Abstract
IMPORTANCE
There is an immediate need to develop local intraoperative adjuvant treatment strategies to improve outcomes in patients with cancer who undergo head and neck surgery.
OBJECTIVES
To determine the safety of photodynamic therapy with 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a (HPPH) in combination with surgery in patients with head and neck squamous cell carcinoma.
DESIGN, SETTING, AND PARTICIPANTS
Nonrandomized, single-arm, single-site, phase 1 study at a comprehensive cancer center among 16 adult patients (median age, 65 years) with biopsy-proved primary or recurrent resectable head and neck squamous cell carcinoma.
INTERVENTIONS
Intravenous injection of HPPH (4.0 mg/m2), followed by activation with 665-nm laser light in the surgical bed immediately after tumor resection.
MAIN OUTCOMES AND MEASURES
Adverse events and highest laser light dose.
RESULTS
Fifteen patients received the full course of treatment, and 1 patient received HPPH without intraoperative laser light because of an unrelated myocardial infarction. Disease sites included larynx (7 patients), oral cavity (6 patients), skin (1 patient), ear canal (1 patient), and oropharynx (1 patient, who received HPPH only). The most frequent adverse events related to photodynamic therapy were mild to moderate edema (9 patients) and pain (3 patients). One patient developed a grade 3 fistula after salvage laryngectomy, and another patient developed a grade 3 wound infection and mandibular fracture. Phototoxicity reactions included 1 moderate photophobia and 2 mild to moderate skin burns (2 due to operating room spotlights and 1 due to the pulse oximeter). The highest laser light dose was 75 J/cm2.
CONCLUSIONS AND RELEVANCE
The adjuvant use of HPPH-photodynamic therapy and surgery for head and neck squamous cell carcinoma seems safe and deserves further study.
Head and neck squamous cell carcinoma (HNSCC) represents a diverse group of malignant neoplasms with varying clinical presentations.1 The treatment paradigm for HNSCC has evolved during the past 2 decades, with increased use of chemoradiotherapy for stage III and stage IV disease in the oropharynx and in the larynx.2 Nevertheless, radical salvage surgery remains the standard criterion for patients who failed nonsurgical therapies.3 Despite advancements in surgical techniques, local recurrence rates after salvage surgery continue to be problematic.4 These recurrences are a major cause of treatment failure (>50%), followed by the development of distant metastases and the occurrence of second primary cancers.5 Hence, there is an immediate need to develop local intraoperative adjuvant treatment strategies to improve outcomes in these patients.
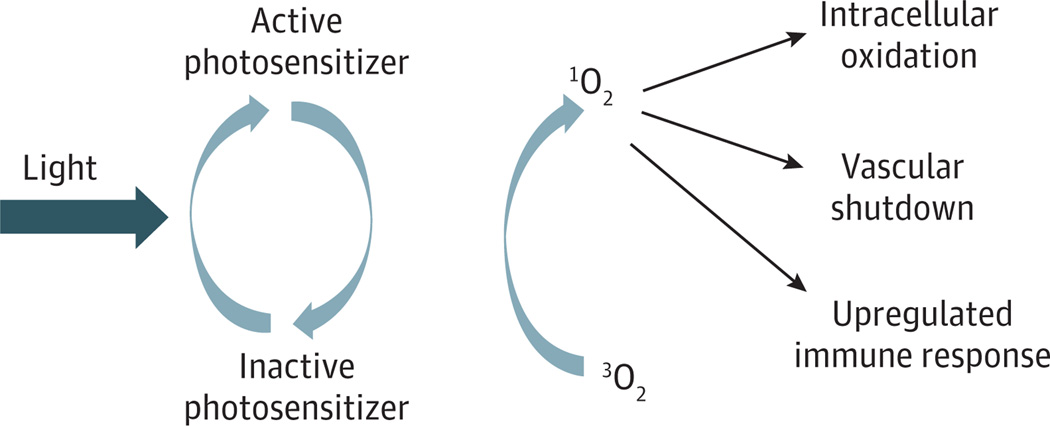
Photodynamic therapy (PDT) is a minimally invasive treatment that involves localized photoactivation of a drug that generates cytotoxic reactive oxygen species, resulting in direct damage to tumor cells.6,7 A schematic illustration of the PDT process is shown in Figure 1.Tumor destruction following PDT is also accomplished by microvascular collapse within the tumor, as well as intense stimulation of the innate and adaptive immune responses.7 Photodynamic therapy using the photosensitizer porfimer sodium (Photofrin) is approved by the Food and Drug Administration for several clinical indications, including obstructing esophageal cancer, high-grade dysplasia in Barrett esophagus, and early-stage and advanced-stage endobronchial cancer.9,10 Clinical studies10–13 have also high-lighted the potential usefulness of PDT in the management of head and neck cancer. While porfimer sodium–mediated PDT is effective, the persistence of the photosensitizer in skin necessitates protection of patients from sunlight and other sources of bright light for long periods (up to 90 days). Another limitation is that its longest absorption peak is 630 nm, and this interferes with tissue light penetration as a result of absorption by hemoglobin.
Figure 1. Photodynamic Therapy Illustrated by Process Layout.
Photodynamic therapy requires the 3 elements of light, photosensitizer, and oxygen. Light of a specific wavelength activates a specific photosensitizer. This activation results in the creation of singlet oxygen, which in turn destroys tissue by intracellular oxidation, shutdown of the microvasculature, and concomitant upregulated immune response at the tumor site and humorally.
Given the prolonged and sometimes severe cutaneous phototoxic effects associated with the use of porfimer sodium, there has been widespread interest in the development of newer photosensitizers with more favourable photophysical and pharmacokinetic properties.14 The chlorin-based compound 2-(1-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH), developed at Roswell Park Cancer Institute (RPCI), is one such sensitizer that has been shown to exhibit potent antitumor activity in several experimental tumor models.15 Phase 1 and phase 2 studies16,17 conducted in patients with lung and esophageal cancer have also revealed good response rates. It has been shown at RPCI that HPPH at clinically effective antitumor doses is associated with significantly reduced cutaneous photosensitivity that rapidly declines during several days.18 In this phase 1 study, the primary objectives were to determine the safety of intraoperative adjuvant HPPH-mediated PDT immediately following tumor resection and to determine the highest laser light dose that can be safely used in patients with HNSCC.
Methods
Patients
This was a single-institution phase 1 clinical study in patients with primary or recurrent histologically confirmed HNSCC. All patients were deemed treatable and had surgically resectable tumors. We studied the safety profile of HPPH-mediated PDT by treating the operative field before wound closure. Therefore, broad enrollment criteria were allowed to include a heterogeneous sample population.
The study was performed at RPCI between November 28, 2006, and October 28, 2011. The protocol was approved by the RPCI Institutional Review Board and was overseen by the RPCI Data and Safety Monitoring Board. All patients provided written informed consent.
The primary inclusion criteria were adult men or nonpregnant, nonlactating women who had an Eastern Cooperative Oncology Group performance status of 0 to 2,with resectable primary or recurrent HNSCC who were undergoing head and neck surgery with the expectation of achieving clear tumor margins. The primary exclusion criteria were as follows: prothrombin time at least 1.5 times above the upper normal limit, porphyria or hypersensitivity to porphyrin or porphyrinlike compounds, platelet count of less than 100 ×103/µL (to convert platelet count to ×109/L, multiply by 1.0), alkaline phosphatase (hepatic) or aspartate aminotransferase level exceeding 3 times the upper normal limit, white blood cell count of less than 4000/µL (to convert white blood cell count to ×109/L, multiply by 0.001), and impaired renal or hepatic function (total serum bilirubin level of >2.0 mg/dL and serum creatinine level of >2.0 mg/dL) (to convert bilirubin level to micromoles per liter, multiply by 17.104; to convert creatinine level to micromoles per liter, multiply by 88.4), as well as patients who had received chemotherapy, radiation therapy, or other biological therapy during the past 30 days. Eligibility was based on medical history, physical examination, laboratory test findings, and the results of endoscopy, electrocardiography, magnetic resonance imaging, and computed tomography with contrast (when needed). Stratification was not required, and all patients were added sequentially.
Statistical Analysis
The objectives of this phase 1 study were to evaluate the safety of this therapy and the tolerability of a range of laser light doses that has been shown to produce good clinical outcomes in other PDT studies. The sample size for this phase 1 trial was determined according to the basic design of phase 1 trials using a standard 3–3 dose escalation scheme. This design is a special case of the A + B design described by Lin and Shih.19 The rationale behind the design is nested in the assumption that both the probabilities of toxic effects and efficacious response are continuous monotonic nondecreasing functions of the dose. The dose is escalated using cohorts of 3 patients until 2 or more dose-limiting toxic effects are observed at a dose level. At this point, escalation stops, and doses are deescalated until no more than 1 of 6 patients experiences a dose-limiting toxic effect. This level is then defined as the maximum tolerated dose. In this study, the laser light dose was escalated through multiple predefined levels, which were determined by analyzing the results obtained in previous studies14,20,21 of HPPH in esophageal and lung cancer. These results indicated that HPPH (4.0 mg/m2) may be as effective as porfimer sodium (2 mg/kg). The highest laser light dose used in intraoperative porfimer sodium–mediated PDT is 75 J/cm2 for HNSCC.22,23 We expected the same laser light dose to be effective in HPPH-mediated PDT. However, the intraoperative use of PDT in HNSCC demands exceptional caution because of the presence of vital structures exposed by the preceding surgery. Therefore, we chose to escalate the laser light dose from 30 J/cm2 to a maximum of 75 J/cm2.
Treatment Protocol
Four patient cohorts were enrolled, with 3 individuals per cohort in the first 3 cohorts and with 6 individuals in the last cohort (highest laser light dose); the respective laser light doses were escalated per cohort from 30 to 50 and 60 J/cm2, up to a maximum of 75 J/cm2. A tunable dye laser was used to deliver light with 665-nm wavelength at 21 to 27 hours after infusion of HPPH. The HPPH was administered as a single intravenous infusion during approximately 1 hour. The laser light was delivered to the operative field immediately following tumor resection. The treatment field was illuminated through an optical fiber with a microlens. The power output was 0.150 W/cm2. The power was measured with an integrating sphere immediately before laser light delivery. The laser light dose (joules per centimeter squared equal watts per centimeter squared times seconds) was increased by varying the illumination time from 200 seconds (to deliver 30 J/cm2) to 500 seconds (to deliver 75 J/cm2). The beam diameter was changed as a function of the target tissue by altering the distance between the microlens and the operative field. Larger areas were illuminated by moving the laser light beam in the radial direction (with about 10%–15% overlap) to treat the entire surgical bed.
This phase 1 study did not have strict criteria for the treatment of tumor margins because the primary objective was the assessment of safety variables. In general, the treated field was proportional to the size of the surgical field. Mucosal margins were included in the illuminated field whenever they were within the surgical field, and nodal basins were treated after neck dissection.
The treatment field ranged from 2 to 14 cm in diameter. Neurovascular structures (carotid artery and cranial nerves) were not intentionally shielded from the laser light.
Patient Follow-up Care, Safety, and Outcome Measures
All patients were instructed to avoid direct exposure to sunlight or bright incandescent light for at least 7 days after drug injection by wearing protective clothing and specific sunglasses provided by the RPCI Photodynamic Therapy Center. The patients were also asked to expose a small area of skin to sunlight for 10 minutes to determine any remaining photosensitivity after 8 days. All patients were monitored for systemic toxic effects at the time of HPPH administration, laser light treatment, and each follow-up visit. Definitions of criteria for safety were used (Common Toxicity Criteria, version 3.0; National Cancer Institute). The expected complications were grouped into HPPH related and PDT related. The expected drug-related adverse event (AE) was skin photosensitivity (erythema, edema, and necrosis), which could occur within 1 week of HPPH administration. The expected PDT complications were the same as the drug-related ones, as well as wound breakdown, fistula formation, and hemorrhage that could occur within 30 days of the laser light illumination.
Adverse events were documented as to onset and resolution date, classification of intensity, relationship to treatment, action taken, and patient outcome. Study follow-up visits occurred at 1 month, then every 3 months for 2 years, and every 6 months thereafter. All AEs were recorded using medical terminology codes (Medical Dictionary for Regulatory Activities; www.meddramsso.com).
Results
Tumor sites and staging are summarized in Table 1. There were equal numbers of primary and recurrent tumors at early and advanced disease stages. Fifteen patients received the full course of treatment, and 1 patient received HPPH without intraoperative laser light because of an unrelated myocardial infarction.
Table 1.
TNM, Tumor Sites, and Type of Surgery
| Patient No. |
Primary vs Recurrent |
TNM | Tumor Site | Type of Surgery |
|---|---|---|---|---|
| 1 | Recurrent | T4 N0 M0 | Laryngeal | Wide local excision of neck skin, chest wall advancement flap, bilateral selective neck dissection (levels 2–4), right thyroid lobectomy, extended total laryngectomy, intraoperative PDT |
| 2 | Primary | T2 N0 M0 | FOM, lower alveolar ridge | Wide local excision with carbon dioxide laser, marginal mandibulectomy, split-thickness skin graft from left anterior thigh, sentinel lymph node biopsy on right side of neck (n = 2), intraoperative PDT to the neck wound |
| 3 | Primary | T1 N0 M0 | Supraglottic | Carbon dioxide laser excision of supraglottic with transoral endoscopic supraglottic laryngectomy, intraoperative PDT to the supraglottic excision site, bilateral levels 2–4 selective neck dissections |
| 4 | Recurrent | T3 NX | Buccal mucosa, FOM, hard palate | Left marginal mandibulectomy; wide local excision of FOM, cheek, and hard palate; sentinel lymph node biopsy (n = 2), one at level 1 and another at level 4 on the left side of the neck; intraoperative PDT to the neck operative field; split-thickness skin graft to the oral cavity |
| 5 | Primary | T3 N2a M0 | Retromolar trigone | Comprehensive extended right neck dissection (levels 1–5), pectoralis major myocutaneous flap for neck reconstruction, intraoperative PDT to the right neck |
| 6 | Primary | T4 N3 M0 | Laryngeal, pharyngeal | Right modified radical neck dissection, intraoperative PDT to the right neck, total laryngectomy, near-total pharyngectomy, pectoralis major myocutaneous flap, pharyngeal reconstruction |
| 7 | Recurrent | T1 | Oral tongue | Left hemiglossectomy with carbon dioxide laser, sentinel lymph node biopsy (levels 2 and 3 left neck and level 2 right neck), intraoperative PDT to oral tongue, tracheostomy |
| 8 | Recurrent | NA | Larynx | Right modified radical neck dissection (levels 1–5), intraoperative PDT to surgical site in the neck |
| 9 | Primary | T1 | Tongue base | No surgery, no PDT |
| 10 | Recurrent | NA | External auditory canal | Right lateral temporal bone resection, right superficial parotidectomy with facial nerve dissection, right neck dissection (levels 2 and 3), right total auriculectomy, intraoperative PDT, reconstruction with right sternocleidomastoid flap and left free radial forearm flap, antebrachial cutaneous nerve to greater auricular nerve neurorrhaphy, split-thickness skin graft to left forearm |
| 11 | Primary | T3 N2-C M0 | Buccal mucosa | Tracheostomy with division of thyroid isthmus, right marginal mandibulectomy, buccal and FOM resection, bilateral selective neck dissection (levels 1–3 on the right and levels 1 and 2 on the left), intraoperative PDT |
| 12 | Recurrent | T1b | Larynx | Percutaneous gastrostomy, supracricoid partial laryngectomy with cricohyoidopexy, tracheostomy, intraoperative PDT |
| 13 | Primary | T4 N1 M0 | Supraglottis, glottis | Total laryngectomy and bilateral selective neck dissections, intraoperative PDT |
| 14 | Recurrent | NA | Larynx | Total laryngectomy, left pectoralis major myocutaneous flap reconstruction of the pharynx, esophagogastroduodenoscopy with percutaneous placement of gastrostomy tube, intraoperative PDT |
| 15 | Primary | T4 N1 M0 | Right lower gingival and mandible | Right neck dissection, right segmental mandibulectomy, resection of posterior FOM and buccal mucosa, intraoperative PDT |
| 16 | Recurrent | NA | Preauricular skin | Right total parotidectomy with facial nerve sacrifice, right selective neck dissection, intraoperative PDT, implantation of 1-g gold weight to right upper eyelid, right static facial sling, right digastric tendon autograft, ligation of right external carotid artery |
Abbreviations: FOM, floor of mouth; NA, not available; PDT, photodynamic therapy.
The most frequent AEs at least possibly related to PDT were grade 1 to grade 2 edema (9 patients) and pain (3 patients). The edema was usual postoperative edema lasting 7 to 10 days. One patient had edema that lasted about 6 weeks. Postoperative pain lasted 2 to 4 weeks, as expected. One patient had a carcinoma of the facial skin with extensive intraparotid nodal metastases and facial nerve paralysis. After surgery, this individual developed facial chronic pain syndrome. A second patient had a salvage temporal bone resection neck dissection and free flap reconstruction for a recurrent squamous cell carcinoma of the external auditory canal. She was initially seen with deep-seated cephalgia, which worsened after surgery. Eventually, she developed recurrent disease with extensive perineural spread and died. We reported these 2 cases of atypical postoperative pain as AEs possibly related to the use of intraoperative HPPH-mediated PDT because these patients were enrolled in the study. Nevertheless, the pain could have been related to the recurrent cancer and the extensive ablative salvage operations. A summary of the AEs and the corresponding laser light doses and management is given in Table 2.
Table 2.
Serious Adverse Events
| Adverse Event | Laser Light Dose, J/cm2 |
Treatment | Outcome |
|---|---|---|---|
| Fistula, previous chemotherapy and radiation therapy | 30 | Local wound care and antibiotics | Closed by secondary intention |
| Mandible fracture, marginal mandibulectomy and neck dissection | 75 | Completed mandibulectomy and antibiotics | Healed with no further problems |
| Wound infection | 75 | Wound drainage and antibiotics | Healed with no further problems |
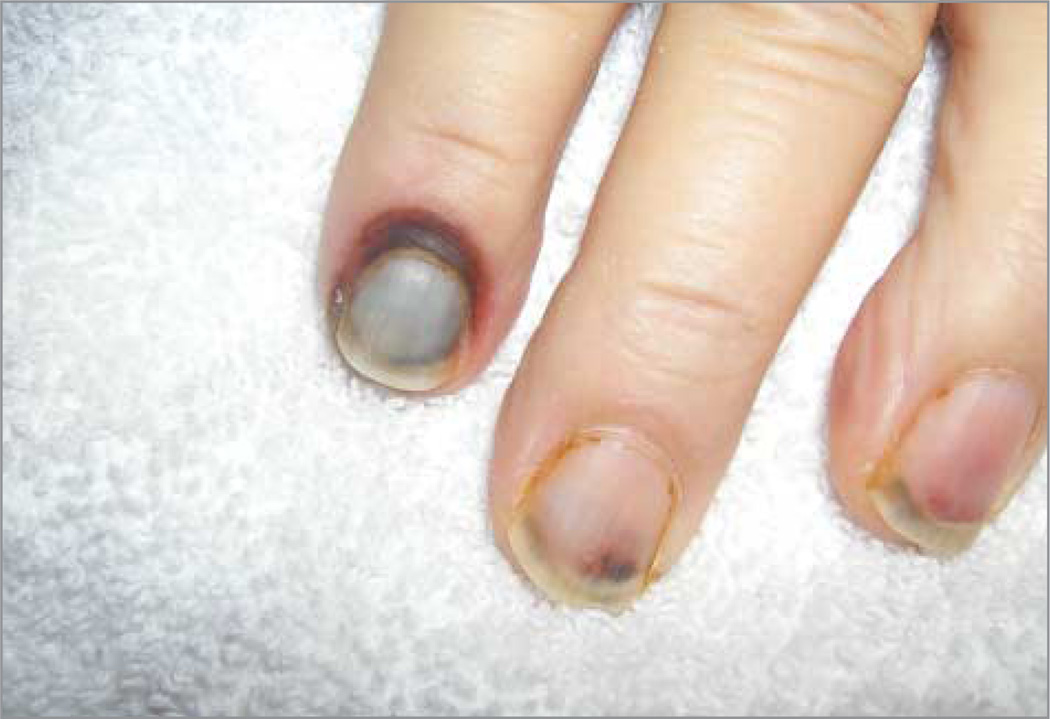
One patient treated surgically for primary oral squamous cell carcinoma via marginal mandibulectomy and neck dissection developed an orocutaneous fistula and bone fracture at the marginal mandibulectomy site several weeks after surgery. This complication was treated by debriding the necrotic bone and closing the fistula using local advancement flaps. Another wound fistula occurred in an individual with recurrent laryngeal cancer previously treated with concurrent chemoradiotherapy. He underwent a total laryngectomy, bilateral neck dissection, and flap reconstruction. Phototoxicity reactions related to HPPH included a grade 2 photophobia and 2 skin burns. One skin burn was due to prolonged exposure to operating room spotlights, which healed completely by secondary intention without permanent wound contraction, and the other was a skin burn of a finger that was exposed to red light from a pulse oximeter for an extended period (Figure 2).
Figure 2. Finger and Nail Burn From Prolonged Exposure to Red Pulse Oximeter Light.
Laser Light Dose
The AEs were unrelated to the level of the laser light dose. We observed no dose-limiting toxic effects in the range of laser light dose (30–75 J/cm2) that was used in this study. The highest laser light dose used (75 J/cm2) was found to be safe.
Clinical Outcomes
In this phase 1 trial, clinical outcomes have limited significance. Nevertheless, clinical follow-up visits at 48 months showed overall survival of 10 patients and progression-free survival of 7 patients. The median survival has not been established because 10 patients are alive to date.
Discussion
Effective management of HNSCC demands a multidisciplinary team approach, often involving the combination of surgery, radiation therapy, and chemotherapy. Recurrent disease at the site of the primary tumor continues to be a significant cause of treatment failure. Although these recurrences have been attributed to overexpression of specific oncogenes and to disease stage, it is well accepted that residual cancer cells that remain undetected by pathological examination will induce local recurrence and reduce survival.24–27 Therefore, there is a need for the development of intraoperative local adjuvant therapies, particularly for patients with advanced disease.
In this study, we report the use of HPPH-mediated PDT in patients with head and neck cancer, to our knowledge, for the first time to date. Traditionally, pain, treatment-related edema, and long-term phototoxic effects are the most common AEs associated with PDT. The HPPH induces limited and short-term (few days) phototoxic effects.2 We observed no long-term phototoxic effects in this study. In an extensive study6 of skin photosensitivity in 48 patients with HPPH-mediated PDT receiving drug doses of 2.5 to 6.0 mg/m2 and solar simulator light doses from 44.4 to 133.2 J/cm2, it was demonstrated that even 1 day after drug administration the highest drug and light doses elicited a response of only erythema, without edema. Therefore, it is reasonable to assume that the edema observed in the present study was related to the surgical procedure. The reported pain was controlled with standard postoperative medication, suggesting that HPPH-mediated PDT is well tolerated and safe.
Another complication was a fistula that occurred after a salvage laryngectomy in an individual who had been previously treated with concurrent chemoradiotherapy for advanced laryngeal squamous cell carcinoma. Unfortunately, this complication is common in patients who are seen with local recurrence following chemoradiotherapy and undergo salvage surgery.28 It is possible that PDT contributed to the development of a fistula in our patient, but it is unlikely that it was the sole cause. The second fistula developed as a result of necrotic mandibular bony sequestrum. This complication may have been related to PDT because the mandible was in the treated field.
In this study, we observed excellent secondary healing of skin burns in 2 patients due to phototoxic effects (Figure 2). The good healing could be explained by the fact that HPPH is not retained in fibroblasts.29 The PDT-induced damaged cells are replaced by native tissue that regains its normal functions, significantly limiting function loss and minimizing scar formation following PDT. This outcome is in agreement with other investigations reporting excellent skin healing following PDT with other drugs (such as temoporfin) for patients with HNSCC.10 However, proper shielding of adjacent skin from operating room spotlights and during lengthy procedures is necessary to prevent skin phototoxic effects. In addition, pulse oximeter sensors should be moved every 15 to 20 minutes to prevent skin and nail damage.
It has been postulated that PDT can also spare healthy vital structures such as nerves and major blood vessels.30 Our results support this hypothesis for HPPH-mediated PDT. The carotid artery and cranial nerves were illuminated with the therapeutic laser light, but no injuries were observed to these vital structures. We hypothesize that this outcome resulted from low uptake of the photosensitizer in these critical regions.
The highest laser light dose used in our study was 75 J/cm2. This light dose has been found to be safe and effective in studies22,23 using porfimer sodium–mediated PDT for HNSCC. This similarity can be explained by comparing the optical properties and doses of these 2 photosensitizers. The therapeutic dose of porfimer sodium is 2 mg/kg, and its laser light absorption coefficient is 3000 M−1cm−1 at 630 nm. The absorption coefficient of HPPH is 47 500 M−1cm−1 at 665 nm (ie, 16 times greater than that of porfimer sodium). Because the photodynamic dose equals the drug dose times the laser light dose, when keeping the laser light dose the same as that used with porfimer sodium, we would expect to deliver the same photodynamic dose with an HPPH dose of one-sixteenth of the porfimer sodium dose (ie, 4.0 mg/m2 of HPPH).
Although of limited significance, the clinical outcomes of this study (overall survival of 10 patients and progression-free survival of 7 patients at 48 follow-up months) are in agreement with earlier clinical studies13,31 of intraoperative adjuvant porfimer sodium–mediated PDT for recurrent head and neck cancer after ablative surgery. One study13 included 17 cases in which the entire tumor resection bed was exposed to PDT. Only 6 patients developed recurrent or metastatic disease, 2 inside the field of PDT and 4 outside the field of surgery or PDT, during a follow-up period of 66 to 97 months. The postoperative course of all patients was uncomplicated, and the duration of hospitalization and the total healing time did not change with the use of PDT compared with surgery alone. It was concluded that the addition of intraoperative PDT immediately after tumor resection may improve the cure rates of recurrent head and neck cancer by allowing larger tumor-free margins while preserving normal structures.
In conclusion, the results of this phase 1 study suggest that intraoperative adjuvant HPPH-mediated PDT is feasible and safe. Future phase 2 trials are needed to assess the efficacy of this novel treatment modality.
Acknowledgments
Funding/Support: This work was supported by grant PO1CA055791 from the National Institutes of Health (Drs Rigual and Henderson).
Footnotes
Author Contributions: Study concept and design: Rigual, Wilding, Sullivan.
Acquisition of data: Rigual, Frustino, Cooper, Sullivan.
Analysis and interpretation of data: Rigual, Shafirstein, Seshadri, Wilding, Henderson.
Drafting of the manuscript: Rigual, Shafirstein, Frustino, Seshadri, Wilding, Sullivan, Henderson.
Critical revision of the manuscript for important intellectual content: Rigual, Shafirstein, Seshadri, Cooper, Henderson.
Statistical analysis: Seshadri, Wilding, Sullivan.
Obtained funding: Henderson.
Administrative, technical, and material support: Rigual, Shafirstein, Frustino, Seshadri, Cooper, Sullivan, Henderson.
Study supervision: Henderson.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This study was presented at the American Head & Neck Society Eighth International Conference on Head and Neck Cancer; July 23, 2012; Toronto, Ontario, Canada.
Contributor Information
Nestor R. Rigual, Department of Head and Neck Surgery, Roswell Park Cancer Institute, Buffalo, New York; Department of Cell Stress Biology, Roswell Park Cancer Institute, Buffalo, New York; Photodynamic Therapy Center, Roswell Park Cancer Institute, Buffalo, New York.
Gal Shafirstein, Department of Head and Neck Surgery, Roswell Park Cancer Institute, Buffalo, New York; Department of Cell Stress Biology, Roswell Park Cancer Institute, Buffalo, New York; Photodynamic Therapy Center, Roswell Park Cancer Institute, Buffalo, New York.
Jennifer Frustino, Division of Oral Medicine and Dentistry, Brigham and Women’s Hospital, Boston, Massachusetts.
Mukund Seshadri, Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Buffalo, New York.
Michele Cooper, Photodynamic Therapy Center, Roswell Park Cancer Institute, Buffalo, New York.
Gregory Wilding, Department of Biostatistics and Bioinformatics, Roswell Park Cancer Institute, Center of Excellence, Buffalo, New York.
Maureen A. Sullivan, Dentistry and Maxillofacial Prosthetics, Roswell Park Cancer Institute, Buffalo, New York.
Barbara Henderson, Department of Cell Stress Biology, Roswell Park Cancer Institute, Buffalo, New York; Photodynamic Therapy Center, Roswell Park Cancer Institute, Buffalo, New York.
REFERENCES
- 1.Gibson MK, Forastiere AA. Multidisciplinary approaches in the management of advanced head and neck tumors: state of the art. Curr Opin Oncol. 2004;16(3):220–224. doi: 10.1097/00001622-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal JP, Gupta T, Kalyani N, et al. Cetuximab with radiotherapy in patients with loco-regionally advanced squamous cell carcinoma of head and neck unsuitable or ineligible for concurrent platinum-based chemo-radiotherapy: ready for routine clinical practice? Indian J Cancer. 2011;48(2):148–153. doi: 10.4103/0019-509X.82872. [DOI] [PubMed] [Google Scholar]
- 3.Zafereo ME, Hanasono MM, Rosenthal DI, et al. The role of salvage surgery in patients with recurrent squamous cell carcinoma of the oropharynx. Cancer. 2009;115(24):5723–5733. doi: 10.1002/cncr.24595. [DOI] [PubMed] [Google Scholar]
- 4.Oksuz DC, Prestwich RJ, Carey B, et al. Recurrence patterns of locally advanced head and neck squamous cell carcinoma after 3D conformal (chemo)-radiotherapy. [Accessed May 17, 2013];Radiat Oncol. 2011 6:e54. doi: 10.1186/1748-717X-6-54. www.ncbi.nlm.nih.gov/pmc/articles/PMC3127781/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Licitra L, Vermorken JB. Is there still a role for neoadjuvant chemotherapy in head and neck cancer? Ann Oncol. 2004;15(1):7–11. doi: 10.1093/annonc/mdh001. [DOI] [PubMed] [Google Scholar]
- 6.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 7.Henderson BW, Bellnier DA. Tissue localization of photosensitizers and the mechanism of photodynamic tissue destruction. Ciba Found Symp. 1989;146:112–130. doi: 10.1002/9780470513842.ch8. [DOI] [PubMed] [Google Scholar]
- 8.Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5(8):497–508. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 9.Wilson BC, Patterson MS. The physics, biophysics and technology of photodynamic therapy. Phys Med Biol. 2008;53(9):R61–R109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 10.Karakullukcu B, van Oudenaarde K, Copper MP, et al. Photodynamic therapy of early stage oral cavity and oropharynx neoplasms: an outcome analysis of 170 patients. Eur Arch Otorhinolaryngol. 2011;268(2):281–288. doi: 10.1007/s00405-010-1361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lou PJ, Jäger HR, Jones L, Theodossy T, Bown SG, Hopper C. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br J Cancer. 2004;91(3):441–446. doi: 10.1038/sj.bjc.6601993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lajer CB, Specht LK, Kirkegaard J, Homøe P. Photodynamic therapy for head and neck cancer [in Danish] Ugeskr Laeger. 2006;168(23):2227–2231. [PubMed] [Google Scholar]
- 13.Biel MA. Photodynamic therapy of head and neck cancers. Methods Mol Biol. 2010;635:281–293. doi: 10.1007/978-1-60761-697-9_18. [DOI] [PubMed] [Google Scholar]
- 14.Bellnier DA, Greco WR, Loewen GM, et al. Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Cancer Res. 2003;63(8):1806–1813. [PubMed] [Google Scholar]
- 15.Lobel J, MacDonald IJ, Ciesielski MJ, et al. 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) in a nude rat glioma model: implications for photodynamic therapy. Lasers Surg Med. 2001;29(5):397–405. doi: 10.1002/lsm.10001. [DOI] [PubMed] [Google Scholar]
- 16.Zumsteg A, Christofori G. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Opin Oncol. 2009;21(1):60–70. doi: 10.1097/CCO.0b013e32831bed7e. [DOI] [PubMed] [Google Scholar]
- 17.Loewen GM, Pandey R, Bellnier D, Henderson B, Dougherty T. Endobronchial photodynamic therapy for lung cancer. Lasers Surg Med. 2006;38(5):364–370. doi: 10.1002/lsm.20354. [DOI] [PubMed] [Google Scholar]
- 18.Bellnier DA, Greco WR, Nava H, Loewen GM, Oseroff AR, Dougherty TJ. Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemother Pharmacol. 2006;57(1):40–45. doi: 10.1007/s00280-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y, Shih WJ. Statistical properties of the traditional algorithm-based designs for phase I cancer clinical trials. Biostatistics. 2001;2(2):203–215. doi: 10.1093/biostatistics/2.2.203. [DOI] [PubMed] [Google Scholar]
- 20.Nava HR, Allamaneni SS, Dougherty TJ, et al. Photodynamic therapy (PDT) using HPPH for the treatment of precancerous lesions associated with Barrett’s esophagus. Lasers Surg Med. 2011;43(7):705–712. doi: 10.1002/lsm.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40(1):340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- 22.Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol. 2007;83(5):1063–1068. doi: 10.1111/j.1751-1097.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 23.Rigual NR, Thankappan K, Cooper M, et al. Photodynamic therapy for head and neck dysplasia and cancer. Arch Otolaryngol Head Neck Surg. 2009;135(8):784–788. doi: 10.1001/archoto.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan CO, Amirghahri N, Rice C, Abreo FW, Shi R, Stucker FJ. Molecular analysis of surgical margins in head and neck squamous cell carcinoma patients. Laryngoscope. 2002;112(12):2129–2140. doi: 10.1097/00005537-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Spiro RH, Guillamondegui O, Jr, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21(5):408–413. doi: 10.1002/(sici)1097-0347(199908)21:5<408::aid-hed5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier P, Audet N, Guertin L, et al. Complete frozen section margins (with measurable 1 or 5 mm thick free margin) for cancer of the tongue, part 2: clinical experience. J Otolaryngol Head Neck Surg. 2010;39(1):20–27. [PubMed] [Google Scholar]
- 27.Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sándor GK. What is the adequate margin of surgical resection in oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):625–629. doi: 10.1016/j.tripleo.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Sewnaik A, Keereweer S, Al-Mamgani A, et al. High complication risk of salvage surgery after chemoradiation failures. Acta Otolaryngol. 2012;132(1):96–100. doi: 10.3109/00016489.2011.617779. [DOI] [PubMed] [Google Scholar]
- 29.Tracy EC, Bowman MJ, Pandey RK, Henderson BW, Baumann H. Cell-type selective phototoxicity achieved with chlorophyll-a derived photosensitizers in a co-culture system of primary human tumor and normal lung cells. Photochem Photobiol. 2011;87(6):1405–1418. doi: 10.1111/j.1751-1097.2011.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biel M. Advances in photodynamic therapy for the treatment of head and neck cancers. Lasers Surg Med. 2006;38(5):349–355. doi: 10.1002/lsm.20368. [DOI] [PubMed] [Google Scholar]
- 31.Biel MA. Photodynamic therapy as an adjuvant intraoperative treatment of recurrent head and neck carcinomas. Arch Otolaryngol Head Neck Surg. 1996;122(11):1261–1265. doi: 10.1001/archotol.1996.01890230105018. [DOI] [PubMed] [Google Scholar]