Abstract
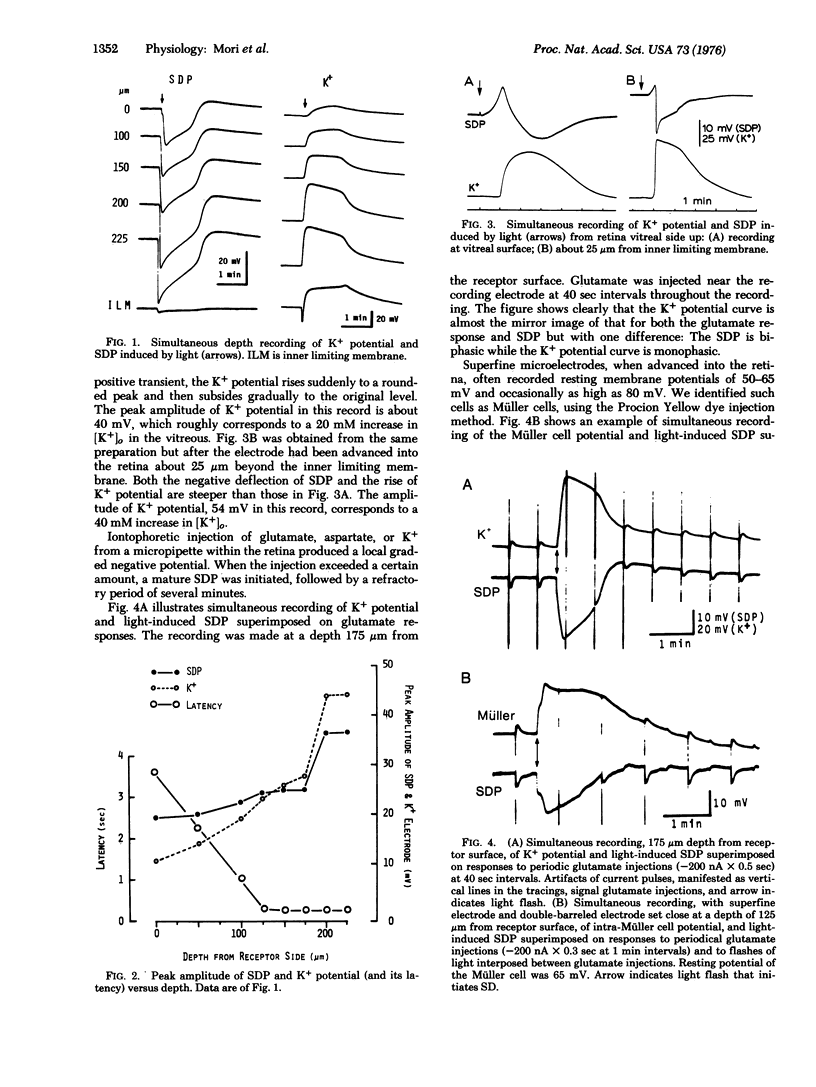
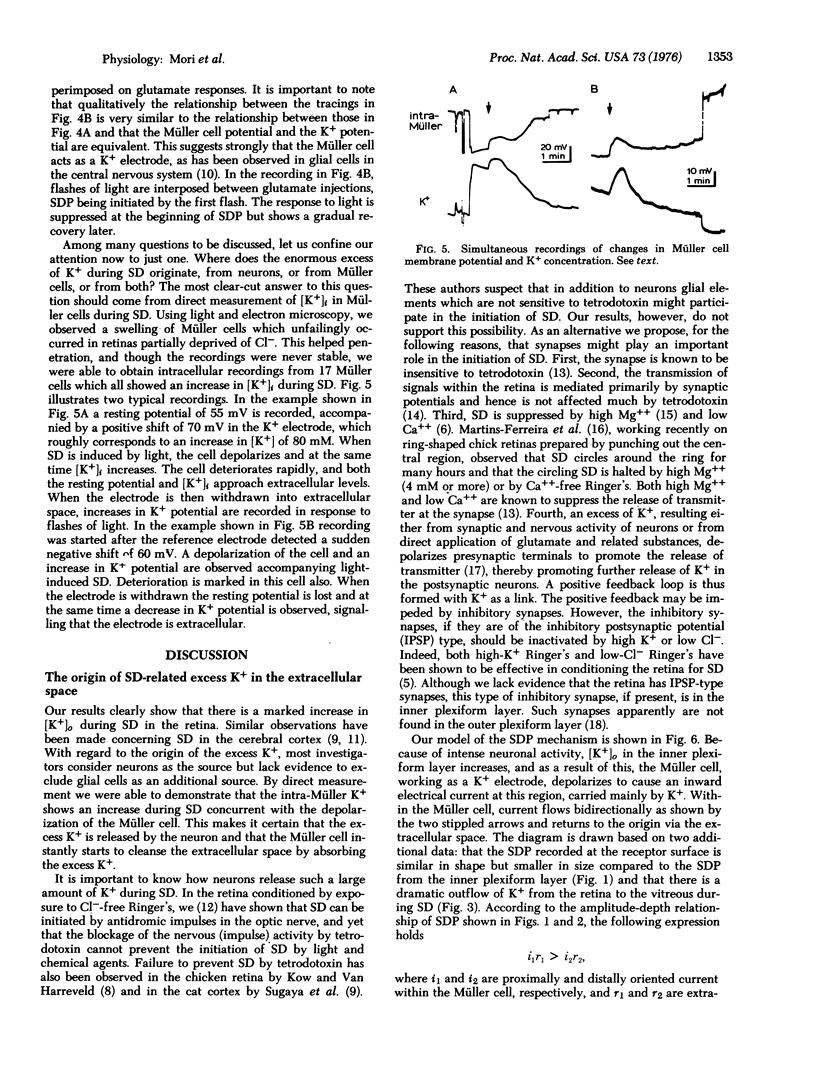
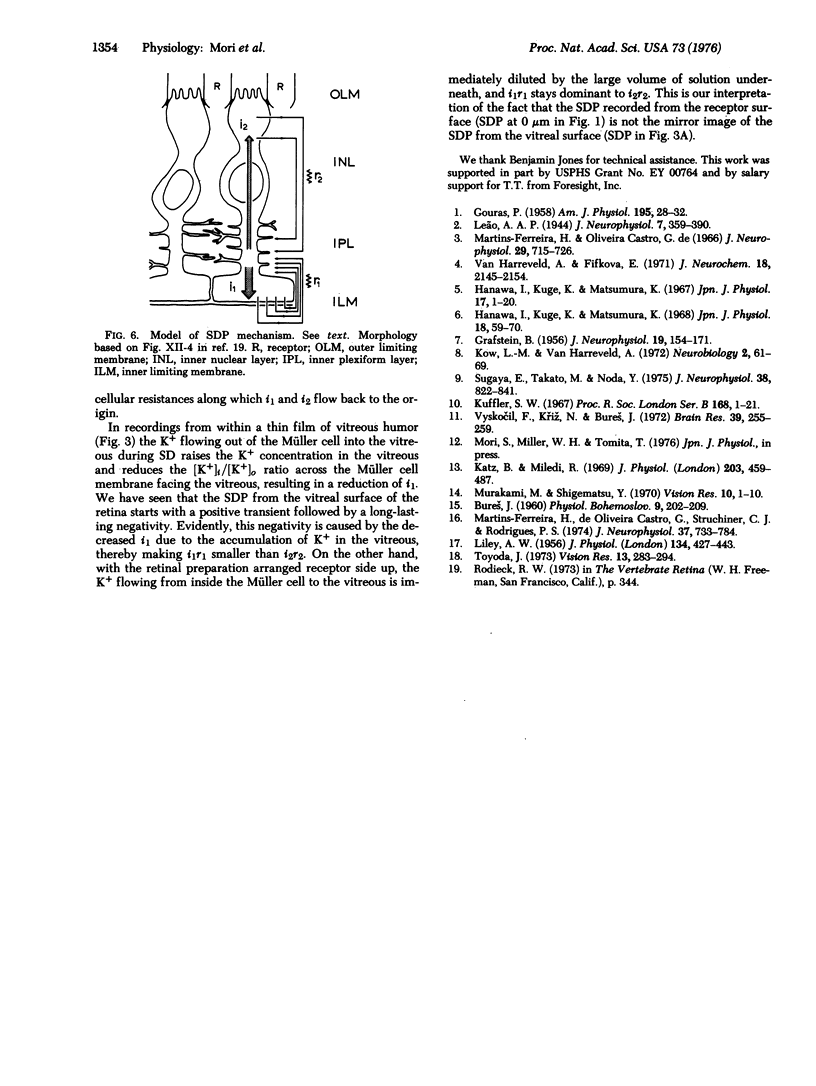
K+ potentials were investigated using K+ microelectrodes in frog (Rana pipiens) retinas conditioned for spreading depression (SD) by Cl--free Ringer's solution. A marked increase in outside K+ concentration, [K+]O, maximal in the inner plexiform layer, was observed during SD. This [K+]o change resembled the simultaneously recorded membrane potential change in Müller cells, suggesting that these cells act as K+ electrodes. Intracellular recording with K+ electrodes in Müller cells showed that upon SD the [K+]i (inside) of Müller cells increases and therefore immediately starts to cleanse the extracellular space of the excess of K+ which, evidence suggests, is mainly caused by pathologically enhanced synaptic activity in the inner plexiform layer.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GOURAS P. Spreading depression of activity in amphibian retina. Am J Physiol. 1958 Oct;195(1):28–32. doi: 10.1152/ajplegacy.1958.195.1.28. [DOI] [PubMed] [Google Scholar]
- GRAFSTEIN B. Mechanism of spreading cortical depression. J Neurophysiol. 1956 Mar;19(2):154–171. doi: 10.1152/jn.1956.19.2.154. [DOI] [PubMed] [Google Scholar]
- Hanawa I., Kuge K., Matsumura K. Mechanism of the slow depressive potential production in the isolated frog retina. Jpn J Physiol. 1968 Feb 15;18(1):59–70. doi: 10.2170/jjphysiol.18.59. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow L. M., van Harreveld A. Ion and water movements in isolated chicken retinas during spreading depression. Neurobiology. 1972;2(2):61–69. [PubMed] [Google Scholar]
- Kuffler S. W. Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci. 1967 Jun 6;168(1010):1–21. doi: 10.1098/rspb.1967.0047. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Ferreira H., De Oliveira Castro G., Struchiner C. J., Rodrigues P. S. Circling spreading depression in isolated chick retina. J Neurophysiol. 1974 Jul;37(4):773–784. doi: 10.1152/jn.1974.37.4.773. [DOI] [PubMed] [Google Scholar]
- Martins-Ferreira H., de Castro G. O. Light-scattering changes accompanying spreading depression in isolated retina. J Neurophysiol. 1966 Jul;29(4):715–726. doi: 10.1152/jn.1966.29.4.715. [DOI] [PubMed] [Google Scholar]
- Murakami M., Shigematsu Y. Duality of conduction mechanism in bipolar cells of the frog retina. Vision Res. 1970 Jan;10(1):1–10. doi: 10.1016/0042-6989(70)90057-x. [DOI] [PubMed] [Google Scholar]
- Sugaya E., Takato M., Noda Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J Neurophysiol. 1975 Jul;38(4):822–841. doi: 10.1152/jn.1975.38.4.822. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Fifkova E. Effects of glutamate and other amino acids on the retina. J Neurochem. 1971 Nov;18(11):2145–2154. doi: 10.1111/j.1471-4159.1971.tb05073.x. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Kritz N., Bures J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 1972 Apr 14;39(1):255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]



