-Jun N-terminal kinase (JNK). Wnt5a, which activates the planar cell polarity signaling pathway52 is crucial for anorectal development as Wnt5a knockout mice display an imperforate anus and rectourethral fistula53. In zebrafish, defects in planar cell polarity signaling are accompanied by cloaca malformation54, suggesting that oriented cell division and cell polarity are important processes in cloaca septation.
Embryology correlation of the histology findings in cloaca malformations
Our recent published work in mice and fourteen human specimens55 has implicated Bone Morphogenetic Protein (BMP) and Shh as having a role in cloaca malformations resulting in defects in epithelial differentiation (human specimen findings summarized in Table 1). This altered Shh and BMP signaling occurs early in cloaca development, and then continues to persist. We saw a nearly identical epithelial and stromal phenotype in the mouse Shh knockout common channel. In the current review article we have further elaborated upon the histology of the epithelial and stromal defects found in fourteen human cloaca specimens with emphasis on the embryological correlation of these findings.
Table 1. Epithelial and stromal defects in human cloaca patients (see Ref 55).
Samples from area “a”, “b” and “c” (see Fig. 2) in 14 cloaca patients have been analyzed histologically by Hematoxylin and Eosin. All samples show hypervascularity and contain indeterminate epithelium (both highlighted in gray). +, presence; -, absence.
| Case | Hypervascular stroma | Loose connective tissue | Colonic-like epithelium | Urothelial-like epithelium | Vaginal-like epithelium | Indeterminate epithelium | Length common channel (cm) | Months | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Area | ||||||||
| 1 | + | + | - | + | + | + | 2 | 6 | a |
| 2 | + | + | + | - | + | + | 1.5 | 48 | a |
| 3 | + | + | + | + | + | + | 2 | 8 | a |
| 4 | + | - | + | - | + | + | 3 | 7 | a |
| 5 | + | + | + | - | - | + | 3.5 | 18 | b |
| 6 | + | + | + | + | - | + | 4.5 | 8 | b |
| 7 | + | + | - | - | - | + | 3 | 11 | b |
| 8 | + | + | - | + | - | + | 2 | 24 | b |
| 9 | + | + | + | - | - | + | 3 | 6 | b |
| 10 | + | + | - | + | - | + | 4 | 10 | c |
| 11 | + | + | - | + | - | + | 4 | 15 | c |
| 12 | + | + | - | + | + | + | 3 | 11 | c |
| 13 | + | + | - | + | - | + | 3.1 | 11 | c |
| 14 | + | + | - | + | - | + | 3.1 | 21 | c |
Epithelial defects in the common channel of cloaca patients
Owing to the inability to directly investigate human embryonic cloaca development, current research has heavily relied on the use of mouse models of anorectal malformations. In our previous study55, human cloaca malformation specimens were analyzed from three different areas (Fig. 2): “a”, near the vagina (n=4), “b”, near the rectum (n=5), and “c”, distal common channel (n=5). All three areas were histologically examined via step-sections, and stained with hematoxylin and eosin.
Histology from area “a” (n=4) revealed stratified squamous epithelium in all cases, colonic mucosa in three cases, and urothelium in two cases. All four cases had foci of indeterminate epithelium (not colonic, urothelium, vaginal, urethral, or transitional epithelium). Area “b” (n=5) all had indeterminate epithelium, and colonic mucosa and urothelium was identified in 3/5 and 2/5 cases respectively. Stratified squamous mucosa consistent with vaginal type mucosa was absent. There was no evidence of colonic or transitional epithelium in any specimens from area “c” (n=5). Instead, we found the distal common channel was composed of urothelium, urethral epithelium, indeterminate epithelium, and one case also showed vaginal mucosa. The indeterminate region did not contain goblet cells, squamous cells, or umbrella cells.
The mesonephric (wolffian) duct fuses with the cloaca by the 24th day in a human embryo and remains with the urogenital sinus during the cloaca separation. The entrance of the mesonephric duct into the primitive urogenital sinus serves as a landmark distinguishing the cephalad vesicourethral canal which gives rise to the bladder and pelvic urethra from the caudal urogenital sinus which gives rise to the distal vaginal vestibule.
Our in-depth study55 revealed the absence of vaginal mucosa from area “b” suggesting alteration in the cloaca occurs after the sixth week when the urogenital sinus and hindgut are separated. In addition, there is likely failure of the vaginal bulbs/plate to proliferate caudally resulting in the lack of separation from the urogenital bulb which in normal circumstances should have canalized by the 5th month of development56.
Normally the terminal part of the hindgut is an endoderm-lined chamber that is in contact with the surface ectoderm at the cloaca membrane. This membrane is composed of endoderm of the cloaca, and ectoderm of the anal pit. The cloaca is divided into dorsal and ventral parts by the urorectal septum which develops in the angle between the allantois and hindgut. As the septum grows toward the cloaca membrane, it develops forklike extensions that produce infoldings of the lateral walls of the cloaca. These folds grow toward each other and fuse to form a partition that divides the cloaca into two parts. The rectum and cranial part of the anal canal dorsally and urogenital sinus ventrally57-61. By the 7th week of gestation, the urorectal septum should fuse with the cloacal membrane forming the perianal body in adults.
Upon further elaboration and speculation, the lack of transitional/colonic mucosa in area “c” suggests 1) abnormal proximal dorsal fusion of the urorectal septum, urorectal septum arrest, and/or 2) abnormal septal infoldings. The histology findings support the etiology of cloaca malformations as the result of septal anomalies likely due to abnormal patterning of paracrine factors like SIX1 and SIX2, BMP7, and Shh that mediate cross-talk between mesenchyme and epithelium37-39; 44-48; 55 and/or possibly primary ciliopathies54. However, in the latter, structural or primary cilia dyskinesia would be a generalized process and should be identified in other regions including the respiratory tract as well.
The absence of vaginal mucosa from area “b” suggests alterations in cloaca occur after the 6th week when the urogenital sinus and hindgut are separated. The lack of a developed urogenital cavity is also likely from arrest and abnormal coordinated epithelial-mesenchymal signalling as described in study by Haraguchi et al47 in sonic hedgehog mutants. Aggarwal et al has suggested the timing of the insult to the urogenital septum probably determines the severity of the manifestations61.
Stromal defects in the common channel of cloaca patients
Our previous study55 showed the stroma in all cloaca samples analyzed was more hypervascular than normal urothelium and vaginal mucosa stroma. The foci of smooth muscle was disorganized and haphazardly arranged not resembling the muscularis mucosa or propria layers of the colon or concentric smooth muscle layer of the urothelium. The stromal hypervascularity and abnormal musculature may be the result of urogenital sinus and cloaca membrane fusion likely the result of abnormal signaling pathway. This unbalanced mesenchymal growth may also be due in part to abnormal paracrine factors. The hypervascularity we detected in the stroma of cloaca patients and in the Shh knockout mouse model may be due to an abnormal cross talk between Shh and BMP4, known to regulate blood formation in zebrafish62.
Our comparative analysis of the nature of the epithelial and the stromal defects found in the cloaca of Shh deficient mice with surgical tissues from human cloaca patients suggests that defects in Shh signaling correlate with the pathology of the disease in humans55. Our histology/embryology correlative analysis supports the concept that human cloaca malformations are likely the result of maturation arrest and signalling pathway anomalies.
Implications for surgical procedures
Understanding the intrinsic molecular mechanisms that control septation and differentiation of the three main types of tissue (urothelium, gastrointestinal and vaginal), as well as the abnormal process that results in cloaca malformation, hopefully will guide us into future research paths to elucidate the best way to grow vaginal tissue to be used clinically in cases that require vaginal replacement.
Currently, all the available options for vaginal replacement such as: skin, buccal mucosal, small bowel, colon, and rectum offer some risks, disadvantages, and complications30-32; 63-67; since none of those work as well as native vaginal tissue. Future complications include malignant potential31-32, no growth of a neovagina constructed during infancy due to lack of hormonal response of the neovaginal tissue during puberty, neovaginal prolapse63-64, diversion colitis63-65, ulcerative colitis in the neovagina30, and excessive mucous production64.The fact that the vagina is an organ that does not require much functionality (contraction, continence, and peristalsis) makes it an ideal cavity for tissue engineering. A recent report described a tissue engineered autologous vagina that was successfully used in 4 patients offering a good promise for patients with cloacal anomaly that require vaginal replacement68.
The present study represents a preliminary step to provide foundation for tissue engineering efforts that in the future could reflect better surgical reconstruction in patients with cloacal malformation.
Acknowledgments
We would like to thank all of our colleagues who are not part of this review but who participated in the work discussed here which include Dr. Marc Levitt, Dr. Margaret Collins, Dr. Shiva Shanmukhappa, Dr. James Wells and Anna Method.
Financial support is provided part by Cincinnati Children's Hospital Medical Center and by National Institutes of Health (NIH) Digestive Health Center P30 DK078392 (G.G).
Footnotes
Disclosure: Conflict of interest: none.
References
- 1.Fritsch H, Aigner F, Ludwikowski B, Reinstadler-Zankl S, Illig R, Urbas D, Schwarzer C, Longato S. Epithelial and muscular regionalization of the human developing anorectum. Anat Rec (Hoboken) 2007;290:1449–58. doi: 10.1002/ar.20589. [DOI] [PubMed] [Google Scholar]
- 2.Kluth D. Embryology of anorectal malformations. Semin Pediatr Surg. 2010;19:201–8. doi: 10.1053/j.sempedsurg.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Warne SA, Hiorns MP, Curry J, Mushtaq I. Understanding cloacal anomalies. Arch Dis Child. 2011 doi: 10.1136/adc.2009.175034. [DOI] [PubMed] [Google Scholar]
- 4.Bulmer J. The development of the human vagina. JAnat. 1957;91:490–509. [PMC free article] [PubMed] [Google Scholar]
- 5.Robboy SJ, Bentley RC. In: Vagina Histology for Pathologists. 3. Mills SE, editor. Philadelphia: Lippincott Williams &Wilkins; 2011. pp. 999–1010. [Google Scholar]
- 6.Ruchelli ED, Huff DS. In: Vagina Color Atlas of Fetal and Neonatal Histology. Ernst LM, Ruchelli ED, Huff DS, editors. New York: Springer; 2011. pp. 195–198. [Google Scholar]
- 7.van der Putte SC. The development of the human anorectum. Anat Rec (Hoboken) 2009;292:951–4. doi: 10.1002/ar.20914. [DOI] [PubMed] [Google Scholar]
- 8.Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Gargollo P, Guo C, Tang T, Mingin G, Sun Y, Li X. Six1 and Eya1 are critical regulators of peri-cloacal mesenchymal progenitors during genitourinary tract development. Dev Biol. 2011;360:186–94. doi: 10.1016/j.ydbio.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt MA, Pena A. Anorectal malformations. Orphanet J Rare Dis. 2007;2:33. doi: 10.1186/1750-1172-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler NS, Kennedy AM, Woodward PJ. Cloacal malformation: embryology, anatomy, and prenatal imaging features. J Ultrasound Med. 2012;31(11):1843–55. doi: 10.7863/jum.2012.31.11.1843. [DOI] [PubMed] [Google Scholar]
- 12.Jo Mauch T, Albertine KH. Urorectal septum malformation sequence: insights into pathogenesis Anat Rec. 2002;268:405–410. doi: 10.1002/ar.10180. [DOI] [PubMed] [Google Scholar]
- 13.Williams DHIV, Fitchev P, Policarpio-Nicolas ML, Wang E, Brannigan RE, Crawford SE. Urorectal septum malformation sequence. Urology. 2005;66:657.e5–657.e7. doi: 10.1016/j.urology.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Burgu B, Duffy PG, Cuckow P, Ransley P, Wilcox DT. Long-term outcome of vaginal reconstruction: comparing techniques and timing. J Pediatr Urol. 2007;3:316–320. doi: 10.1016/j.jpurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Levitt MA, Pena A. Cloacal malformations: lessons learned from 490 cases. Semin Pediatr Surg. 2010;19:128–38. doi: 10.1053/j.sempedsurg.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Bischoff A, Levitt MA, Breech L, Louden E, Peña A. Hydrocolpos in Cloacal Malformations. J Pediatr Surg. 2010;45(6):1241–1245. doi: 10.1016/j.jpedsurg.2010.02.097. [DOI] [PubMed] [Google Scholar]
- 17.Manzella A, Filho PB. Hydrocolpos, uterus didelphys, and septate vagina in association with ascites: antenatal sonographic detection. J Ultrasound Med. 1988;17:465–468. doi: 10.7863/jum.1998.17.7.465. [DOI] [PubMed] [Google Scholar]
- 18.Levitt MA, Stein DM, Peña A. Gynecological Concerns in the Treatment of Teenagers with Cloaca. J Pediatr Surg. 1998;33(2):188–193. doi: 10.1016/s0022-3468(98)90429-8. [DOI] [PubMed] [Google Scholar]
- 19.Breech L. Gynecologic concerns in patients with anorectal malformations. Sem Pediatr Surg. 2010;19:139–145. doi: 10.1053/j.sempedsurg.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Warne SA, Wilcox DT, Creighton S, Ransley PG. Long-term gynecological outcome of patients with persistent cloaca. J Urol. 2003;170:1493–1496. doi: 10.1097/01.ju.0000086702.87930.c2. [DOI] [PubMed] [Google Scholar]
- 21.Hamai Y, Fujii T, Iwasaki M, Muronosono E, Taketani Y. A case of pregnancy in a woman with cloacal dysgenesis and a rudimentary uterine horn. Hum Reprod. 1997;12:1103. doi: 10.1093/humrep/12.5.1103. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg JA, Hendren WH. Vaginal delivery after cloacal malformation repair. Obstet Gynecol. 1997;90:666–667. doi: 10.1016/s0029-7844(97)00407-9. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y, Murakami T, Kadowaki M, Konno R, Yoshida S, Okamura K. A remnant tubal pregnancy after cloacal malformation repair. Fertil Steril. 2001;75:440–441. doi: 10.1016/s0015-0282(00)01722-2. [DOI] [PubMed] [Google Scholar]
- 24.Greenber JA, Wu JM, Rein MS, Hendren WH. Triplets after cloacal malformation repair. J Pediatr Adolesc Gynecol. 2003;16:43–44. doi: 10.1016/s1083-3188(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 25.Shrim A, Podymow T, Breech L, Dahan MH. Term delivery after in vitro fertilization in a patient with cloacal malformation. J Obstet Gynaecol Can. 2011;33:952–954. doi: 10.1016/S1701-2163(16)35021-6. [DOI] [PubMed] [Google Scholar]
- 26.Torres R, Levitt MA, Tovilla JM, Rodriguez G, Pena A. Anorectal malformations and Down's syndrome. J Pediatr Surg. 1998;33:194–7. doi: 10.1016/s0022-3468(98)90430-4. [DOI] [PubMed] [Google Scholar]
- 27.Falcone RA, Jr, Levitt MA, Pena A, Bates M. Increased heritability of certain types of anorectal malformations. J Pediatr Surg. 2007;42:124–7. doi: 10.1016/j.jpedsurg.2006.09.012. discussion 127-8. [DOI] [PubMed] [Google Scholar]
- 28.Wong EH, Ng CL, Lui VC, So MT, Cherny SS, Sham PC, Tam PK, Garcia-Barcelo MM. Gene network analysis of candidate Loci for human anorectal malformations. PLoS One. 2013;8:e69142. doi: 10.1371/journal.pone.0069142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pena A, Devries PA. Posterior sagittal anorectoplasty: important technical considerations and new applications. J Pediatr Surg. 1982;17:796–811. doi: 10.1016/s0022-3468(82)80448-x. [DOI] [PubMed] [Google Scholar]
- 30.Gabarain G, Garcia-Naveiro R, Ponsky TA, Boulanger SC, Parry RL. Ulcerative colitis of the neovagina as a postsurgical complication of persistent cloaca. J Pediatr Surg. 2012;47:e19–22. doi: 10.1016/j.jpedsurg.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 31.Idrees MT, Deligdisch L, Altchek A. Squamous papilloma with hyperpigmentation in the skin graft of the neovagina in Rokitansky syndrome: literature review of benign and malignant lesions of the neovagina. J Pediatr Adolesc Gynecol. 2009;22:e148–55. doi: 10.1016/j.jpag.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Schober JM. Cancer of the neovagina. J Pediatr Urol. 2007;3:167–70. doi: 10.1016/j.jpurol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.McNairn AJ, Guasch G. Epithelial transition zones: merging microenvironments, niches, and cellular transformation. Eur J Dermatol. 2011;21 Suppl 2:21–8. doi: 10.1684/ejd.2011.1267. [DOI] [PubMed] [Google Scholar]
- 34.Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–27. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Runck LA, Kramer M, Ciraolo G, Lewis AG, Guasch G. Identification of epithelial label-retaining cells at the transition between the anal canal and the rectum in mice. Cell Cycle. 2010;9:3039–45. doi: 10.4161/cc.9.15.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li FF, Zhang T, Bai YZ, Yuan ZW, Wang WL. Spatiotemporal expression of Wnt5a during the development of the hindgut and anorectum in human embryos. Int J Colorectal Dis. 2011;26(8):983–8. doi: 10.1007/s00384-011-1191-y. [DOI] [PubMed] [Google Scholar]
- 37.Wang C, Gargollo P, Guo C, Tang T, Mingin G, Sun Y, Li X. Six1 and Eya1 are critical regulators of peri-cloacal mesenchymal progenitors during genitourinary tract development. Dev Biol. 2011;360(1):186–94. doi: 10.1016/j.ydbio.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Wang JY, Borer J, Li X. Embryonic Origin and Remodeling of the Urinary and Digestive Outlets. Plos One. 2013;8:e55587, 1–11. doi: 10.1371/journal.pone.0055587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr Patterns. 2009;9(4):224–30. doi: 10.1016/j.gep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–90. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Rowitch DH, S-Jacques B, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–65. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr Biol. 1999;9:1304–14. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- 43.Locker M, Agathocleous M, Amato MA, Parain K, Harris WA, Perron M. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 2006;20:3036–48. doi: 10.1101/gad.391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifert AW, Bouldin CM, Choi KS, Harfe BD, Cohn MJ. Multiphasic and tissue-specific roles of sonic hedgehog in cloacal septation and external genitalia development. Development. 2009;136(23):3949–57. doi: 10.1242/dev.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Kim P, Hui CC. The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet. 2001;59:306–15. doi: 10.1034/j.1399-0004.2001.590503.x. [DOI] [PubMed] [Google Scholar]
- 46.Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 2001;159:765–74. doi: 10.1016/S0002-9440(10)61747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134(3):525–33. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 48.Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136(23):3959–67. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roegiers F, Jan YN. Asymmetric cell divisions. Curr Opin Cell Biol. 2004;16(2):195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437(7056):275–80. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu K, Wu X, Shapiro E, Huang H, Zhang L, Hickling D, Deng Y, Lee P, Li J, Lepor H, Grishina I. Bmp7 functions via a polarity mechanism to promote cloacal septation. Plos One. 2012;7(1):e29372. doi: 10.1371/journal.pone.0029372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harb Perspect Biol. 2009;1(3):a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tai CC, Sala FG, Ford HR, Wang KS, Li C, Minoo P, Grikscheit TC, Bellusci S. Wnt5a knock-out mouse as a new model of anorectal malformation. J Surg Res. 2009;156:278–82. doi: 10.1016/j.jss.2009.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bubenshchikova E, Ichimura K, Fukuyo Y, Powell R, Hsu C, Morrical SO, Sedor JR, Sakai T, Obara T. Wtip and Vangl2 are required for mitotic spindle orientation and cloaca morphogenesis. Biol Open. 2012;15:588–96. doi: 10.1242/bio.20121016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Runck LA, Method A, Bischoff A, Levitt M, Pena A, Collins MH, Gupta A, Shanmukhappa S, Wells JM, Guasch G. Defining the molecular pathologies in cloaca malformation: similarities between mouse and human. Dis Model Mech. 2014;7:483–93. doi: 10.1242/dmm.014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadler TW. Langerman's Medical Embryology. 12. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 57.Martinez-Frias ML, Bermejo E, Rodriguez-Pinilla E. Anal atresia, vertebral, genital, and urinary tract anomalies: a primary polytopic developmental field defect identified through an epidemiological analysis of associations. Am J Med Genet. 2000;95:169–73. doi: 10.1002/1096-8628(20001113)95:2<169::aid-ajmg15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 58.Jo Mauch T, Albertine KH. Urorectal septum malformation sequence: Insights into pathogenesis. Anat Rec. 2002;268:405–10. doi: 10.1002/ar.10180. [DOI] [PubMed] [Google Scholar]
- 59.Padmanabhan R, Naruse I, Shiota K. Caudal dysgenesis in staged human embryos: Carnegie stages 16-23. Am J Med Genet. 1999;87:115–27. doi: 10.1002/(sici)1096-8628(19991119)87:2<115::aid-ajmg2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 60.Pauli RM. Lower mesodermal defects: a common cause of fetal and early neonatal death. Am J Med Genet. 1994;50:154–72. doi: 10.1002/ajmg.1320500206. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal S, Phadke SR. Recurrence of urorectal septum malformation sequence spectrum anomalies in siblings: time to explore the genetics. Am J Med Genet A. 2013;161A:1718–21. doi: 10.1002/ajmg.a.35950. [DOI] [PubMed] [Google Scholar]
- 62.Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development. 2006;133(11):2275–84. doi: 10.1242/dev.02388. [DOI] [PubMed] [Google Scholar]
- 63.Burgu B, Duffy PG, Cuckow P, Ransley P, Wilcox DT. Long-term outcome of vaginal reconstruction: comparing techniques and timing. J Pediatr Urol. 2007;3:316–20. doi: 10.1016/j.jpurol.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Hensle TW, Shabsigh A, Shabsigh R, Reiley EA, Meyer-Bahlburg HF. Sexual function following bowel vaginoplasty. J Urol. 2006;175:2283–6. doi: 10.1016/S0022-5347(06)00337-5. [DOI] [PubMed] [Google Scholar]
- 65.Syed HA, Malone PS, Hitchcock RJ. Diversion colitis in children with colovaginoplasty. BJU Int. 2001;87:857–60. doi: 10.1046/j.1464-410x.2001.02180.x. [DOI] [PubMed] [Google Scholar]
- 66.Krege S, Walz KH, Hauffa BP, Korner I, Rubben H. Long-term follow-up of female patients with congenital adrenal hyperplasia from 21-hydroxylase deficiency, with special emphasis on the results of vaginoplasty. BJU Int. 2000;86:253–8. doi: 10.1046/j.1464-410x.2000.00789.x. discussion 258-9. [DOI] [PubMed] [Google Scholar]
- 67.Cali RW, Pratt JH. Congenital absence of the vagina. Long-term results of vaginal reconstruction in 175 cases. Am J Obstet Gynecol. 1968;100:752–63. [PubMed] [Google Scholar]
- 68.Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P, Ordorica-Flores R, Soker S, Yoo JJ, Atala A. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;26:329–36. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]