Abstract
Sleep is implicated in cognitive functioning in young adults. With increasing age there are substantial changes to sleep quantity and quality including changes to slow wave sleep, spindle density, and sleep continuity/fragmentation. A provocative question for the field of cognitive aging is whether such changes in sleep physiology affect cognition (e.g., memory consolidation). We review nearly a half-century of research studies across 7 diverse correlational and experimental literature domains, which historically have had little crosstalk. Broadly speaking, sleep and cognitive functions are often related in advancing age, though the prevalence of null effects (including correlations in the unexpected, negative direction) in healthy older adults indicates that age may be an effect modifier of these associations. We interpret the literature as suggesting that maintaining good sleep quality, at least in young adulthood and middle age, promotes better cognitive functioning and serves to protect against age-related cognitive declines.
Keywords: memory consolidation, epidemiology, napping, sleep deprivation, actigraphy, polysomnography, neuropsychology, sleep pharmacology
Introduction
Across an 85-year lifespan, an individual may sleep nearly 250,000 hours, or over 10,000 full days. People often disparage time spent sleeping as “lost” time, but the persistent internal drive to sleep and its presumed universality across species would suggest that sleep is purposeful. Sleep science pioneer Allan Rechtschaffen put it most eloquently: “If sleep does not serve an absolutely vital function, then it is the biggest mistake the evolutionary process ever made” (University of Chicago Sleep Laboratory, Smithsonian Institute, November, 1978).
Sleep does serve many functions, and these range from tissue restoration (Adam & Oswald, 1977) to brain metabolite clearance (Xie et al., 2013). Of particular interest to psychological scientists is sleep’s role in cognitive functioning. Sleep loss has long been recognized to impair performance on attention and executive control tasks (see Bonnet, 2011, for a review). The more exciting possibility, however, is that normal sleep might actively promote memory stabilization and integration (see Table 1 for theories of the relation between sleep and memory) and this hypothesis has been supported across a diversity of psychological tests in young adults (Appendix). A topic of current interest is whether aging moderates the association between sleep and memory.
Table 1.
Influential Theories of the Relation between Sleep and Memory.
| Theory Name | Description | References |
|---|---|---|
| Interference | Sleep passively protects memories against daytime interference | Jenkins & Dallenbach (1924) |
| System Consolidation | The hippocampus reactivates memories and transfers them to neocortical regions, primarily during sleep | Marr (1971); McClelland et al. (1995) |
| Synaptic Consolidation | Hippocampal long-term potentiation, primarily induced during REM, strengthens synaptic representations of memories | Bramham & Srebro (1989) |
| Dual Stage Consolidation | SWS promotes episodic memory consolidation and REM sleep promotes procedural memory consolidation | Plihal & Born (1997) |
| Multiple Trace | Each memory reactivation results in a new, but altered and distributed, memory trace rendering retrieval increasingly easier | Nadel & Moscovitch (1997); Nadel et al. (2012) |
| Synaptic Homeostasis | Sleep promotes global downscaling of synaptic weights, resulting in an improved signal-to-noise ratio for memories | Tononi & Cirelli (2003) |
| Permissive/Opportunistic Consolidation | Sleep affords an environment conducive to, but not necessary for, consolidation to occur | Wixted (2004); Mednick et al. (2011) |
| Recovery and Stabilization | Sleep stabilizes memories and recovers performance following daytime interference | Brawn et al. (2010) |
| Selective Consolidation | Only memories tagged as “relevant” during encoding are reactivated during sleep and consolidated | Stickgold & Walker (2013) |
Theories are listed chronologically. For further critical review of these theories, see Ellenbogen, Payne, and Stickgold (2006) and Frankland and Bontempi (2005).
Abbreviations. SWS: slow wave sleep; REM: rapid eye movement sleep
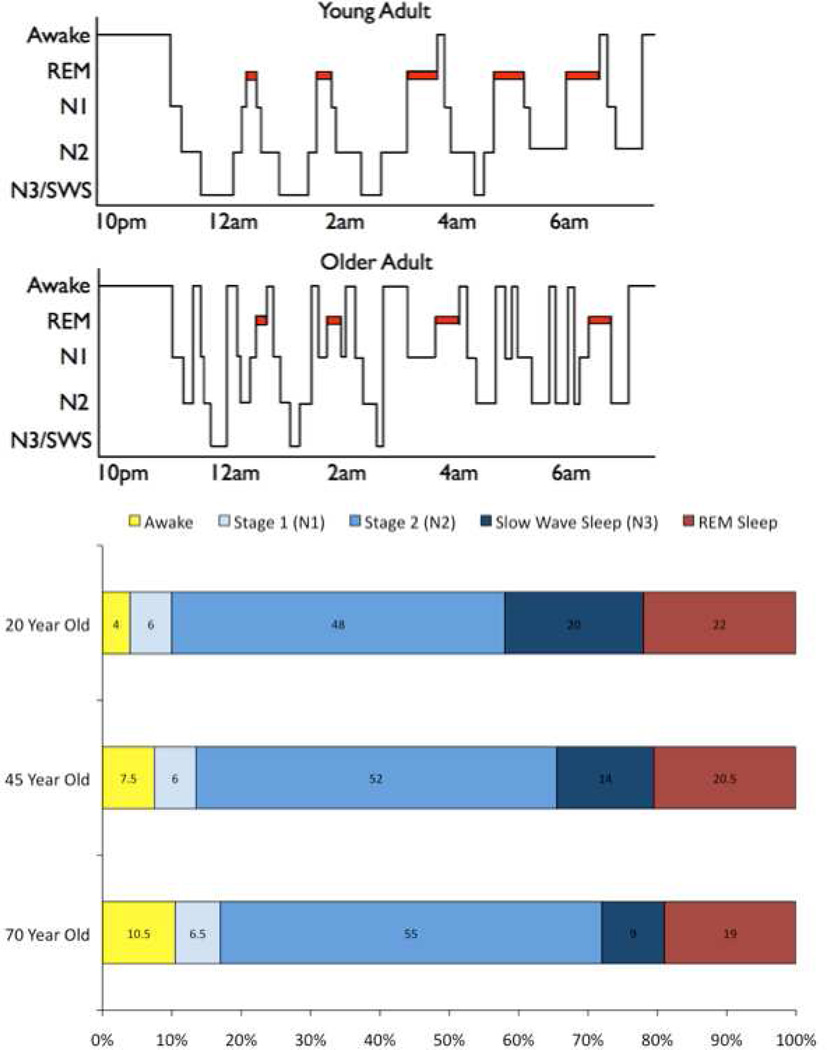
The present article focuses on sleep’s implications for cognitive aging. Referring back to the estimate of 250,000 hours of sleep in a lifetime, a few assumptions become evident. First, this estimate assumed 8 hours of sleep per night, but sleep duration often declines across the lifespan (Bliwise, 1993). Furthermore, as depicted in Figure 1, sleep quality may change dramatically from young to older age: Sleep becomes more fragmented (i.e., older adults wake up more at night; e.g., Bliwise et al., 2009) and there is a decline in the quantity and quality of the “deep” stages of sleep such as slow-wave sleep and rapid eye movement sleep (Ohayon, Carskadon, Guilleminault, & Vitiello, 2004).
Figure 1.
If sleep functions to benefit memory and cognition in young adults, but is substantially altered in quantity/quality across the lifespan, then an alluring question is whether lifespan changes in sleep cause the widespread changes in cognitive functioning commonly observed in older adults (for overview of cognitive aging, see Cabeza, Nyberg, & Park, 2005). If so, then improving sleep might delay or reverse cognitive aging, as many authors have alluded (Altena, Ramautar, Van Der Werf, & Van Someren, 2010; Bruce & Aloia, 2006; Buckley & Schatzberg, 2005; Cipolli, Mazzetti, & Plazzi, 2013; Cirelli, 2012; Engel, 2011; Fogel et al., 2012; Goder & Born, 2013; Harand et al., 2012; Hornung, Danker-Hopfe, & Heuser, 2005; Kronholm, 2012; Pace-Schott & Spencer, 2011; Rauchs, Carrier, & Peigneux, 2012; Vance, Heaton, Eaves, & Fazeli, 2011; Wilckens, Erickson, & Wheeler, 2012). This “sleep—cognition hypothesis” (Feinberg & Evarts, 1969) has previously been challenging to verify because sleep, cognition, and aging represent three topics that are individually extremely rich, deeply broad, and diversely complex.
To fully address the question of whether age-related changes in sleep may be associated with age-related changes in cognition we have taken an integrative, multidisciplinary approach that incorporates experimental, clinical neuropsychological, and epidemiological literatures. Here we review 7 distinct and seldom cross-referenced domains, ranging from large-scale correlational studies that assessed self-reported sleep to experimental studies that deprived or extended sleep duration/quality. Table 2 provides an overview of the breadth of this review and the depth of each literature included. To foreshadow, some literatures produce curious findings (e.g., sleep deprivation affects young adults more than older adults) whereas other literatures highlight the potential for augmenting sleep (e.g., afternoon naps) to benefit cognitive functioning in middle-aged adults. We contend that these seven literatures provide complementary perspectives on how sleep and cognition interact as we age.
Table 2.
Overview of Sleep, Cognition, and Normal Aging Literatures Reviewed.
| Literature | # of Studies |
Typical Sample |
Comments on Young Age |
Comments on Middle Age |
Comments on Older Adults |
|---|---|---|---|---|---|
| Self-Reported Sleep Correlational Studies | Many | Large-to-Very Large Samples | Cross-sectional and longitudinal correlations | Cross-sectional and longitudinal correlations | Fewer correlations |
| Motor Activity (Actigraphy) and Neuropsychology Correlational Studies | Few-Medium | Medium-to-Large Samples | N/A (too few studies) | Activity and cognition often correlate | Activity and cognition often correlate |
| Sleep Brain Wave (PSG) and Neuropsychology Correlational Studies | Medium | Small-to-Medium Samples | Some sleep— cognition correlations | Some sleep— cognition correlations | Some sleep— cognition correlations |
| Sleep Deprivation Experiments | Medium | Small Samples | Many adverse cognitive consequences | Some adverse cognitive consequences | Minimal or no cognitive consequences |
| Napping Experiments | Few | Small Samples | Naps benefit cognitive functioning | Naps may benefit cognitive functioning | Naps have few or no benefits to cognition |
| Sleep-Dependent Memory Consolidation Experiments | Medium-Many | Small Samples | Sleep promotes memory consolidation | Consolidation may be reduced | Consolidation is often absent |
| Nocturnal Sleep Intervention Experiments | Medium | Small Samples | Better/more sleep benefits cognition | Some cognitive benefits observed | Few cognitive benefits observed |
This table provides an introduction to each literature and is not intended to capture the nuances, exceptions, and moderating variables existent in each domain. Number of studies that used healthy middle-aged or healthy older adults are estimated as few (e.g., 10), medium (e.g., 20–30), and many (e.g., >50). Typical group sample size is approximated as small (e.g., n = 20), medium (e.g., n = 100), large (e.g., n = 1,000) and very large (e.g., n = 10,000).
Abbreviations. PSG: Polysomnography
Wherever possible, we discuss findings separated across young (<30 years old), middle-aged (30–60 years old), and healthy older (≥60 years old) adult groups (Roebuck, 1979). By doing so we can begin to address whether age modifies sleep—cognition associations. Given our focus on “normal” aging, we consider studies of abnormal aging (e.g., dementia, insomnia, sleep apnea; e.g., Cipolli et al., 2013) as well as developmental studies (e.g., Kopasz et al., 2010) to be beyond the scope of this review. Finally, to ensure that positive findings constitute strong supportive evidence, we have employed the conservative approach of reporting results following adjustment for demographics and comorbidities, whenever possible.
Self-Report Studies
We can begin to address sleep, cognition, and aging relationships by examining the most fully developed literature in this review: studies that simply asked adults how well they usually sleep. These self-report studies ask how many hours one typically sleeps, how long it takes one to fall asleep, how often one wakes up in the middle of the night, and how sleepy one feels during the day. The limitations of these studies will be evident in their reliance on subjective sleep measures and insensitive cognitive measures (e.g., mini mental state examination; MMSE), but their advantages in statistical power, attempts to capture habitual sleep patterns, adjustment for potentially confounding variables, and use of both cross-sectional and longitudinal designs are laudable.
Cross-Sectional Studies
Table 3 summarizes >40 studies that correlated self-reported sleep measures and cognitive functioning at a single time point. Studies conducted in middle-aged adults consistently linked sleep duration (e.g., short sleep) and waking up at night to poorer executive control (Regestein et al., 2004), working memory (Sternberg et al., 2013), episodic memory (Kronholm et al., 2009), attention (e.g., Krieg et al., 2001), and greater cognitive complaints (e.g., Roane et al., 2014). One explanation for these associations is that night-to-night sleep quality dictates day-to-day cognitive performance in middle-aged adults. For example, when maintaining a sleep diary and repeating a cognitive battery for 2–3 weeks, cognitive composite scores were lower the day after getting either less sleep or more sleep than normal (Gamaldo, Allaire, & Whitfield, 2010). Another interesting potential mechanism is that poor sleep in middle-aged adults could cause neurobiological impairments that summate over time. Consistent with this hypothesis, short sleep duration in cognitively-normal adults was recently linked to greater cortical β-amyloid burden (Spire et al., 2013), which is a precursor to cognitive declines (Bateman et al., 2012). We elaborate on this potential mechanism in the conclusions section.
Table 3.
Cross-Sectional Studies on Self-Reported Sleep Disturbances and Cognitive Function.
| Reference | Sample | Age | Cognitive Measure |
Short Sleep |
Long Sleep |
SOL | Early Waking |
Night Waking |
Daily Naps |
EDS | PSQI/ SMHS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roth 1999 | 1,000 | ≥18 | Self-report | − | |||||||
| Sternberg 2013d | 127,048 | ≥18 | Battery | − | − | ||||||
| Stenfors 2013d,c | 8,362 | ≥20 | Self-report | − | |||||||
| Spiegelhalder 2008 | 60 | ≥24 | Stroop | ns | |||||||
| Jennum 1994d,c | 1,504 | ≥30 | Self-report | − | − | − | − | ||||
| Kronholm 2009d,c | 5,171 | ≥30 | Battery | − | − | ||||||
| Regestein 2004c | 75 | ≥45 | Battery | − | |||||||
| Xu 2011d,c | 28,670 | ≥50 | MMSE, VerM | − | − | ||||||
| Gildner 2014d | 32,142 | ≥50 | Battery | − | − | ||||||
| Parsey 2012d | 48 | ≥50 | EPT | ns | − | ns | |||||
| Ramos 2013d,c | 927 | ≥50 | MMSE | ns | − | ||||||
| Miller 2014d,c | 8,789 | ≥50 | Battery | − | − | + | |||||
| Lovato 2013 | 49 | ≥51 | Double Span | ns | |||||||
| Foley 2004d,c | 1,506 | ≥55 | Self-report | ns | − | ns | − | ns | |||
| Dealberto 1996d,c | 1,389 | ≥60 | Battery | ns | |||||||
| Faubel 2009d,c | 3,212 | ≥60 | MMSE | ns | − | ||||||
| Ohayon 2002,2005d,c | 1,026 | ≥60 | Self-report | − | ns | ns | − | − | |||
| Amer 2013 | 100 | ≥60 | MMSE | − | |||||||
| Miyata 2013d | 78 | ≥60 | N-Back, CPT | ns | |||||||
| Wan 2013d | 88 | ≥60 | Battery | − | − | ns | |||||
| Cross 2013 | 43 | ≥60 | MMSE | ns | |||||||
| Hoch 1994 | 50 | ≥61 | MMSE | ns | |||||||
| Maggi 1998d,c | 2,398 | ≥65 | MMSE | + | ns | ||||||
| Adam 2014d,c | 2,287 | ≥65 | MMSE | ns | ns | ns | |||||
| Habte-Gabr 1991d | 3097 | ≥65 | VerM | +1 | ns | ||||||
| Whitney 1998d,c | 4,578 | ≥65 | MMSE | ns | |||||||
| Foley 1995 | 9,282 | ≥65 | SPMSQ | + | |||||||
| Auyeung 2013d,c | 2,947 | ≥65 | MMSE | ns | − | − | ns | ||||
| Blackwell 2011ad,c | 3,132 | ≥65 | MMSE,TMT,DV | − | − | ns | ns | ||||
| Cricco 2001d,c | 6,444 | ≥65 | SPMSQ | ns | |||||||
| Saint Martin 2012d,c | 272 | ≥65 | Battery | ns | ns | ns | ns | ||||
| Mary 2013 | 16 | ≥65 | VerM | ns | − | ||||||
| Nebes 2009c | 157 | ≥65 | Battery | ns | − | − | |||||
| Gamaldo 2008d,c | 174 | ≥65 | Battery | − | |||||||
| Newman 1997d,c | 5,201 | ≥65 | MMSE | ns | ns | ns | ns | ||||
| Gooneratne 2003d,c | 76 | ≥65 | FOSQ-V | − | |||||||
| ***Ward 2013d | 84 | ≥66 | Battery | ns | |||||||
| Tworoger 2006d,c | 1,844 | ≥70 | Battery | − | ns | − | |||||
| Hayward 1992 | 124 | ≥70 | Battery | ns | − | ||||||
| Foley 1999d | 2,905 | ≥71 | CASI | − | |||||||
| Schmutte 2007d,c | 375 | ≥75 | Battery | ns | − | − | ns | ||||
| Chang-Quan 2012dc | 660 | ≥90 | MMSE | ns | − | − | |||||
Studies are sorted by age (lower limit). Significant effects after adjustment for demographic (d) and comorbidity (c) variables are indicated when more sleep complaints correlate with poorer (−) or better (+) cognitive scores. Merged cells indicate combined measures, blank cells indicate not collected/reported measures, and ns indicates p > .05.
Might also or instead indicate that long sleep is associated with poorer memory
Sleep Abbreviations. EDS: excessive daytime sleepiness; PSQI: Pittsburgh Sleep Quality Index (total score); SMHS: St. Mary’s Hospital Sleep Questionnaire; SOL: sleep onset latency (difficulty falling asleep)
Cognitive Test Abbreviations. CASI: Cognitive Abilities Screening Instrument; CPT: continuous performance test; DWRT: delayed word recall test; DV: Digit Vigilance; EPT: Everyday Problems Test; FOSQ-V: functional outcomes of sleep questionnaire – vigilance subscale; MMSE: mini mental state examination; SPMSQ: short portable mental status questionnaire; TMT: trail making test; VerM: verbal memory
Several cross-sectional studies have restricted their samples to older adults. Table 3 clearly shows that, as a whole, these studies have produced weaker results. First consider the evidence for short sleep duration. After controlling for demographic and/or health-related variables, most studies showed non-significant associations between cognitive measures and short sleep in older adults (see also Ramos et al., 2014). Interestingly, recent work indicates that the association between short sleep and episodic memory changes with increasing age such that middle-aged, but not older, adults showed this short-sleep—cognition association (Miller, Wright, Ji, & Cappuccio, 2014).
Perhaps sleep fragmentation rather than short sleep is the critical correlate of cognition in older adults. Increased nighttime awakenings were associated with poor memory in one small sample study (Mary, Schreiner, & Peigneux,, 2013; see also Sampaio, Sampaio, Yamada, Tsuboyama, & Arai, 2013), but puzzlingly, at least four studies have found the opposite pattern (Foley et al., 1995; Maggi et al., 1998; McCrae, Vatthauer, Dzierzewski, & Marsiske, 2012; Miller et al., 2014). The peculiar finding that greate wake time at night could be related to better cognitive performance in older adults is in notable contrast to the findings in young and middle-aged adults. Such unexpected results could be interpreted to mean: a) a hyperarousal mechanism (i.e., less sleep leads to hyperarousal which increases participant effort to perform the task); b) better self-awareness of sleep in adults who are more cognitively-intact (e.g., Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008), or c) Type I error in older adults. With regard to the above explanations, we note that some similar correlations emerge in studies that objectively-measured sleep.
One frequent cognitive association in older adults is with long sleep duration (e.g., ≥10 hours). Long sleep can indicate several diverse factors including underlying diseases and failing health (Grandner & Drummond, 2007). Additional consistent cognitive associations in older adults are difficulty falling asleep and daytime sleepiness (measured subjectively or as the frequency of needing to take daytime naps; but cf. Bliwise, Carskadon, Seidel, Nekich, & Dement, 1991). The conundrum here is how to cohesively explain such associations as sleep-specific effects when most supportive studies simultaneously found no correlation with short or fragmented sleep.
Sleep epidemiology studies typically incorporate 1–4 sleep questions, but the complexity and diversity of sleep symptoms might require more extensive questionnaires. The Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is a 19-item questionnaire that uses a cutoff score to distinguish poor and good sleepers. In young adults, poorer executive function and attention performance is associated with poorer PSQI-defined sleep, independent of potentially confounding variables such as depression (Benitez, & Gunstad, 2012). In older adults, some provocative research suggests an association between PSQI and global cognition scores (e.g., MMSE; Amer, Hamza, El Akkad, & Abdel Galeel, 2013; Chang-Quan, Bi-Rong, & Yan, 2012; Lo, Loh, Zheng, Sim, & Chee, 2014; Potvin et al., 2012), executive control (Blackwell et al., 2014; Nebes, Buysse, Halligan, Houck, & Monk, 2009), and even spectroscopy estimates of glial functioning in brain regions associated with memory (hippocampus; Cross et al., 2013). However, some of the above studies did not control for cognitive status (i.e., combined healthy adults and patients), and a more perspicacious inspection of the literature reveals no shortage of null PSQI effects (≥10 studies) across a range of attention, executive control, working memory, problem solving, global cognition, and episodic memory tasks (Table 3). To presage a re-occurring theme in this review, Sutter, Zöllig, Allemand, and Martin (2012) concluded that, in healthy older adults, “poor sleep quality per se seems not to lead to changes in cognitive performance” (p. 773).
Longitudinal Studies
Does poor sleep in recent months predict cognitive decline years later? Table 4 summarizes the prospective epidemiological studies that have assessed self-reported sleep complaints as predictors of subsequent cognitive decline. At least eight such studies that included middle-aged adults have reported significant cognitive associations with short and/or fragmented sleep (e.g., waking up at night). Increased wake time at night predicted increased cognitive complaints and instrumental disabilities two years later (Stenfors, Hanson, Oxenstierna, Theorell, & Nilsson, 2013) and 28 years later (Kulmala et al., 2013), respectively. Moreover, short sleep duration predicted poorer performance on telephone-based cognitive tests 22 years later (Virta et al., 2013). Complementary and supportive evidence arises from four studies that indicated that poor sleep at baseline predicted development of cognitive disorders including mild cognitive impairment and Alzheimer’s disease (Table 4; Lobo et al., 2008). These studies, however, did not always control for comorbidities.
Table 4.
Longitudinal Studies on Self-Reported Sleep and Cognition.
| Reference | Sample | Age | Cognitive Measure |
F/U (yrs) |
Short Sleep |
Long Sleep |
SOL | Early Waking |
Night Waking |
Daily Naps |
EDS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Osorio 2011c | 346 | ≥24 | Diagnosis | 7.7 | − | ||||||
| Stenfors 2013d,c | 3,264 | ≥20 | Self-report | 2 | − | ||||||
| Loerbroks 2010d,c | 689 | ≥40 | TICS | 8.5 | ns | − | |||||
| Kulmala 2013d,c | 2,994 | ≥44 | IADL | 28 | − | ||||||
| Ferrie 2011d | 5,431 | ≥45 | Battery | 5.4 | − | − | |||||
| Jelicic 2002d,c | 838 | ≥46 | MMSE | 3 | ns | ns | |||||
| Virta 2013d,c | 2,336 | ≥49 | TICS, TELE | 22.1 | − | − | |||||
| Xu 2012d,c | 11,196 | ≥50 | MMSE, VerM | 3.8 | − | ns | ns | ns | |||
| Sterniczuk 2013d | 28,697 | ≥50 | Diagnosis | 4 | ns | ns | |||||
| Devore 2014d,c | 15,385 | ≥56 | Battery | 6 | − | − | |||||
| Quesnot 1999d,c | 1,389 | ≥59 | MMSE | 4 | ns | ||||||
| Keage 2012d | 2012 | ≥65 | MMSE | 10 | − | ns | ns | ns | + | − | |
| Potvin 2012d,c | 1,664 | ≥65 | MMSE | 1 | − | − | − | ||||
| Benito-León 2009d | 3,286 | ≥65 | MMSE | 3 | ns | − | |||||
| Benito-León 2013d,c | 2,715 | ≥65 | MMSE | 3 | ns | − | |||||
| Cricco 2001d,c | 6,444 | ≥65 | SPMSQ | 3 | − | ||||||
| Jaussent 2012d,c | 4,894 | ≥65 | MMSE, VisM | 8 | ns | ns | + | − | |||
| Blackwell 2014d,c | 2,822 | ≥65 | MMSE, TMT | 3.4 | ns | ns | |||||
| Tworoger 2006d,c | 1,844 | ≥70 | Battery | 2 | ns | ns | ns | ||||
| Foley 2001d,c | 2,242 | ≥71 | CASI | 3 | ns | − | |||||
| Pedraza 2012d,c | 1085 | ≥75 | MMSE | 3 | ns | ns | + | ||||
| Hahn 2013d,c | 214 | ≥75 | Diagnosis | 9 | ns | ||||||
Significant effects following adjustment for demographic (d) and comorbidity (c) variables (if reported) are indicated when more sleep complaints are associated with poorer (−) or better (+) cognitive functioning. F/U refers to follow-up time (in years), ns indicates p > .05, merged cells indicate combined sleep measures, and blank cells indicate that the measure was not collected or reported. Studies are listed by chronological age (lower limit).
Sleep Abbreviations. EDS: excessive daytime sleepiness; F/U: follow-up (years); PSQI: Pittsburgh Sleep Quality Index; SOL: sleep onset latency (difficulty falling asleep)
Cognitive Test Abbreviations. CASI: Cognitive Abilities Screening Instrument; Diagnosis: Patient received dementia diagnosis (including Alzheimer’s disease); IADL: instrumental activities of daily living; MMSE: mini mental state examination; TICS: telephone interview for cognitive status; TMT-B: Trail making task Version B; SPMSQ: short portable mental status questionnaire; VerM: verbal memory; VisM: visual memory
The Whitehall II Study (Ferrie et al., 2011) provides some of the strongest evidence in support of a role for sleep in middle-aged adults as a protector against cognitive declines in normal aging. In this epidemiologically-famous longitudinal study, a shift from sleeping 6–8 hours/night at baseline to shorter sleep duration 5 years later was associated with lower cognitive performance on most cognitive measures (the surprising exception was episodic memory; cf. Devore et al., 2014). Thus, there is support for the hypothesis that sleeping well in middle age promotes sustained cognitive integrity.
Poor sleep may be a weaker predictor of declining cognition when baseline mesures were assessed in older adults. Similar to the cross-sectional studies, sleep fragmentation (i.e., nighttime awakenings) was not a strong correlate of cognitive performance in older adult samples, and two studies even reported that nighttime awakenings (“difficulty maintaining sleep”) was associated with significantly better cognitive preservation (Jaussent et al., 2012; Pedraza, Al Snih, Ottenbacher, Markides, & Raji, 2012). Such findings are somewhat surprising given much of the zeitgeist of sleep—cognition findings in young adults (Rasch & Born, 2013; Rolls et al., 2011).
Though difficulty falling asleep correlated with cognitive functioning in older adults in cross-sectional studies (Table 3), 5 of 7 longitudinal studies in older adults suggested no association (see Table 4 column labeled “SOL”). Subjective sleepiness is a moderately consistent predictor of cognitive decline (see Table 4 column labeled “EDS”), but we would stress to the reader that even this finding becomes non-significant after controlling for depression (Quesnot & Alperovitch, 1999) and general health (Blackwell et al., 2014; Sterniczuk, Theou, Rusak, & Rockwood, 2013). The strongest evidence for short sleep duration in old age leading to speedier cognitive decline arises from the Nurses’ Health Study (N>15,000; Devore et al., 2014), but readers might suspend judgment on this issue because at least five papers failed to show this effect (Table 4).
Summary, Critique, and Future Research Directions
In middle-aged adults, short and poor quality sleep is often associated with, and can even precede, cognitive declines. In older adults, self-reported sleep measures have been less consistently linked to poorer cognitive functioning. This pattern sets the stage for predicting age-related modification of sleep—cognition associations, a theme that we consider in the remaining sections.
Some of the modest effects in this literature might reflect our conservative approach of emphasizing effects following correction for demographic and health-related variables, including depression. The interplay of sleep and depression is fascinating in that even though depression might independently explain cognitive effects (Lichtenberg, Ross, Millis, & Manning, 1995), sleep deprivation has sometimes been experimentally linked to increased depressive symptoms (Kahn-Greene, Killgore, Kamimori, Balkin, & Killgore, 2007). Therefore, future research in aging populations should attempt to disentangle causality (perhaps using structural equation modeling; Olaithe, Skinner, Hillman, Eastwood, & Bucks, 2014) amongst sleep, depression, and cognitive variables (cf. Vanderlind et al., 2014).
The sleep epidemiology literature provides a foundation for beginning to understand sleep, cognition, and aging associations. Despite its strengths, this literature is limited in several important ways. Cognitive psychologists will lament the overuse of the MMSE, which may be insensitive to cognitive variability in healthy adults. Furthermore, sleep neuroscientists are likely to question the validity of the subjective nature of the sleep measures. In the remaining sections we discuss attempts to more objectively measure sleep and more precisely measure cognitive performance.
Motor Activity (Actigraphy) and Neuropsychological Testing
Actigraphy is a small device that measures motor activity and is typically worn as a wristband (e.g., Fitbit®, Actiwatch®). Some sleep researchers have capitalized on actigraphy-defined periods of little or no movement as being a proxy for sleep. The benefits of actigraphy are it places minimal burden on the participant and researcher and it can be worn for weeks (i.e., to assess habitual sleep patterns). Though actigraphy estimates sleep/wake state it cannot measure sleep stages, and importantly, as sleep quality gets worse the reliability and validity of actigraphy diminishes (de Souza et al., 2003; Paquet, Kawinska, & Carrier, 2007; Sivertsen et al., 2006). Put bluntly, it is possible to lie motionless for hours and still be unable to sleep (but actigraphy may score as sleep; e.g., Montgomery-Downs, Insana, & Bond, 2012). Nonetheless, actigraphy is an attractive option for attempting some objective measurement of sleep.
Studies with small-to-medium sized samples of cognitively-normal older adults have produced varied associations between actigraphy variables and episodic memory, problem solving, executive function, and processing speed performance (Cochrane, Robertson, & Coogan, 2012; Miyata et al., 2013; Oosterman, van Someren, Vogels, Van Harten, & Scherder, 2009; Parsey et al., 2012; Regestein et al., 2004; Scullin, 2013; Westerberg et al., 2010; Wilckens, Woo, Erickson, & Wheeler, 2014). These studies typically did not control for demographic or health-related variables (cf. Olaithe et al., 2014). In one study that controlled for age and depression, Cochrane et al. (2012) found one significant unexpected correlation (shorter sleep duration was associated with better episodic memory), one significant expected correlation (greater wake time at night was associated with poorer Stroop performance), and many null correlations.
Three epidemiology studies collectively involving thousands of participants—Rush Memory and Aging, Study of Osteoporotic Fractures, and the Osteoporotic Fractures in Men Study—have reported several significant correlations (Blackwell et al., 2006; 2011a; 2014; Lim et al., 2012, 2013). Though their findings consistently demonstrated that individuals with poorer actigraphy-defined “sleep” (i.e., rest-activity variability) showed poorer cognitive performance (sometimes even using longitudinal data; Lim et al., 2013), these studies typically used very old age groups and they often included mild cognitive impairment and dementia patients. Thus, their meaning for “normal” aging is uncertain. On the one hand, these results could be viewed as supportive to findings that poor sleep is associated with β-amyloid deposition (Spira et al., 2013). On the other hand, collapsing across control and dementia groups that are known to differ both on sleep and cognition (possibly due to a third unrecognized process) might bias one to find actigraphy—cognitive correlations, even if an association did not exist in the healthy controls. Consider, for example, that in the Osteoporotic Fractures in Men Study, age significantly moderated the cognitive association with nighttime awakenings such that these correlations were minimal/absent in 65–79 year old adults (i.e., the group more likely to include cognitively-normal adults; Blackwell et al., 2011a).
Summary, Critique, and Future Research Directions
The results of large-scale actigraphy studies suggest an association between motor activity (“sleep”) and cognitive measures. Age modification of sleep—cognition associations was not a strong theme in this literature (cf. Section A), though many of the actigraphy studies focused on very old sample groups (cf. Blackwell et al., 2011a). Because most of the actigraphy studies were cross-sectional we need to consider direction of causation: Evidence that cognitive declines can precede changes in sleep (Yaffe, Blackwell, Barnes, Ancoli-Israel, & Stone, 2007) may indicate that a common neurobiological substrate (e.g., amyloid deposition; Ju et al., 2013) underlies both cognitive and sleep impairments in older adults. Thus, the major, unanswered question is whether common neurobiological underpinnings are caused by poor sleep, poor cognition, both factors, or a related “third” process.
Actigraphy is a low-cost and non-invasive tool for measuring sleep in population-based studies, but its use remains highly controversial. Correlations between actigraphy-and electroencephalography(EEG)-defined sleep variables in older adults are lamentably low (rs<.25) and may reflect underlying disorders (Mehra et al., 2008), which can independently explain poorer cognitive functioning (e.g., Kushida et al., 2012; Pearson et al., 2006). Prudence thus requires us to look for converging evidence for cognitive associations using the gold standard in sleep measurement, polysomnography.
Polysomnography and Neuropsychological Testing
Polysomnography (PSG) is the gold standard in capturing the complexity of sleep physiology. PSG measurement involves recording electrophysiological data from electrodes attached to the scalp (at frontal, central, and occipital EEG sites), beside the eyes (to identify eye movements), and on the chin (to evaluate movements and muscle tone). More elaborate PSG recordings are possible, but even this simple approach allows one to measure sleep stages, microarchitecture (e.g., sleep spindles), and sleep continuity/fragmentation (Figure 1).
One might hypothesize that several PSG variables should correlate with cognitive functioning. First, sleep duration (and nighttime awakenings) can be precisely measured using PSG; if the subjective- and actigraphy-based reports (see the previous sections) validly reflect associations between sleep and cognition, then they should replicate with PSG. Second, PSG allows measurement of slow-wave sleep (SWS), which has a neurophysiological signature suggestive of cortical plasticity and memory processing in young adults. In SWS, brain regions that are recognized to be important to memory functioning (hippocampus, frontal cortex) are believed to “dialogue” against a quiet subcortical background (Buzsáki, 1996; Buzsáki & Peyrache, 2013; Logothetis et al., 2012; Massimini, Huber, Ferrarelli, Hill, & Tononi, 2004). Third, “sleep spindles,” suggested to reflect synaptic plasticity mechanisms (Rosanova & Ulrich, 2005), are also detected with PSG. Fourth, one can isolate periods of rapid eye movement (REM) sleep, which is characterized by vivid dreaming, increased cerebral blood flow in several regions (e.g., the amygdala; Maquet et al., 1996), and increased cholinergic activity which might promote long term potentiation (Diekelmann & Born, 2010).
Table 5 summarizes studies that have evaluated overnight PSG in relation to neuropsychological testing in older adults. These studies sometimes included patients with dementia or psychiatric conditions and cross-sectional differences in PSG variables relative to healthy older adults are well recognized (e.g., Cipolli et al., 2013; Foley et al., 2003; Loewenstein et al., 1982; Palma, Urrestarazu, & Iriarte, 2013; Prinz et al., 1982a; Reynolds et al., 1985). In this section, we focus on normal aging and whether neuropsychological test performance—which is presumably “trait-like,” but may incorporate some “state” effects— is associated with PSG measures of sleep duration, SWS, REM sleep, or spindle density. To foreshadow, early studies (First Wave) did not consistently link PSG variables to cognitive measures, but more recent studies (Second Wave) have suggested a potential age-related modification of PSG—cognitive associations.
Table 5.
Polysomnography and Neuropsychological Testing Studies.
| Reference | N | Age | TST | Wake | N1/N2 | N3/SWS | REM | Spindle | (NS) Measures |
|---|---|---|---|---|---|---|---|---|---|
| “First Wave” of Studies | |||||||||
| Berry 1985 | 119 | 58 | ns | − AA7,RPM | + AA7, RPM(N1) | ns | ns | VIQ, PIQ, WMS | |
| Spiegel 1981 | 57 | 64 | +/− VIQ | +/− VIQ, RPM | − VIQ, RPM | PIQ | |||
| Spiegel 1986 | 43 | 69 | ns | VIQ, PIQ, RPM | |||||
| Guazzelli 1986 | 48 | 72 | ns | VerM, VisM, VIQ, PIQ,TMT,VF,speed | |||||
| Hoch 1994d,c | 50 | 75 | − MMSE | ||||||
| Feinberg 1967d | 15 | 77 | + RPM | − RPM | ns ns |
− RPM | + PIQ,WMS | ns | VIQ |
| Spiegel 1999 | 30 | 77 | + MMSE, ERFC | ns | ns | +/− MMSE, Vocab,ERFC | |||
| Kahn 1969 | 16 | 80 | + Digit | WMS, VIQ, PIQ | |||||
| Prinz 1977 | 12 | 82 | ns ns |
ns | + PIQ, WMS | VIQ | |||
| “Second Wave” of Studies | |||||||||
| Edinger 2008 | 84 | 49 | − SRT, SAT | CPT | |||||
| Lafortune 2014d | 58 | 63 | ns | ns | ns | + VF | + VerM | +VerM,VF CPT, Bells | N-Back |
| Bastien 2003 | 20 | 63 | ns | − VerM, WCST,PB | + VerM(N2) | ns | ns | VisM, TMT-A/B, WRT, DS (F/B) | |
| Anderson 2003d | 24 | 67 | +WCST, VF CFI, ToL | PVT | |||||
| Kim 2011d,c | 30 | 67 | − VerM, VF, CP | + BNT | + VisM | ||||
| Hita-Yañez 2012/2013d | 25 | 67 | ns | ns | ns | WMS VerM | |||
| Seeck-Hirschner 2012d | 19 | 68 | ns | ns | ns | ns | ns | ns | MMSE, TMT-A |
| Mander 2013ad | 14 | 72 | + VisM/ VerM | ||||||
| Blackwell 2011bd,c | 2,909 | 76 | -TMTB,DV MMSE(N1) | ns | + TMT-B, DV | ||||
| Cole 2009 | 90 | 81 | − MMSE | − MMSE | |||||
| Yaffe 2011d,c | 298 | 82 | ns | ns | VerM, DS, VF, MMSE, TMT-B | ||||
Statistically significant positive (+) or negative (−) correlations following adjustment for demographic (d) and comorbidity (c) variables (if reported). The cognitive correlate is listed in each cell, and additional (ns) cognitive measures are listed in the rightmost column. Missing cells indicate that those data were not collected or reported. Studies are sorted by timing as in the main text (e.g., “first wave”), and chronological age (mean age rounded).
Sleep Abbreviations. N1: Stage 1 sleep; N2: Stage 2 sleep; N3/SWS: slow wave sleep duration, slow wave density/slope, or delta spectral power; REM: rapid eye movement sleep; SOL: sleep onset latency; TST: total sleep time; Wake indicates number of nighttime awakenings, sleep efficiency, arousal index, Non-REM shifts, or total time awake.
Cognitive Abbreviations. AA7: Army Alpha Test 7; BNT: Boston Naming Test; CP: Constructional Praxis; CPT: continuous performance test; CFI: Cattell Fluid Intelligence; Digit: Wechsler Adult Intelligence Scale – digit symbol; DS: Digit Span (Forward and Backward); DV: Digit Vigilance; ERFC: Évaluation rapide des fonctions cognitives; MMSE: mini mental state examination (including modified [3MS] versions); PB: Purdue Board; PIQ: Wechsler Adult Intelligence Scale – Performance Intelligence Quotient; PVT: psychomotor vigilance task; SAT: switching attention; SRT: simple reaction time; TMT: Trail Making Task (part A and/or B); VerM: verbal memory; VF: verbal fluency; VisM: visual memory; VIQ: Wechsler Adult Intelligence Scale – Verbal Intelligence Quotient; Vocab: Wechsler Adult Intelligence Scale – vocabulary subtest; WCST: Wisconsin Card Sorting Task; WMS: Wechsler Memory Scale; WRT: Wilkinson reaction time
First Wave (1967–1999)
Feinberg, Koresko, and Heller (1967) pioneered the study of whether PSG variables correlate with performance on intelligence tests in older adults. They found that performance correlated positively with REM sleep (but also negatively with SWS). Most early PSG—cognition studies were limited by sample size. However, in one impressive early study, 119 middle-to-older-aged adults underwent five consecutive nights of PSG recording (Berry & Webb, 1985). Consistent with the conclusions from the Self-Reported Sleep literature (e.g., Sutter et al., 2012), “sleep and cognitive variables known to be sensitive to aging processes failed to intercorrelate robustly” (p. 334, Berry & Webb).
It is impressive that the “first wave” of PSG and neuropsychological studies included four longitudinal studies (Guazzelli et al., 1986; Hoch et al., 1994; Prinz, 1977; Spiegel, 1981). Two studies suggested that baseline cognitive levels, or longitudinal changes in cognition, predicted poorer PSG-measured sleep (rather than vice versa; Hoch et al.; Prinz), but most studies reported very few significant effects with cognitive measures. For example, Feinberg and colleagues (1986) found that the correlations expected based on contemporary memory studies (Rasch & Born, 2013)—such as those between frontal spindle density and face recognition and memory for names—were consistently near zero or nominally negative (even in a 3-year follow-up; I. Feinberg, personal communication, June 23, 2013). Likewise, a 14-year study produced sporadic correlations (i.e., in both positive and negative directions) with REM measures (Spiegel, Herzog, & Koberle, 1999; Spiegel, Koberle, & Allen, 1986). Counterintuively, older adults who showed greater awakenings from sleep/SWS at baseline showed more preserved cognition.
Second Wave (1999-present)
With a few exceptions, interest in correlating PSG and neuropsychological measures in healthy older adults dwindled in the 1980s (Bliwise, 1989). The last decade, however, has seen a “second wave” (p. 135, Vertes, 2004) of interest. One advancement is the utilization of more technologically sophisticated analyses of PSG data. One approach is to calculate the number of microarousals (i.e., very brief awakenings) that occurred during SWS and REM sleep (Hita-Yañez, Atienza, Gil-Neciga, & Cantero, 2012), but these precise sleep fragmentation measures did not correlate with episodic memory in cognitively-normal older adults after controlling for age (J. Cantero, personal communication, February 15, 2013).
Another approach is to analyze EEG spectral power so as to capture not only the quantity, but presumably also the quality, of SWS. For example, analyzing spectral power in the delta range (0.5–4 Hz) provides a measure that conflates the incidence and amplitude of slow waves. Null effects are still sometimes observed (e.g., Seeck-Hirschner et al., 2012), however, in one study (Anderson & Horne, 2003) that analyzed spectral power in only the 0.5–1.0 Hz frequency range (motivated by Steriade, Nunez, & Amzica’s, 1993, studies) during the first ~42 minutes of sleep, spectral power correlated positively with performance on many attention, executive function, and intelligence tests in older adults (cf. Mathias, Zihl, Steiger, & Lancel’s, 2005, experimental study).
Similarly-focused attempts to extract precise components of sleep physiology include computer-automated analyses of sleep spindles. Sleep spindle counts have been associated with cognitive performance in young adults (Fogel & Smith, 2011; Nader & Smith, 2001), and two studies reported that episodic memory correlated with spindle density in aging adults (Table 5). Thus, early studies (“first wave”) may have missed some PSG—cognition correlations because they primarily focused on sleep stage quantity, rather than on early-sleep delta spectral power or sleep spindles. Another worthwhile consideration is that many studies in this literature used broad age ranges. Returning to an overarching theme initially prompted by the findings of the self-report literature, could age be a modifier of the PSG—cognition relationship?
Three studies examined SWS correlates of a vigilance task in different age groups. In young adults, poorer vigilance task performance was associated with less SWS (Jurado, Luna-Villegas, & Buela-Casal, 1989), and similar findings emerged in middle-aged adults, albeit perhaps less consistently (Edinger, Glenn, Bastian, & Marsh, 2000). By contrast, in healthy older adults, no such correlations were observed (Crenshaw & Edinger, 1999). These findings suggested that “the degree to which slow-wave sleep restores neurocognitive processes among normal sleepers changes as a function of aging” (p. 127, Edinger et al.).
An exciting possibility is that it is age-related physiological changes—and not age per se—that modifies SWS—cognition relations. As an initial step toward addressing this possibility, one study measured cerebral oxygen reserve (or, more broadly, cerebrovascular risk) during SWS-rich sleep in 112 older adults without sleep apnea (Carlson, Neelon, Carlson, Hartman, & Bliwise, 2011). During early SWS, cerebral oxygenation increases in most young adults, but decreases or does not change in most older adults (Carlson, Neelon, Carlson, Hartman, & Dogra, 2008). Critically, older adults who showed a similar increase (as young adults) in cerebral oxygenation during SWS showed relatively preserved episodic memory performance.
Summary, Critique, and Future Research Directions
The PSG and neuropsychological testing literature has a rich, but often forgotten, history. Across nearly half a century of research, PSG studies have failed to provide converging evidence for self-report- and actigraphy-based findings that short sleep duration correlates with cognitive performance (Table 5). Furthermore, there was little evidence for specific relations between particular cognitive abilities and particular PSG variables in older adults. Consider, for example, studies that used tasks dependent on executive function (N-back, Wisconsin’s Card Sorting Task, Trail Making Task B, switching attention): The predominant finding was no PSG correlation with executive function, and when significant correlations were observed, the particular PSG correlate was observed only in a single study.
Does preserved SWS correlate with preserved episodic memory in older adults? This popular hypothesis was not supported by the studies in Table 5 that included tests of visual memory, verbal memory, face-name pair learning, or the Wechsler Memory Scale (see Table 5 for correlations with spindle density, REM sleep, and nighttime awakenings, though null correlations were most common). Verbal fluency, which is presumably a measure of semantic memory that relies on frontal and temporal lobes (Baldo, Schwartz, Wilkins, & Dronkers, 2006), was the only cognitive ability to demonstrate any replicability in correlating with SWS measures in older adults.
One important technical consideration for SWS is that there is a strong reduction in slow-wave EEG amplitude in older adults, and traditional SWS scoring criteria (Rechtschaffen & Kales, 1968) dictate that slow-wave amplitude must exceed 75µV. Therefore, one might be concerned that SWS duration does not correlate with cognitive variables only because of the scoring method used (i.e., using amplitude rather than frequency). This explanation does not account for null SWS findings in studies in which the analyses ignored traditional amplitude criteria (e.g., Berry & Webb, 1985).
A weakness of the PSG literature is the overreliance on small sample sizes (except Blackwell et al., 2011b; Yaffe et al., 2011), which is commonly assumed to decrease statistical power, but also increases risk of Type I errors (Button et al., 2013; Yarkoni, 2009). Another limitation is that computer-automated spindle detection has been extensively validated in healthy young adults, but only minimally in older adults. A third challenge for the field will be to demonstrate that spectral power correlations with cognitive measures represent sleep-specific effects, and not trait-based EEG correlations that could be observed during wakefulness (Finnigan & Robertson, 2011; Vlahou, Thurm, Kolassa, & Schlee, 2014).
Despite equivocal evidence for short sleep and SWS quantity, REM sleep often correlated with cognitive performance in older adults. Such findings seem to converge with early animal studies (Markowska et al., 1989; Stone, Altman, Berman, Caldwell, & Kilbey, 1989) and with reports of cross-sectional differences in REM sleep between healthy controls and dementia patients (Allen, Seiler, Stahelin, & Spiegel, 1987; Dykierek et al., 1998; Feinberg et al., 1967; Prinz et al., 1982a/b; Reynolds et al., 1985/1988; Vitiello et al., 1984). The crucial, unanswered question here is whether the reduction in REM and the development of dementia are both epiphenomenal to other mechanisms such as decreased cholinergic neurotransmission (cf. Stone, Rudd, Parsons, & Gold, 1997; Yaffe et al., 2007) or if loss of REM sleep drives cognitive changes (via, e.g., reduced long term potentiation).
Multiple studies suggested associations between cognitive measures and time awake after going to bed (Table 5; cf. Tables 3–4). The correlational findings with time awake converge with some of the actigraphy literature’s findings. However, in the next section, readers will likely be surprised by the striking contrast between these correlational findings and the findings of the experimental sleep deprivation literature.
Experimental Sleep Deprivation Studies
A typical finding in healthy young adults is that sleep deprivation causes poorer cognitive performance (Williams, Lubin, & Goodnow, 1959; for reviews, see Durmer & Dinges, 2005; Killgore, 2010), which some have pointed out to be reminiscent of cognitive impairments with increasing age (Harrison, Horne, & Rothwell, 2000). Sleep deprivation has numerous neurobiological and psychological effects (e.g., stress) that could potentially mediate sleep—cognition associations (Bonnet, 2011), but the general approach in the literature is to view loss of sleep as a manipulation of sleep per se. The experimental sleep deprivation literature therefore provides a reasonable test of the hypothesis generated by the correlational studies in all three previous sections that short sleep (or increased wake time at night) causes poorer cognitive functioning in older adults.
Several behavioral studies examined sleep deprivation in only middle-aged or only older adult groups. These studies suggested some detrimental consequences of sleep deprivation/fragmentation to “state” cognition (Bonnet, 1985; Carskadon & Dement, 1985; Froberg, Karlsson, Levi, & Lidberg, 1975; Webb; 1986; Williams et al., 1959; Williams, Gieseking, & Lubin, 1966). For example, Van Der Werf et al. (2009) reported that in 12 middle-to-older-aged adults that fragmenting SWS led to visual memory encoding impairments.
Table 6 lists experimental studies that compared young adults to middle-aged or healthy older adults following normal sleep versus sleep deprivation/fragmentation. Webb and colleagues’ (1982; 1985) early studies conducted with middle-aged faculty members raised the possibility that the cognitive effects of sleep deprivation increase with age; yet the more common theme that emerges from 30 years of experimental research is that sleep deprivation/fragmentation affects cognitive functioning in young adults, but has less of an effect, no effect, or even a facilitating effect on cognitive functioning in older adults (16 of the 20 studies in Table 6). It is possible that some of the reduced effects in older adults reflect diminished baseline performance in older adults (i.e., floor effects), but this explanation cannot account for studies in which older adults outperformed their younger counterparts following sleep deprivation (e.g., Duffy, Willson, Wang, & Czeisler, 2009; Stenuit & Kerkhofs, 2005). Another concern is the overreliance on vigilance tasks, but similar age modification effects have been observed with episodic memory (Bonnet & Rosa, 1987) and multitasking (Nesthus, Scarborough, & Schroeder, 1998).
Table 6.
Experimental Sleep Deprivation/Restriction Studies That Assessed Whether Cognitive Impairment Varied By Age.
| Paper | Young Adults |
Middle Adults |
Older Adults |
Length of Manipulation |
Outcome Measure |
Age Effect Interpretation |
|---|---|---|---|---|---|---|
| Total Sleep Deprivation Procedures | ||||||
| Brezinova 1969 | N=5 (M=22) | N=5 (M=40) | 64 hours | EEG | Young impaired more than older | |
| Webb 1982 | N=6 (18–22) | N=10 (40–49) | 41 hours | Cognitive Battery | Young impaired less than older | |
| Webb 1985 | N=6 (20–25) | N=12 (50–60) | 2 nights | Cognitive Battery | Age effect depended on task | |
| Nesthus 1998a/b | N=14 (M=27) | N=13 (M=51) | 34 hours | Cognitive battery | Young impaired more than older | |
| Killgore 2006 | N=34 (19–39) | 49.5 hours | IGT | Young impaired less than older | ||
| Bonnet 1987 | N=12 (18–28) | N=12 (55–71) | 64 hours | Memory, RT | Young impaired more than older | |
| Philip 2004 | N=10 (M=23) | N=10 (M=58) | 1 night | RT | Young impaired more than older | |
| Lowden 2009 | N=10 (18–24) | N=10 (55–64) | 4 AM testing | EEG | Young impaired more than older | |
| Brendel 1990 | N=14 (M=20) | N=10 (M=80) | 1 night | Vigilance | Young impaired more than older | |
| Smulders 1997 | 12 YA (M=21) | N=12 (M=67) | 28 hours | Multiple RT tasks | Young impaired more than older | |
| Mertens 1986 | N=16 (M=21) | N=14 (M=67) | 1 night | Cognitive battery | Young impaired more than older (in morning) | |
| Adam 2006 | N=12 (M=25) | N=11 (M=66) | 40 hours | PVT | Young impaired more than older | |
| Blatter 2006 | N=16 (M=25) | N=11 (M=65) | 40 hours | PVT | Young impaired more than older | |
| Duffy 2009 | N=26 (M=22) | N=11 (M=68) | 26 hours | PVT | Young impaired more than older | |
| Sagaspe 2012 | N=14 (M=23) | N=11 (M=68) | 40 hours | Go/No-Go, RT | Young impaired more than older (some tests) | |
| Sleep Restriction and Fragmentation Procedures | ||||||
| Bliese 2006 | N=65 (M=38) | 3, 5, 7, or 9 hours for 7 days | PVT | Young impaired slightly more than older | ||
| Stenuit 2005 | N=11 (M=23) | N=10 (M=60) | 4 hours for 3 nights | PVT, MWT | Young impaired more than older | |
| Stenuit 2008 | N=10 (M=23) | N=10 (M=60) | 4 hours for 3 nights | Cognitive battery | Young impaired same as older | |
| Bonnet 1989 | N=12 (M=22) | N=12 (M=63) | 14 arousals/hour for 2 nights | Addition Task | Young impaired more than older | |
| Filtness 2012 | N=20 (M=23) | N=19 (M=67) | 5 hours for 1 night | Driving simulator | Young impaired more than older | |
Sample sizes and mean age (rounded) or range are separated by age groups. Papers are sorted by age comparison group and split by total sleep deprivation versus sleep restriction and fragmentation procedures.
Abbreviations. IGT: Iowa Gambling Task; MWT=Maintenance of Wakefulness Test; PVT: Psychomotor Vigilance Task; RN=recovery night; RT=reaction time task
Summary, Critique, and Future Research Directions
Sleep deprivation’s minimalistic impact on cognition in older adults is somewhat surprising in light of the studies that correlated short sleep and/or increased time awake at night with poorer cognitive performance (Tables 3–5). First consider non-sleep-specific mechanisms for this age dissociation. Some argue that sleep deprivation primarily impairs cognitive performance in young adults at the nadir of their body temperature rhythm (i.e., a circadian, not a sleep, effect; Bonnet, 2011); the reduced change in cognitive performance in older adults might therefore reflect that the strength of such body temperature fluctuations diminishes with aging. A second possibility is that the cognitive repercussions of sleep deprivation are mediated by sleep-loss-induced cortisol elevations (Spiegel, Leproult, & Van Cauter, 1999). Elevated cortisol is a known correlate of cognitive impairments (McEwan & Sapolsky, 1995), but cortisol would be unlikely to explain the age interactions discussed herein (Maggio et al., 2013) because age does not moderate the cortisol and sleep-loss relationship or the cortisol—cognition relationship (Lee et al., 2007; Vgontzas et al., 2003).
Next consider sleep-specific interpretations. One possibility is that older adults are chronically sleep deprived and depriving of them of additional sleep will have minimal effects. However, age-dissociations are still present in studies that selected for good-sleeping older adults (e.g., Adam, Retey, Khatami, & Landolt, 2006). Additional possibilities are that older adults need less sleep than young adults (e.g., Bliwise, 2000; 2011) or that sleep is less restorative to cognitive functions (Edinger et al., 2000), and thus, less detrimental to cognition when lost. The behavioral results of the sleep deprivation and aging literature are clear, but the explanation for such effects requires further attention.
Experimental Napping and Sleep Extension Studies
Humans are encouraged to sleep 8 hours per night, but often fail to achieve this standard. If chronic sleep loss plagues modern American society, and if even mild sleep deprivation impairs cognition (Van Dongen, Maislin, Mullington, & Dinges, 2003), then extending sleep would be expected to improve cognitive functioning (cf. Buysse, Grunstein, Horne, & Lavie, 2010). Could taking a daily nap be our society’s solution? The practice of regular napping has been observed in “Blue Zones” (Buettner, 2012), which are areas such as Ikaria, Greece where adults commonly live healthy lives into their 90s (“nonagenarians”; see also Asada, Motonaga, Yamagata, Uno, & Takahashi, 2000; cf. Table 3). Furthermore, in infants, children, adolescents, and young adults, daytime naps have been linked to improved cognitive performance and memory consolidation (for reviews, see Kopasz et al., 2010; Mednick, 2006; Milner & Cote, 2009). Similar cognitive benefits of prophylactic naps (Schweitzer, Randazzo, Stone, Erman, & Walsh, 2006) and naps during a night shift (Purnell, Feyer, & Herbison, 2002) have been documented in middle-aged shift-workers (Ficca, Axelsson, Mollicone, Muto, & Vitiello, 2010). In this section, we evaluate experimental studies that investigated whether napping on a single day or across several weeks boosts cognitive functioning in (non-shift-worker) aging adults.
Nap Experiments (1–2 Days)
Some early napping, cognition, and aging work was well-designed but limited by ceiling effects (Tamaki, Shirota, Hayashi, & Hori, 2000; Tamaki, Shirota, Tanaka, Hayashi, & Hori, 1999). Subsequent work, however, has provided compelling evidence that an afternoon nap benefits middle-aged adults’ cognitive functioning. When 32 healthy middle-to-older-aged adults were given a two-hour early afternoon nap or rest opportunity, napping led to improved reaction time and Stroop performance (Campbell, Murphy, & Stauble, 2005). Similarly, 10 young adults, 10 middle-aged adults, and 12 middle-to-older-aged adults completed cognitive testing before and after short naps (20-min), long naps (60-min), and no-nap reading conditions (Milner & Cote, 2008). Some nap-related cognitive benefits were observed (e.g., serial addition/subtraction performance), and these benefits did not significantly interact with age group. However, statistical power may have been a limiting factor; for example, in the 60-minute nap condition, serial addition/subtraction accuracy increased pre-to-post nap in the young adults and middle-aged adults, but decreased pre-to-post nap in the middle-to-older-aged adults. In another study that focused only on older adults (N=24), there were no significant differences in episodic memory, attention, working memory, or procedural memory across 60-minute nap versus rest conditions (Wan, 2013). Thus, there is persuasive evidence for the cognitive value of napping in young and middle-aged adults, but such benefits may decrease with increasing age.
Nap-based Sleep Extension Interventions (Weeks)
Some napping studies have been designed to increase 24-hour sleep across several days or weeks (Creighton, 1995). These studies have failed to demonstrate an experimental benefit of increased sleep on cognitive functioning. In one study, 21 middle-to-older-aged adults adhered to a month-long short- or long-nap regimen (there was not a no-nap control group; Campbell, Stanchina, Schlang, & Murphy, 2011), and in another study, nine healthy older adults took a 90-minute early afternoon nap or rested (no-nap) across 17 days (Monk, Buysse, Carrier, Billy, & Rose, 2001). The experimental manipulation increased 24-hour sleep duration in both studies, but no experimental group differences were observed across a range of executive control, intelligence, and attention tasks.
Summary, Critique, and Future Research Directions
Taking an afternoon nap improves cognitive functioning in middle-aged adults. Such benefits might not extend to older adults and this finding converges with evidence that older adults’ cognitive functioning is minimally impacted by sleep deprivation. Future research should use no-nap control conditions and control the amount of nocturnal sleep prior to the nap. This literature would also benefit from disentangling whether naps improve overall cognitive ability (transfer), or alternatively, consolidation of trained tasks, the topic to which we next turn our attention.
Experimental Studies of Memory Consolidation
The newest frontier in sleep, cognition, and aging research focuses on memory consolidation. There exist several theories of memory consolidation (see Table 1), but a consistent theme is that after a memory is encoded (i.e., learned or perceived) it must undergo a process of stabilization and integration (i.e., consolidation) if it is to later be retrieved (i.e., recollected). For at least 50 years, psychological scientists have hypothesized that memory consolidation declines with increasing age (Doty & Doty, 1964), but researchers have only recently focused on sleep-dependent memory consolidation and aging.
Sleep’s role in memory consolidation has been elegantly demonstrated in human and animal studies that showed that memories are “replayed” and strengthened during sleep (Rasch, Buchel, Gais, & Born, 2007; Wilson & McNaughton, 1994; Yang et al., 2014). Though the sleep and memory consolidation field is not without some controversy and debate (Rickard, Cai, Rieth, Jones, & Ard, 2008; Siegel, 2001; Vertes, 2004; Vertes & Siegel, 2005) our interpretation is that the diversity of supportive empirical evidence (Appendix) is sufficiently compelling to conclude that there exists an active role for sleep in memory in young adults (Hennevin, Huetz, & Edeline, 2007; Oudiette & Paller, 2013; Rasch & Born, 2013).
In a typical memory consolidation study, participants study verbal materials or learn a motor memory task in the evening, then sleep and are re-tested in the morning. Memory consolidation is a “state-dependent” effect that is inferred following sleep relative to wake-only intervals when procedural (non-declarative) memory performance increases, when episodic (declarative) memory forgetting is reduced, or when a qualitative change in the memory trace is observed (e.g., integration). The procedures in the memory consolidation literature are typically derived from experimental psychology (Appendix) rather than from clinical neuropsychology or epidemiology.
The most straightforward prediction for aging is that as sleep becomes shortened, fragmented, and less “deep” (i.e., lower SWS), the sleeping brain may engage in less memory consolidation. If so, then the loss of memory consolidation during sleep might be one reason for the weakening evidence for sleep—cognition associations in older adults: If active cognitive processes are not occurring during sleep then sleep variables would not be expected to correlate with cognitive variables (Tables 3–6). Indeed, in animal studies, both young rodents and “middle-aged” rodents tend to show “replay” of memories during sleep (i.e., reactivation of learned hippocampal sequences; Huxter, Miranda, & Dias, 2012), but sleep-dependent memory replay is diminished in older rodents (Gerrard, Burke, McNaughton, & Barnes, 2008). Behavioral evidence from animal models also supports the idea of an age-related decline in memory consolidation (e.g., Hermann et al., 2007; Oler & Markus, 1998; Ward, Oler, & Markus, 1999) In the following section, we address whether there is an age-related change in sleep-dependent procedural memory (Table 7) and episodic memory (Table 8) consolidation in aging humans.
Table 7.
Procedural Memory Consolidation Studies in Healthy Middle-Aged and Older Adults.
| Reference | Samples (Age) | Procedural Memory Tests |
Retention Interval |
Sleep- Related Benefit? |
PSG-Memory Improvement Correlations |
|---|---|---|---|---|---|
| Studies that Primarily Included Middle-Aged Adults (Mean Age <60) | |||||
| Djonlagic 2014 | N=20 (M=35.3) | PVT, MST | ~10 hrs (sleep-only) | Yes (MST) | Arousals (−)(Age) |
| Backhaus 2006 | N=13 (M=40.1) | MT | ≥8 hrs (sleep only) | Yes | |
| Genzel 2014 | N=16 (M=41.8) | MST fast or paced (w/in-sub) | ≥9 hrs (sleep only) | Yes | Spindle trend(+) |
| Manoach 2010 | N=15 (M=42) | MST | 9 hrs w/in-sub wake vs. sleep | Yes (late) | Null effect(Age), 1 |
| Manoach 2004 | N=14 (M=44) | MST | 24 hrs (wake + sleep) | Yes | N/A |
| Deak 2011 | N=9 (M=44.7) | MST | 12 hrs w/in-sub wake vs. sleep | Yes (late) | |
| Nissen 2006 | N=7 (M=44.9) | MT | 10 hrs (sleep-only) | Yes | Null effects |
| Nissen 2011 | N=53 (M=46.6) | MT | 12 hrs b/n-sub wake vs. sleep | Yes | REM density (+) |
| Kloepfer 2009 | N=20 (M=47.4) | MT | 10.5 hrs (sleep-only) | Yes | Null effects |
| Nemeth 2013 | N=17 (M=57.8) | Implicit ASRT | 24 hrs (wake + sleep) | Yes | N/A |
| Oudiette 2011 | N=18 (M=57.9) | Modified SRRT | 13 hrs (sleep only) | Yes | |
| Studies that Primarily Included Older-Aged Adults (Mean Age >60) | |||||
| Siengsukon 2009a/b | N=40 (M=62.3) | Explicit or implicit CTT (b/n-sub) | 12 hrs b/n-sub wake vs. sleep | No | N/A |
| Siengsukon 2008 | N=18 (M=65.6) | Implicit CTT | 12 hrs b/n-sub wake vs. sleep | No | N/A |
| Terpening 2013 | N=20 (M=66.1) | MST | ~10 hrs (sleep-only) | Yes (late) | SWS(+Late)(Age) |
| Hornung 2007 | N=107 (M=66.1) | MT | 10 hrs AChE-I, rem-reb, rem-/nrem-depr, sleep | Yes | Null effects(Exp) |
| Studies that Compared Multiple Age Groups | |||||
| Wilson 2012 | -N=24 (M=25.9) -N=32 (M=44.0) -N=31 (M=63.1) |
10-item SRTT (explicit) | 12 hrs w/in-sub wake vs. sleep | -Yes -Reduced -No |
N/A |
| Dresler 2010 | -N=12 (M=25.3) -N=38 (M=47.0) |
MST | 24 hrs (wake + sleep) | -Yes -Reduced |
N/A |
| Brown 2009 | -N=14 (M=20.4) -N=12 (M=58.3) |
SRTT | 24 hrs (wake + sleep) | -Yes -No |
N/A |
| Spencer 2007 | -N=38 (M=20.8) -N=32 (M=59.0) |
10-item SRTT Explicit, Implicit | 12 hrs w/in-sub wake vs. sleep | -Yes -No |
N/A |
| Pace-Schott 2013 | -N=62 (M=20.1) -N=50 (M=62.0) |
Goal- vs. Muscle-based (b/n-sub) | 12 hrs b/n-sub wake vs. sleep | -Yes (goal) -No |
N/A |
| Fogel 2014 | -N=28 (M=24.0) -N=29 (M=62.6) |
MST | 90-min b/n-sub nap vs no-nap | -Yes -No |
|
| Tucker 2011 | -N=15 (M=20.1) -N=16 (M=68.0) |
MST | 12/24 hr w/in-s, wake vs. sleep | -Yes -Yes (late) |
-N/A -Null effects |
| Nemeth 2010 | -N=25 (M=21) -N=24 (M=69.8) |
Implicit ASRT | 12 hrs b/n-sub wake vs. sleep | -No -No |
N/A |
| Peters 2008 | -N=14 (M=20.1) -N=14 (M=69.8) |
Pursuit rotor | Up to 1 week (wake + sleep) | -Yes -Yes |
Non-significant trends |
Sample refers only to the healthy (control) group. PSG correlations (if reported) with overnight memory improvement are listed after adjusting for performance baseline, chronological age (Age), & experimental group (Exp). “Late” indicates that plateau, not immediate, improvement was observed. Papers are sorted by those with a single versus multiple age groups and then sorted by mean age.
The authors reported this correlation following removing an outlier.
Retention Interval Abbreviations. Depr; deprivation; Reb: rebound; AChE-I: Acetylcholinesterase Inhibitor Donepezil.
Memory Task Abbreviations. ASRT: alternating serial reaction time; CTT: Continuous Tracking Task; MST: Finger Tapping Motor Sequence Test; MT: Mirror Tracing; PVT: Psychomotor Vigilance Task; SRTT: Serial Reaction Time Task
Table 8.
Declarative Memory Consolidation Studies in Healthy Middle-Aged and Older Adults.
| Reference | Samples (Age) |
Declarative Memory Tests |
Retention Interval |
Sleep-Related Effect? |
PSG-retention Correlations |
|---|---|---|---|---|---|
| Studies that Primarily Included Middle-Aged Adults (Mean Age <60) | |||||
| Backhaus 2006 | N=13 (M=40.1) | PAL (40 pairs to 60%) | ≥8 hrs (sleep only) | (no wake control) | SWS (+)(Age) |
| Mawdsley 2014 | N=40 (M=40.3) | Item and source memory | 12 hrs b/n-sub wake vs. sleep | Marginal age reduction | N/A |
| Deak 2011 | N=9 (M=44.7) | Selective Reminding task | 12 hrs w/in-sub wake vs. sleep | No | SWS (+ trend) |
| Nissen 2011 | N=53 (M=46.6) | VVM | 12 hrs b/n-sub wake vs. sleep | Yes | Null effects |
| Kloepfer 2009 | N=20 (M=47.4) | VVM | 10.5 hrs (sleep-only) | (no wake control) | Null effects |
| Studies that Primarily Included Older-Aged Adults (Mean Age >60) | |||||
| Mazzoni 1999 | N=30 (M=68) | PAL: 20 pairs (no immediate test) | Sleep only | (no wake control) | Sleep cycles (+) SWS (− trend) |
| Hornung 2007 | N=107 (M=66.1) | PAL (34 pairs studied 2×) | 10 hrs b/n-sub sleep, rem-depr, rem-reb, nrem-depr, AChE-I | No group effects | Null effects(Exp) |
| Schredl 2001 | N=8 (M=66.5) | 40 words (read, recall 8×) | 11 hrs baseline sleep vs AChE-I | (no wake control) | REM% (+) on AChE-I night |
| Lo 2014 | N=14 (M=66.6) | DRM lists (lures, studied words) | 8.3 hrs w/in-sub wake vs. sleep | No (study item) Yes (lure item) |
Studied: Null Lures: SWS (−) |
| Seeck-Hirschner 2012 | N=19 (M=68) | Rey-Osterrieth Complex Figure | ≥10 hrs (sleep only) | (no wake control) | SWS(−),wake(+), spindles(+)(Age) |
| Conte 2012 | N=16 (M=72.5) | PAL (study 168 pairs 3×) | >9 hrs (sleep only) | (no wake control) | arousals (−), transitions (−) |
| Westerberg 2012 | N=16 (M=72.7) | PAL (study 44 pairs 2×); face-fact pairs | Sleep only (two nights) | (no wake control) | PAL: delta, theta (+), Face: Null |
| Hot 2011 | N=14 (M=76.7) | 15 words (studied 5×) | Sleep only | Ceiling effects | N/A (ceiling effects) |
| Studies that Compared Multiple Age Groups | |||||
| Wilson 2012 | -N=24 (M=25.9) -N=32 (M=44.0) -N=31 (M=63.1) |
PAL (32 pairs to 62.5%) | 12 hrs w/in-sub wake vs. sleep | -Yes -Yes -Yes |
N/A |
| Backhaus 2007 | -N=16 (M=20.4) -N=12 (M=50.0) |
PAL (40 pairs to 60%) | 3.5 hrs w/in-sub early vs late sleep | Early > Late sleep (both) | SWS (+)(Age) |
| Giambra 1993 Exp 1 | -N=24 (18–21) -N=24 (55–64) |
48 Sentences (Studied 2×) | Up to 24 hours | Similar forgetting rates | N/A |
| Giambra 1993 Exp 2 | -N=24 (17–21) -N=24 (65–74) |
48 Sentences (Studied 3×) | Up to 24 hours | Forgetting rate was faster in OA | N/A |
| Cherdieu 2014 | -N=20 (M=22.1) -N=20 (M=68.9) |
Object location task | 12 hrs b/n-sub wake vs. sleep | -Yes -No |
Cycle time/Sleep time |
| Mary 2013 | -N=16 (M=21.0) -N=16 (M=69.7) |
PAL (Study 28 pairs 5× or 100%) | 7 days | Forgetting rate was faster in OA | N/A |
| Scullin 2013 | -N=57 (M=19.7) -N=41 (M=70.7) |
PAL (20 pairs to 80% criterion) | 12/24 hr, b/n-sub wake vs. sleep | -Yes -No |
-SWS (young) -Null (older) |
| Mander 2013b | -N=32 YA (M=20.7) -N=26 OA (M=73.4) |
PAL (120 pairs to 100%) | 10 hrs b/n-sub wake vs. sleep | -Yes -No |
Relative delta (+) |
| Rauchs 2008 | -N=14 (M=23.4) -N=14 (M=75.1) |
Study 15 words 3× or 5×); Stories | (sleep only) | Age reduction trend (ceiling) | Null effects |
| Aly 2010 | -N=10 (M=22.8) -N=12 (M=75.2) |
WMS stories; Personal memory | 12 hrs w/in-sub wake vs. sleep | -Yes (both tests) -Yes; Reduced |
N/A |
Sample refers only to the healthy (control) group. PSG correlations with overnight retention are listed after adjusting for baseline, chronological age (Age), & experimental group (Exp). Sleep-related benefits are reported relative to wake-control conditions. Papers are sorted by those with a single group versus multiple age groups.
Sample Abbreviations. MA: middle-aged (30–60); OA: older-aged (>60); YA: younger-aged (18–30).
Retention Interval Abbreviations. Depr; deprivation; Reb: rebound. AChE-I: Acetylcholinesterase Inhibitor Donepezil.
Memory Task Abbreviations. DRM: Deese-Roediger-McDermott lists; PAL: paired associate word learning; VVM: visual and verbal memory task; WMS: Wechsler Memory Scale
Procedural Memory Consolidation
In young adults, consolidation of procedural memories is most often linked to sleep spindles and REM sleep (Plihal & Born, 1997; Walker, 2009). These aspects of sleep change in quantity and quality across the lifespan (e.g., Martin et al., 2013; Ohayon et al., 2004). Therefore, one would predict less procedural memory consolidation during sleep in older adults.
One of the largest experimental studies on procedural memory consolidation in older adults, compared REM fragmentation, non-REM fragmentation, REM rebound (i.e., increased REM sleep following a night of REM deprivation), and pharmacologically-enhanced sleep (cholinesterase inhibitor) conditions to a placebo, normal-sleep control group (Hornung, Regen, Danker-Hopfe, Schredl, & Heuser, 2007). The major finding was that only the cholinergic medication accelerated overnight improvements in the procedural memory task. The characteristics of REM sleep certainly differed across experimental conditions (e.g., REM density), but it was still surprising that relative to the control condition, the sleep fragmentation groups did not affect procedural memory consolidation.
The early conclusion that “REM sleep does not critically affect procedural memory consolidation in old age” (p. 755; Hornung et al., 2007) has been generally supported by subsequent research. Consider Table 7. Whereas at least 10 studies in middle-aged adults showed behavioral evidence for overnight procedural memory improvements (but perhaps with an age-related decline; Dresler, Kluge, Genzel, Schussler, & Steiger, 2010; Roig, Ritterband-Rosenbaum, Lundbye-Jensen, & Nielsen, 2014; Wilson, Baran, Pace-Schott, Ivry, & Spencer, 2012), no sleep-specific improvements were observed in 7 of the 8 studies that experimentally contrasted sleep and wake retention intervals in middle-to-older adult groups. The studies that suggested relatively preserved procedural memory consolidation with aging either mixed wake and sleep over long time intervals or suggested that sleep-related benefits emerge only after repeated testing (i.e., “late/plateau” improvements; e.g., Tucker, McKinley, & Stickgold, 2011).
Current research focuses on how procedural memory consolidation changes with aging. One possibility is that procedural memory consolidation in older adults occurs equally across both wake and sleep intervals (Nemeth et al., 2010; 2013; Pace-Schott & Spencer, 2013). Though young adults are expected to display some consolidation across wake intervals (e.g., Dewar, Alber, Butler, Cowan, & Della Sala, 2012), the absence of additional benefits from sleep might reflect a failure of the sleeping brain to “replay” the procedural memory (cf. animal models; Gerrard et al., 2008). Supportive evidence in humans arises from two studies in which young and older adults were trained on a motor skill and then slept. In one study that used neuroimaging (Fogel et al., 2014), young adults showed post-training activation in frontal, parietal, and hippocampal regions during sleep; these patterns were not observed in older adults. In another study (Peters, Ray, Smith, & Smith, 2008), young adults demonstrated a significant boost in sleep spindle density following motor skill training, which is a reproducible finding (in young adults; Fogel & Smith, 2011). By contrast, older adults did not demonstrate training-related increases in spindle density, but interestingly, they showed a significant increase in SWS quantity. SWS might therefore act as a compensatory mechanism in older adults, but additional work on this intriguing hypothesis is required because SWS did not correlate with memory measures (K. Peters, personal communication, March 6, 2013).
Episodic Memory Consolidation
Episodic memory refers to explicit memories for events, and is often distinguished from other forms of declarative memory such as semantic (general knowledge) memory (Tulving, 1972; 2002). In young adults, episodic memory consolidation has most often been linked to SWS (Diekelmann & Born, 2010) or to spindles (Fogel & Smith, 2011), both features of sleep that change in quantity and quality with aging (Carrier et al., 2011; Ehlers & Kupfer, 1989; Martin et al., 2013). Table 8 illustrates that most (aging) studies using young and middle-aged adults provided some behavioral evidence for sleep-dependent episodic memory consolidation. In older groups, one well-designed study suggested preserved sleep-dependent episodic memory consolidation (Wilson et al., 2012) whereas at least five other studies indicated some age-related impairments for at least some episodic memory tests (Table 8). With few exceptions, these studies used simple verbal learning procedures rather than employing experimental psychology manipulations such as emotional memory trade-off, directed forgetting, or contextual prospective memory procedures, methodologies which have been critical to convincingly demonstrating sleep-dependent memory consolidation in young adults (Appendix). Furthermore, these studies have generally not evaluated possible neurobiological, cardiovascular, or endocrine moderators of consolidation (but see Mander et al., 2013b), which when identified may explain variability across studies (cf. Carlson et al., 2011).
Identifying the PSG correlate(s) of episodic memory consolidation in aging adults has been challenging, perhaps because most work showed no behavioral evidence for consolidation in older adults (i.e., if there is no memory consolidation, then there should be no PSG correlate of the memory consolidation measure). Nevertheless, some work has connected overnight retention of episodic memories to spindle density (Seeck-Hirschner et al., 2012) and ability to maintain sleep (i.e., few awakenings; Conte, Carobbi, Errico, & Ficca, 2012; Mazzoni et al., 1999). Others have failed to observe correlations with spindles (and sigma spectral power; Hornung et al., 2007, 2009; C. Westerberg, personal communication, July 22, 2013) or reported a positive correlation between retention and time awake at night (Seeck-Hirschner et al., 2012).
Much work on episodic memory consolidation and aging has focused on the role of SWS. Table 8 indicates that in some middle-aged adult samples that memory consolidation correlated with SWS duration (Backhaus et al., 2006, 2007; Deak, Stickgold, Pietras, Nelson, & Bubrick, 2011). The SWS picture is still murky in older adults: SWS duration is typically not associated with overnight retention of episodic memories, but some have reported positive correlations with delta spectral power, which quantitatively blends the incidence and amplitude of slow waves (Mander et al., 2013b; Westerberg et al., 2012). These positive findings notwithstanding, we were again surprised to see that three studies have observed negative correlations between SWS duration and retention of veridical memories (Mazzoni et al., 1999; Seeck-Hirschner et al., 2012) and lure (false memory) recognition (Lo et al., 2014b). Perhaps this literature can be cohesively explained by drawing a distinction between SWS quantity and SWS quality (operationalized as delta spectral power), but before drawing this conclusion we encourage researchers to consider the following evidence: a) delta power during wakefulness is associated with cognitive performance in older adults (Vlahou et al., 2014), b) overnight word pair retention was unaffected in older adults when Non-REM sleep was experimentally fragmented (Hornung et al., 2007), and c) overnight episodic memory retention was not affected by pharmacologically increasing delta power in SWS (Hornung et al., 2009).
Summary, Critique, and Future Research Directions
The burgeoning memory consolidation and aging literature is still developing, and therefore a few methodological weaknesses remain to be addressed. The reliance on small sample sizes and the absence of young-adult and wake-only control conditions are frequent limitations. We also must carefully consider the inferences drawn from studies that reported significant correlations between PSG and memory variables but demonstrated no experimental benefit of sleep (relative to wake). Correlating PSG variables with memory performance regardless of experimental effects is a very common practice (e.g., Scullin, 2013), but should we interpret such correlations as sleep-specific effects? Cognitive measures correlate with delta and theta spectral power during wakefulness in older adults (Finnigan & Robertson, 2011; Vlahou et al., 2014), and therefore, we caution against interpreting PSG—memory correlations as evidence for sleep-dependent memory consolidation in the absence of converging experimental evidence for consolidation.
Despite some limitations, several interesting themes are emerging in the aging and memory consolidation field. Middle-aged adults tend to demonstrate somewhat-dampened evidence for episodic and procedural sleep-dependent memory consolidation. In many (if not most) cases, older adults show reduced (or no) evidence for overnight memory consolidation. To the extent that memory consolidation forms the building blocks of optimal cognitive functioning (e.g., Mazzoni et al., 1999), the loss of sleep-dependent memory consolidation might accelerate cognitive aging and result in weaker sleep— cognition associations.
We were again surprised to see multiple reports of negative correlations between memory and SWS quantity (Lo, Sim, & Chee, 2014; Mazzoni et al., 1999; Seeck-Hirschner et al., 2012; refer back to Section C: Bastien et al., 2003; Buechel et al., 2011; Feinberg et al., 1967; Platt et al., 2011; Scullin, 2013; Spiegel, 1981), though some studies did not show a concomitant experimental sleep effect. One interpretation of negative SWS—cognition correlations is “overactive” synaptic downscaling (Table 1), whereby too much SWS might prune synapses that would otherwise support memory in older adults (Scullin, 2013; cf. Chang et al., 2006). Another interpretation is that slow EEG activity represents pathologic neural activity, as suggested by waking EEG data in severe cognitive disorders (Fernandez et al., 2002). Another conceivable alternative is that because SWS duration is negatively associated with REM duration, the negative correlations might potentially reflect a compensatory role for REM sleep in episodic memory consolidation in older adults (cf. Table 5). A fourth interesting possibility is that negative SWS correlations might indicate more gist-like memory processing (e.g., Payne et al., 2009). According to this view, the negative SWS correlations may reflect the tendency for older adults to engage in gist-based rather than detail-based remembering (Adams, 1991; Castel, McGillivray, & Friedman, 2012).
It is important to recognize that not all SWS is created equal (fragmented SWS, low-density SWS, etc.). Understanding the individual and contextual conditions supporting memory-promoting SWS should therefore be a priority for future research on aging. For example, memory consolidation in older adults might depend on initial learning strength (Tucker & Fishbein, 2008), retrieval efficiency (Bizzozero et al., 2008), anxiety or arousal levels (Nielson, Wulff, & Arentsen, 2014; Platano, Fattoretti, Balietti, Bertoni-Freddari, & Aicardi, 2008), prefrontal cortex and hippocampal connectivity (Mander et al., 2013b), cerebral oxygenation (Carlson et al., 2011), cholinergic (e.g., Schredl, Weber, Leins, & Heuser, 2001) and/or dopaminergic modulation (Chowdhury, Guitart-Masip, Bunzeck, Dolan, & Duzel, 2012; Scullin, Trotti, Wilson, Greer, & Bliwise, 2012), but there are likely many other influential factors.
Nocturnal Sleep Interventions for Cognition
An important final question is whether interventions that improve the quantity and/or quality of overnight sleep also improve cognitive functioning (cf. napping). One approach to improving overnight sleep is to extend total time in bed or adjust sleep/nighttime habits (e.g., by routinizing bedtimes). These behavior-based sleep extension manipulations have been linked to positive cognitive outcomes in adolescents, young adults, and middle-aged adults (e.g., Dewald-Kaufmann, Oort, & Meijer, 2013; Lucassen et al., 2014; Mah, Mah, Kezirian, & Dement, 2011; but see Sadeh, Gruber, & Raviv, 2003). One study is suggestive of a similar benefit in healthy older adults (Klerman & Dijk, 2008), but this study did not control for practice effects (Shipstead, Redick, & Engle, 2012). In a similar vein, psychological therapies such as cognitive-behavioral therapy, can be applied to treat insomnia in older adults (Morin, Culbert, & Schwartz, 1994), but such clinical trials typically have not included cognitive performance or memory consolidation as an outcome (cf. Altena, Van Der Werf, Strijers, & Van Someren, 2008; Haimov & Shatil, 2013; Sun, Kang, Wang, & Zeng, 2013).
Another approach is to bolster sleep-dependent memory consolidation, for example, via targeted memory reactivation (Oudiette & Paller, 2013). One method for reactivating memories during sleep is to wait until the participant is in SWS and then play sounds over a speaker that were previously encoded during word learning, such as a “meow” sound if the word “cat” was previously studied (Rudoy, Voss, Westerberg, & Paller, 2009). Enhanced memory consolidation is inferred when recall is greater for cued words than for non-cued (counterbalanced) words. This procedure was successfully applied to increase memory consolidation in a small sample that included middle-aged adults (Fuentemilla et al., 2013), but no such study has been published in older adults. The only published attempt at increasing SWS-dependent episodic memory consolidation in older adults used transcranial current stimulation (Eggert et al., 2013), which when applied to young adults increases SWS density and episodic memory consolidation (Marshall, Molle, Hallschmid, & Born, 2004). However, in older adults, the stimulation neither benefited SWS nor affected memory consolidation.
A fascinating, but controversial question is whether pharmacologically impairing or improving sleep impacts cognitive functioning. Antidepressants have been associated with REM suppression and their (lack of) impact on cognitive function was the subject of much debate a decade ago (Vertes, 2004; Walker & Stickgold, 2004; for a potential resolution, see Dresler, Kluge, Genzel, Schussler, & Steiger, 2010, and Goerke, Cohrs, Rodenbeck, & Kunz, 2014). Furthermore, there is a paradox that sleep hypnotics might improve memory consolidation (e.g., Mednick et al., 2013; but see Hall-Porter, Schweitzer, Eisenstein, Ahmed, & Walsh, 2013), while also causing anterograde amnesia or psychomotor slowing in young adults. Though clearly a complex and controversial area (Vermeeren & Coenen, 2011), we can currently ask whether common sleep medications demonstrate any benefits to memory consolidation or cognitive performance in older adults after the hypnotic effect of the medication has presumably dissipated.
In young adults, zolpidem (Ambien®) increases sleep spindles (Feinberg, Maloney, & Campbell, 2000) and possibly episodic memory consolidation (Mednick et al., 2013). Research is inconclusive regarding zolpidem’s effect on delta spectral power (for review, see Monti, Spence, Pandi-Perumal, Langer, & Hardeland, 2009). In older adults, zolpidem can improve sleep continuity, REM sleep, and SWS duration (e.g., Kummer et al., 1993), but it does not improve next-day memory or cognitive performance (Allain, Bentué-Ferrer, Tarral, & Gandon, 2003; Fairweather, Kerr, & Hindmarch, 1992; Hindmarch, Legangneux, Stanley, Emegbo, & Dawson, 2006; Otmani et al., 2008; Scharf, Mayleben, Kaffeman, & Krall, 1991). Similar null effects (or even reserved direction effects) in older adults have been reported with eszopiclone (Lunesta®; Hemmeter, Müller, Bischof, Annen, & Holsboer-Trachsler, 2000; Leufkens & Vermeeren, 2009).
Pharmacological studies that specifically targeted SWS (tiagabine, sodium oxybate, gaboxodol) produced results consistent with age-modification of SWS—cognition associations. These SWS medications improved cognitive performance in young and middle-aged adults (Walsh et al., 2006, 2010), but did not impact episodic memory and working memory in healthy older adults, even when delta spectral power was enhanced (Mathias et al., 2005; also see Baker & Vitiello, 2013; Benedict, Chapman, & Schiöth, 2013). Finally, controversy surrounds whether melatonin is a “true” hypnotic (van den Heuvel, Ferguson, Mila Macchi, & Dawson, 2005; Zhdanova, 2005), but there is interesting pilot data that melatonin may benefit cognition in healthy older adults (Peck, LeGoff, Ahmed, & Goebert, 2004) and Alzheimer’s disease patients (Cardinali, Furio, & Brusco, 2010).
Summary, Critique, and Future Research Directions
The extent to which increasing memory consolidation on a nightly basis could ameliorate cognitive declines and/or (re)strengthen sleep—cognition associations is an exciting avenue for further research. We expect this line of work to be challenging because the external stimulation techniques used to reactivate memories in young adults (e.g., playing a “meow” sound for the studied word “cat”) might cause disruptions to sleep in older adults because older adults are more likely to awaken in response to auditory stimuli (even in SWS; Zepelin, McDonald, & Zammit, 1984). Age-related neurobiological changes such as decreased hippocampal—neocortical connectivity (Grady, McIntosh, & Craik, 2003) and reduced brain derived neurotrophic factor (Calabrese, Guidotti, Racagni, & Riva, 2013) may also constitute significant barriers to increasing memory consolidation in older adults.
Current attempts at pharmacologically-enhancing sleep, which produce some positive outcomes in young adults, have nearly uniformly failed to improve memory and cognition in older adults. The null findings do not seem to be attributable only to lingering hypnotic effects or to failures to augment SWS duration or delta spectral power. These experimental studies might indicate that sleep physiology (e.g., SWS, sleep continuity) is not causally related to cognitive functioning in older adults; however, this literature still needs to evaluate whether sleep medications do not improve memory because they are not enhancing spindles in the “fast” spindle frequency range (Fogel & Smith, 2011) and whether melatonin can benefit cognitive functioning via improved sleep quality.
The most successful intervention for improving cognitive functioning in aging adults might combine treatments that target not only sleep, but also psychological health, the endocrine system, and exercise (among others; Benloucif et al., 2004; Dzierzewski et al., 2014; Horne, 2013; Naylor et al., 2000; Snigdha, de Rivera, Milgram, & Cotman, 2014; Tanaka & Shirakawa, 2004). Importantly, even if sleep interventions ultimately have no impact on memory in older adults, improving sleep may still have several non-cognitive benefits including improved quality of life (Arakawa, Tanaka, Toguchi, Shirakawa, & Taira, 2002; Faubel et al., 2009; Reid et al., 2010), mental health (Driscoll et al., 2008; Tanaka et al., 2001; 2002), and longevity (Dew et al., 2003; Marin, Carrizo, Vicente, & Agusti, 2005). Sleeping ~250,000 hours across the lifespan is time well-spent!
Conclusions and Interpretations
Nearly half a century of research has investigated sleep—cognition associations in normal aging (Feinberg et al., 1967). One of the most exciting trends in the self-report literature is that poor sleep in middle-age is linked to neurodegeneration-related biomarkers (e.g., amyloid deposition) and subsequent cognitive decline. Similar findings emerge when using movement-based actigraphy and EEG-based polysomnography, but the studies in these literatures often mixed healthy older adults with patient groups. Furthermore, healthy older adults did not always demonstrate clear associations between sleep and cognitive functioning.
In the experimental literatures, several thought-provoking themes have materialized: Sleep deprivation causes greater cognitive impairments in young adults than in older adults, sleep promotes memory consolidation in young adults more so than in older adults, and napping and enhancing nocturnal sleep benefit cognitive functioning in young and middle-aged adults but often not in older adults. Thus, the seven literatures reviewed herein indicate that in older adults inter- and intra-variability in sleep often do not relate to cognitive functioning, and solely improving sleep may not reverse cognitive impairments. We will consider five perspectives that might account for these findings.
The first explanation is that sleep does not relate to cognitive functions—in particular to memory consolidation—even in young adults (Rickard et al., 2008; Siegel, 2001; Vertes, 2004). Therefore, memory consolidation (and trait-like cognition) should not be associated with sleep in older adults. Though we acknowledge that memory consolidation studies sometimes suffer methodological weaknesses such as small sample sizes, analytical missteps, and inflated effect sizes (Button et al., 2013), the quantity and breadth of evidence for a role for sleep in memory consolidation in young adults is very strong (see Appendix and Rasch & Born, 2013). The mechanisms underlying this relationship, however, are still a matter of lively debate (Table 1).
A second explanation is that all sleep—cognition findings in older adults are complementary at a holistic level, and point to specific, preserved associations between particular cognitive domains and particular aspects of sleep quality. For example, one might only predict an association between SWS quality and episodic memory. The null findings in these 7 literatures may therefore be due to misguided selection of cognitive tests, imprecise measurements of cognitive functioning (e.g., MMSE), and/or inadequate measurement and analysis of sleep (self-report versus PSG, sleep quantity versus quality, etc.).
The two above explanations might be considered opposite ends of a spectrum ranging from no cognitive association with sleep per se in older adults to a wholly preserved association that is masked by measurement. We are inclined to believe that the truth lies somewhere in between. If we assume that sleep and cognitive functions are associated in young adults (Appendix), then the 7 sleep, cognition, and aging literatures we reviewed might indicate that there is an age-related change in sleep—cognition associations. The next three perspectives—reduced sleep need, compensation, and functional weakening—consider the possible nature of this change.
One provocative idea is that we need sleep less as we age. Rather than lamenting the loss of, for example, SWS with increasing age some researchers argue that SWS declines simply because it is a remnant of maturational processes from earlier in life (Feinberg, 2000). Or, if degree of daytime learning dictates amount of SWS (rather than vice versa; see synaptic downscaling theory, Table 1), and if learning tendency or ability decreases with aging, then the need for SWS would decrease as a natural consequence (Cirelli, 2012). The “sleep need” view is provocative, but has been difficult to test (e.g., Bliwise, 2000).
Assuming similarity in “sleep need” with increasing age, a fourth possibility draws upon the compensation theory of neurocognitive aging (e.g., Park & Reuter-Lorenz, 2009). By this view, specific cognitive functions (e.g., procedural memory) that were once supported by specific aspects of sleep (e.g., REM) might receive additional support (or become dedifferentiated) from another aspect of sleep (e.g., SWS; cf. Peters et al., 2008). Consider also that in young adults sleep preferentially supports memory consolidation, but consolidation can still occur in the wake state (e.g., Carr, Jadhav, & Frank, 2011; Dewar et al., 2012). Thus, it is possible that in response to age-related changes in sleep physiology, older adults’ brains compensate by increasing the frequency of wake-based memory consolidation (e.g., Nemeth et al., 2013; Pace-Schott & Spencer, 2013; Salas et al., 2014).
A fifth framework is that the sleep—cognition link grows less resilient as we age because the aging brain can no longer efficiently support sleep-specific cognitive processes. Several researchers have noted this “functional weakening” possibility (Edinger et al., 2000; Kronholm, 2012; McCrae et al., 2012; Pace-Schott & Spencer, 2011; Schmidt, Peigneux, & Cajochen, 2012; Scullin, 2013; Spiegel et al., 1986), and some supportive evidence was observed in most of the reviewed literatures. Though functional weakening remains relatively unexplored, this view uniquely predicts that increasing the quantity of sleep in older adults is unlikely to restore cognitive abilities because age-related neurobiological changes (e.g., neural atrophy, decreased plasticity, nocturnal hypoxia, neuroendocrine changes, altered neuromodulation) that are presumably necessary to support sleep-specific cognitive processes will still be impaired. In the context of episodic memory consolidation, the widespread age-related neurobiological changes may cause SWS to no longer be “memory promoting” even when SWS density and amplitude are relatively preserved. Consider functional weakening within the context of the systems consolidation view (Table 1) that memories are reactivated in the hippocampus and transferred to the neocortex during SWS: If the hippocampus, neocortex, or hippocampal—neocortical connections are greatly impaired (Grady, 2012), then hippocampal—neocortical consolidation will not occur regardless of the quantity of SWS.
A particularly intriguing possibility is that sleep disturbances per se cause changes in sleep’s cognitive functions across the lifespan (e.g., prompting compensation attempts and/or weakening sleep—cognition links). Acute sleep deprivation and chronic sleep restriction have diverse effects on allostatic load including increases in blood pressure, evening cortisol levels, insulin, proinflammatory cytokines, and sympathetic tone (McEwen, 2006; Vgontzas et al., 2004), all which are hypothesized to accelerate cognitive aging (McEwen & Sapolsky, 1995). Furthermore, sleep deprivation/restriction in young animals can cause protein misfolding (Naidoo, Ferber, Master, Zhu, & Pack, 2008), decreased tau phosphorylation (Di Meco, Joshi, & Pratico, 2014; Rothman, Herdener, Frankola, Mughal, & Mattson, 2013), and increased amyloid deposition (Kang et al., 2009; Xie et al., 2013), which impair memory consolidation (e.g., Borlikova et al., 2013; Freir et al., 2011) and underpin Alzheimer’s disease (Hardy & Selkoe, 2002). If these effects accrue “for years in a silent but irreversible manner” (Hita-Yanez et al., 2012, p. 293), then sleep disturbances in young and middle-age could be at the root of subsequent cognitive decline, even when no sleep—cognition associations are observed in old age.
Acknowledgments
We are grateful for the generosity of the following scientists who shared unpublished data or helpful comments during the preparation of this paper: Mariam Aly, Julián Benito-León, Carolina Campanella, Jose Cantero, Joe Dzierzewski, Gil Einstein, Irwin Feinberg, Alyssa Gamaldo, Tyler Harrison, Orla Hornung, Nicole Lovato, Christoph Nissen, Kevin Peters, Susan Redline, Quentin Regestein, Jill Shelton, Katie Siengsukon, Rebecca Spencer, René Spiegel, and Carmen Westerberg.
Funding
This work was supported in part by the National Institutes of Health (R01NS050595, F32AG041543).
Appendix
Sample List of Cognitive Tests and Experimental Paradigms Implicated in Sleep and Consolidation Studies.
| Cognitive Test | Reference | Cognitive Test | Reference |
|---|---|---|---|
| Procedural/Perceptual Memory | Episodic Memory | ||
| Visual discrimination | Karni et al., 1994 | Nonsense syllables | Jenkins & Dallenbach, 1924 |
| Mirror-tracing task | Plihal & Born, 1997 | Word associates | Ekstrand, 1967 |
| Semantic priming | Stickgold et al. 1999 | Emotional and neutral texts | Wagner et al., 2001 |
| Finger tapping | Walker et al., 2002a | Virtual maze | Peigneux et al., 2004 |
| Opposition task | Fischer et al., 2002 | Face-name pairs | Clemens et al., 2005 |
| Visuomotor adaptation | Huber et al., 2004 | Remember/know judgment | Hu et al., 2006 |
| Implicit tone structure | Durrant et al., 2011 | Object locations | Rasch et al., 2007 |
| Melody production | Antony et al., 2012 | Face locations | Talamini et al., 2008 |
| Emotional scenes | Payne et al., 2008 | ||
| Creativity and Insight | DRM lists | Fenn et al., 2009 | |
| Anagrams | Walker et al., 2002b | Prospective memory | Scullin & McDaniel, 2010 |
| Number reduction | Wagner et al., 2004 | Retrieval induced forgetting | Racsmány et al., 2010 |
| Transitive inference | Ellenbogen et al., 2007 | Dream content | Wamsley et al., 2010 |
| Remote associates task | Cai et al., 2009 | Microeconomics lecture | Scullin et al., 2011 |
| Unusual uses task | Ritter et al., 2012 | Directed forgetting | Rauchs et al., 2011 |
| IAPS pictures | Baran et al., 2012 | ||
| Working Memory and Updating | Cartoons | Chambers et al., 2013 | |
| N-back | Kuriyama et al., 2008 | Testing effect | Bäuml et al., 2014 |
| Digit span | Scullin et al., 2012 | ||
| Language Acquisition | |||
| Animal Learning | French class | De Koninck et al., 1989 | |
| Inhibitory avoidance | Fishbein, 1971 | Phonemes | Fenn et al., 2003 |
| Vocal learning | Dave et al., 2000 | Abstraction | Gómez et al., 2006 |
| Courtship conditioning | Donlea et al., 2011 | Vocabulary | Gais et al., 2006 |
| Location of food reward | Martin-Ordas et al., 2011 | Lexical competition | Dumay et al., 2007 |
| Auditory classification | Brawn et al., 2013 | Storybooks | Williams et al., 2014 |
References are sorted chronologically within cognitive domains.
Abbreviations. DRM: Deese-Roediger-McDermott; IAPS: International Affective Picture System
References
- Adam K, Oswald I. Sleep is for tissue restoration. Journal of the Royal College of Physicians of London. 1977;11:376–388. [PMC free article] [PubMed] [Google Scholar]
- Adam AM, Potvin O, Callahan BL, Bastien C, Lorrain D, Desjardins S, Hudon C. Subjective sleep quality in non-demented older adults with and without cognitive impairment. International Journal of Geriatric Psychiatry. 2014 doi: 10.1002/gps.4087. [DOI] [PubMed] [Google Scholar]
- Adam M, Retey JV, Khatami R, Landolt H-P. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep. 2006;29:55–57. doi: 10.1093/sleep/29.1.55. [DOI] [PubMed] [Google Scholar]
- Adams C. Qualitative age differences in memory for text: A life-span developmental perspective. Psychology and Aging. 1991;6:323–336. doi: 10.1037//0882-7974.6.3.323. [DOI] [PubMed] [Google Scholar]
- Allain H, Bentué-Ferrer D, Tarral A, Gandon JM. Effects on postural oscillation and memory functions of a single dose of zolpidem 5 mg, zopiclone 3.75 mg and lormetazepam 1 mg in elderly healthy subjects. A randomized, cross-over, double-blind study versus placebo. European Journal of Clinical Pharmacology. 2003;59:179–188. doi: 10.1007/s00228-003-0591-5. [DOI] [PubMed] [Google Scholar]
- Allen SR, Seiler WO, Stahelin HB, Spiegel R. Seventy-two hour polygraphic and behavioral recordings of wakefulness and sleep in a hospital geriatric unit: comparison between demented and nondemented patients. Sleep. 1987;10:143–159. doi: 10.1093/sleep/10.2.143. [DOI] [PubMed] [Google Scholar]
- Altena E, Ramautar JR, Van Der Werf YD, Van Someren EJ. Do sleep complaints contribute to age-related cognitive decline? Progress in Brain Research. 2010;185:181–205. doi: 10.1016/B978-0-444-53702-7.00011-7. [DOI] [PubMed] [Google Scholar]
- Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: Effects of chronic insomnia and sleep therapy. Journal of Sleep Research. 2008;17:335–343. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010;18:327–334. doi: 10.1080/09658211003601548. [DOI] [PubMed] [Google Scholar]
- Amer MS, Hamza SA, El Akkad RM, Abdel Galeel YI. Does self-reported sleep quality predict poor cognitive performance among elderly living in elderly homes? Aging & Mental Health. 2013;17:788–792. doi: 10.1080/13607863.2013.790930. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal cortex: Links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40:349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nature Neuroscience. 2012;15:1114–1116. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M, Tanaka H, Toguchi H, Shirakawa S, Taira K. Comparative study on sleep health and lifestyle of the elderly in the urban areas and suburbs of Okinawa. Psychiatry and Clinical Neurosciences. 2002;56:245–246. doi: 10.1046/j.1440-1819.2002.01015.x. [DOI] [PubMed] [Google Scholar]
- Asada T, Motonaga T, Yamagata Z, Uno M, Takahashi K. Associations between retrospectively recalled napping behavior and later development of Alzheimer's disease: association with APOE genotypes. Sleep. 2000;23:629–634. [PubMed] [Google Scholar]
- Auyeung TW, Lee JSW, Leung J, Kwok T, Leung PC, Woo J, Wing YK. Cognitive deficit is associated with phase advance of sleep–wake rhythm, daily napping, and prolonged sleep duration—a cross-sectional study in 2,947 community-dwelling older adults. Age. 2013;35:479–486. doi: 10.1007/s11357-011-9366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learning & Memory. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biological Psychiatry. 2006;60:1324–1330. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- Baker LD, Vitiello MV. Growth hormone–releasing hormone improves cognitive function in older adults: Sleep on it—Reply. JAMA Neurology. 2013;70:529–530. doi: 10.1001/jamaneurol.2013.2064. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society. 2006;12:896–900. doi: 10.1017/S1355617706061078. DOI: http://dx.doi.org/10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. Journal of Neuroscience. 2012;32:1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien CH, Fortier-Brochu É, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia: Relationship between objective and subjective measures. Journal of Psychosomatic Research. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. New England Journal of Medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauml K-HT, Holterman C, Abel M. Sleep can reduce the testing effect: It enhances recall of restudied items but can leave recall of retrieved items unaffected. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2014 doi: 10.1037/xlm0000025. [DOI] [PubMed] [Google Scholar]
- Benedict C, Chapman CD, Schioth HB. Growth hormone–releasing hormone improves cognitive function in older adults: Sleep on it. JAMA Neurology. 2013;70:529. doi: 10.1001/2013.jamaneurol.349. [DOI] [PubMed] [Google Scholar]
- Benitez A, Gunstad J. Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. The Clinical Neuropsychologist. 2012;26:214–223. doi: 10.1080/13854046.2012.658439. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: A prospective population-based study. European Journal of Neurology. 2009;16:990–997. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- Benito-León J, Louis ED, Bermejo-Pareja F. Cognitive decline in short and long sleepers: A prospective population-based study (NEDICES) Journal of Psychiatric Research. 2013;47:1998–2003. doi: 10.1016/j.jpsychires.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J, Zee PC. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;27:1542–1551. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- Berry DTR, Webb WB. Sleep and cognitive functions in normal older adults. Journal of Gerontology. 1985;40:331–335. doi: 10.1093/geronj/40.3.331. [DOI] [PubMed] [Google Scholar]
- Bizzozero I, Capitani E, Faglioni P, Lucchelli F, Saetti MC, Spinnler H. Recollection of public events in healthy people: A latent-variable stochastic approach to disentangling retrieval and storage. Cortex. 2008;44:150–160. doi: 10.1016/j.cortex.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Stone KL. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS Sleep Study. Sleep. 2011a;34:1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Stone KL. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: The Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society. 2011b;59:2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Stone KL. Poor sleep is associated with impaired cognitive function in older women: The study of osteoporotic fractures. Journal of Gerontology: Medical Sciences. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, Stone KL. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: The MrOS sleep study. Sleep. 2014;37:655–663. doi: 10.5665/sleep.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter K, Graw P, Münch M, Knoblauch V, Wirz-Justice A, Cajochen C. Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behavioural Brain Research. 2006;168:312–317. doi: 10.1016/j.bbr.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Bliese PD, Wesensten NJ, Balkin TJ. Age and individual variability in performance during sleep restriction. Journal of Sleep Research. 2006;15:376–385. doi: 10.1111/j.1365-2869.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Neuropsychological function and sleep. Clinics in Geriatric Medicine. 1989;5:381–394. [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3rd edition. Philadelphia: Saunders; 2000. pp. 26–42. [Google Scholar]
- Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th edition. Philadelphia: Saunders; 2011. pp. 27–41. [Google Scholar]
- Bliwise DL, Carskadon MA, Seidel WF, Nekich JC, Dement WC. MSLT-defined sleepiness and neuropsychological test performance do not correlate in the elderly. Neurobiology of Aging. 1991;12:463–468. doi: 10.1016/0197-4580(91)90074-t. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Foley DJ, Vitiello MV, Ansari FP, Ancoli-Israel S, Walsh JK. Nocturia and disturbed sleep in the elderly. Sleep Medicine. 2009;10:540–548. doi: 10.1016/j.sleep.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH. Recovery of performance during sleep following sleep deprivation n older normal and insomniac adult males. Perceptual and Motor Skills. 1985;60:323–334. doi: 10.2466/pms.1985.60.1.323. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiology of Aging. 1989;10:21–25. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Acute sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th edition. Philadelphia: Saunders; 2011. pp. 54–66. [Google Scholar]
- Bonnet MH, Rosa RR. Sleep and performance in young adults and older normals and insomniacs during acute sleep loss and recovery. Biological Psychology. 1987;25:153–172. doi: 10.1016/0301-0511(87)90035-4. [DOI] [PubMed] [Google Scholar]
- Borlikova GG, Trejo M, Mably AJ, Mc Donald JM, Sala Frigerio C, Regan CM, Walsh DM. Alzheimer brain-derived amyloid β-protein impairs synaptic remodeling and memory consolidation. Neurobiology of Aging. 2013;34:1315–1327. doi: 10.1016/j.neurobiolaging.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Srebro B. Synaptic plasticity in the hippocampus is modulated by behavioral state. Brain Research. 1989;493:74–86. doi: 10.1016/0006-8993(89)91001-9. [DOI] [PubMed] [Google Scholar]
- Brawn TP, Fenn KM, Nusbaum HC, Margoliash D. Consolidating the effects of waking and sleep on motor-sequence learning. Journal of Neuroscience. 2010;30:13977–13982. doi: 10.1523/JNEUROSCI.3295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn TP, Nusbaum HC, Margoliash D. Sleep consolidation of interfering auditory memories in starlings. Psychological Science. 2013;24:439–447. doi: 10.1177/0956797612457391. [DOI] [PubMed] [Google Scholar]
- Brendel DH, Reynolds CF, Jennings JR, Hoch CC, Monk TH, Berman SR, Kupfer DJ. Sleep stage physiology, mood, and vigilance responses to total sleep deprivation in healthy 80-year-olds and 20-year-olds. Psychophysiology. 1990;27:677–685. doi: 10.1111/j.1469-8986.1990.tb03193.x. [DOI] [PubMed] [Google Scholar]
- Brezinova V, Hort V, Vojtĕchovsky M. Sleep deprivation with respect to age: An EEG study. Activitas Nervosa Superior. 1969;11:182–187. [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS ONE. 2009;4:e6683. doi: 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Aloia MS. Sleep and cognition in older adults. Sleep Medicine Clinics. 2006;1:207–220. [Google Scholar]
- Buckley TM, Schatzberg AF. Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. American Journal of Geriatric Psychiatry. 2005;13:344–352. doi: 10.1176/appi.ajgp.13.5.344. [DOI] [PubMed] [Google Scholar]
- Buechel HM, Popovic J, Searcy JL, Porter NM, Thibault O, Blalock EM. Deep sleep and parietal cortex gene expression changes are related to cognitive deficits with age. PloS ONE. 2011;6:e18387. doi: 10.1371/journal.pone.0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner D. The blue zones: 9 lessons for living longer from the people who've lived the longest. National Geographic Books; 2012. [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Grunstein R, Horne J, Lavie P. Can an improvement in sleep positively impact on health? Sleep Medicine Reviews. 2010;14:405–410. doi: 10.1016/j.smrv.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Peyrache A. A BOLD statement about the hippocampal-neocortical dialogue. Trends in Cognitive Sciences. 2013;17:57–59. doi: 10.1016/j.tics.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Ageing. New York: Oxford Press; 2005. [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences. 2009;106:10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Guidotti G, Racagni G, Riva MA. Reduced neuroplasticity in aged rats: A role for the neurotrophin brain-derived neurotrophic factor. Neurobiology of Aging. 2013;34:2768–2776. doi: 10.1016/j.neurobiolaging.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. Journal of the American Geriatrics Society. 2005;53:48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Stanchina MD, Schlang JR, Murphy PJ. Effects of a month-long napping regimen in older individuals. Journal of the American Geriatrics Society. 2011;59:224–232. doi: 10.1111/j.1532-5415.2010.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Current Neuropharmacology. 2010;8:218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BW, Neelon VJ, Carlson JR, Hartman M, Bliwise DL. Cerebral oxygenation in wake and during sleep and its relationship to cognitive function in community-dwelling older adults without sleep disordered breathing. Journal of Gerontology: Medical Sciences. 2011;66:150–156. doi: 10.1093/gerona/glq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BW, Neelon VJ, Carlson JR, Hartman M, Dogra S. Exploratory analysis of cerebral oxygen reserves during sleep onset in older and younger adults. Journal of the American Geriatrics Society. 2008;56:914–919. doi: 10.1111/j.1532-5415.2008.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: A potential substrate for memory consolidation and retrieval. Nature Neuroscience. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Filipini D. Sleep slow wave changes during the middle years of life. European Journal of Neuroscience. 2011;33:758–766. doi: 10.1111/j.1460-9568.2010.07543.x. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Sleep loss in elderly volunteers. Sleep. 1985;8:207–221. doi: 10.1093/sleep/8.3.207. [DOI] [PubMed] [Google Scholar]
- Castel AD, McGillivray S, Friedman MC. Metamemory and memory efficiency in older adults. In: Naveh-Benjamin M, Ohta N, editors. Memory and Aging: Current Issues and Future Directions. New York, NY: Psychology Press; 2012. pp. 245–270. [Google Scholar]
- Chambers AM, Payne JD. Laugh yourself to sleep: Memory consolidation for humorous information. Experimental Brain Research. 2013:1–13. doi: 10.1007/s00221-013-3779-7. [DOI] [PubMed] [Google Scholar]
- Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, Huerta PT. AMPA receptor downscaling at the onset of Alzheimer’s disease pathology in double knockin mice. Proceedings of the National Academy of Sciences. 2006;103:3410–3415. doi: 10.1073/pnas.0507313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Quan H, Bi-Rong D, Yan Z. Association between sleep quality and cognitive impairment among Chinese nonagenarians/centenarians. Journal of Clinical Neurophysiology. 2012;29:250–255. doi: 10.1097/WNP.0b013e3182570f2e. [DOI] [PubMed] [Google Scholar]
- Cherdieu M, Reynaud E, Uhlrich J, Versace R, Mazza S. Does age worsen sleep-dependent memory consolidation? Journal of Sleep Research. 2014;23:53–60. doi: 10.1111/jsr.12100. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Düzel E. Dopamine modulates episodic memory persistence in old age. Journal of Neuroscience. 2012;32:14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolli C, Mazzetti M, Plazzi G. Sleep-dependent memory consolidation in patients with sleep disorders. Sleep Medicine Reviews. 2013;17:91–103. doi: 10.1016/j.smrv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Cirelli C. Brain plasticity, sleep and aging. Gerontology. 2012;58:441–445. doi: 10.1159/000336149. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cochrane A, Robertson IH, Coogan AN. Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: An actigraphic study. Journal of Neural Transmission. 2012;119:1233–1239. doi: 10.1007/s00702-012-0802-2. [DOI] [PubMed] [Google Scholar]
- Cole CS, Richards KC, Beck CC, Roberson PK, Lambert C, Furnish A, Tackett J. Relationships among disordered sleep and cognitive and functional status in nursing home residents. Research in Gerontological Nursing. 2009;2:183–191. doi: 10.3928/19404921-20090527-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte F, Carobbi G, Errico BM, Ficca G. The effects of pre-sleep learning on sleep continuity, stability, and organization in elderly individuals. Frontiers in Neurology. 2012;3:109. doi: 10.3389/fneur.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton C. Effects of afternoon rest on the performance of geriatric patients in a rehabilitation hospital: A pilot study. American Journal of Occupational Therapy. 1995;49:775–779. doi: 10.5014/ajot.49.8.775. [DOI] [PubMed] [Google Scholar]
- Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiology & Behavior. 1999;66:485–492. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. Journal of the American Geriatrics Society. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- Cross NE, Lagopoulos J, Duffy SL, Cockayne NL, Hickie IB, Lewis SJ, Naismith SL. Sleep quality in healthy older people: Relationship with 1H magnetic resonance spectroscopy markers of glial and neuronal integrity. Behavioral Neuroscience. 2013;127:803–801. doi: 10.1037/a0034154. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Deak MC, Stickgold R, Pietras AC, Nelson AP, Bubrick EJ. The role of sleep in forgetting in temporal lobe epilepsy: A pilot study. Epilepsy & Behavior. 2011;21:462–466. doi: 10.1016/j.yebeh.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dealberto MJ, Pajot N, Courbon D, Alperovitch A. Breathing disorders during sleep and cognitive performance in an older community sample: The EVA Study. Journal of the American Geriatrics Society. 1996;44:1287–1294. doi: 10.1111/j.1532-5415.1996.tb01397.x. [DOI] [PubMed] [Google Scholar]
- De Koninck J, Lorrain D, Christ G, Proulx G, Coulombe D. Intensive language learning and increases in rapid eye movement sleep: Evidence of a performance factor. International Journal of Psychophysiology. 1989;8:43–47. doi: 10.1016/0167-8760(89)90018-4. [DOI] [PubMed] [Google Scholar]
- de Souza L, Benedito-Silva AA, Pires MN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Reynolds CF. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension on sleep and cognitive performance in adolescents with chronic sleep reduction: An experimental study. Sleep Medicine. 2013;14:510–517. doi: 10.1016/j.sleep.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Dewar M, Alber J, Butler C, Cowan N, Della Sala S. Brief wakeful resting boosts new memories over the long term. Psychological Science. 2012;23:955–960. doi: 10.1177/0956797612441220. [DOI] [PubMed] [Google Scholar]
- Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, Schernhammer ES. Sleep duration in midlife and later life in relation to cognition. Journal of the American Geriatrics Society. 2014;62:1073–1081. doi: 10.1111/jgs.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Di Meco A, Joshi YB, Praticò D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer's disease with plaques and tangles. Neurobiology of Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Djonlagic I, Guo M, Matteis P, Carusona A, Stickgold R, Malhotra A. Untreated sleep-disordered breathing: Links to aging-related decline in sleep-dependent memory consolidation. PLoS ONE. 2014;9:e85918. doi: 10.1371/journal.pone.0085918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science. 2011;332:1571–1576. doi: 10.1126/science.1202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty BA, Doty LA. Effect of age and chlorpromazine on memory consolidation. Journal of Comparative and Physiological Psychology. 1964;57:331–334. doi: 10.1037/h0047580. [DOI] [PubMed] [Google Scholar]
- Dresler M, Kluge M, Genzel L, Schussler P, Steiger A. Impaired off-line memory consolidation in depression. European Neuropsychopharmacology. 2010;20:553–561. doi: 10.1016/j.euroneuro.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Driscoll HC, Serody L, Patrick S, Maurer J, Bensasi S, Houck PR, Reynolds CF., III Sleeping well, aging well: A descriptive and cross-sectional study of sleep in “successful agers” 75 and older. American Journal of Geriatric Psychiatry. 2008;16:74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. Journal of the American Geriatrics Society. 2009;57:1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumay N, Gaskell MG. Sleep-associated changes in the mental representation of spoken words. Psychological Science. 2007;18:35–39. doi: 10.1111/j.1467-9280.2007.01845.x. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Durrant SJ, Taylor C, Cairney S, Lewis PA. Sleep-dependent consolidation of statistical learning. Neuropsychologia. 2011;49:1322–1331. doi: 10.1016/j.neuropsychologia.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Dykierek P, Stadtmuller G, Schramma P, Bahro M, Van Calker D, Braus DF, Riemann D. The value of REM sleep parameters in differentiating Alzheimer's disease from old-age depression and normal aging. Journal of Psychiatric Research. 1998;32:1–9. doi: 10.1016/s0022-3956(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Dzierzewski JM, Buman MP, Giacobbi PR, Roberts BL, Aiken-Morgan AT, Marsiske M, McCrae CS. Exercise and sleep in community-dwelling older adults: Evidence for a reciprocal relationship. Journal of Sleep Research. 2014;23:61–68. doi: 10.1111/jsr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Glenn DM, Bastian LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: Findings among middle-aged adults with and without insomnia complaints. Physiology & Behavior. 2000;70:127–134. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimulation. 2013;6:938–945. doi: 10.1016/j.brs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ. Effects of age on delta and REM sleep parameters. Electroencephalography and Clinical Neurophysiology. 1989;72:118–125. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Payne JD, Stickgold R. The role of sleep in declarative memory consolidation: Passive, permissive, active or none? Current Opinion in Neurobiology. 2006;16:716–722. doi: 10.1016/j.conb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Engel P. Does poor sleep quality in late life compromise cognition and accelerate progression of the degenerative dementias? In: McNamara P, editor. Dementia. Vol. 1. 2011. pp. 177–200. [Google Scholar]
- Ekstrand BR. Effect of sleep on memory. Journal of Experimental Psychology. 1967;75:64–72. doi: 10.1037/h0024907. [DOI] [PubMed] [Google Scholar]
- Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. European Journal of Clinical Pharmacology. 1992;43:597–601. doi: 10.1007/BF02284957. [DOI] [PubMed] [Google Scholar]
- Faubel R, López-GarcÍa E, Guallar-Castillón P, Graciani A, Banegas JR, Rodríguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. Journal of Sleep Research. 2009;18:427–435. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Slow wave sleep and release of growth hormone. JAMA. 2000;284:2717–2718. [PubMed] [Google Scholar]
- Feinberg I, Evarts EV. Changing concepts of the function of sleep: Discovery of intense brain activity during sleep calls for revision of hypotheses as to its function. Biological Psychiatry. 1969;1:331–348. [PubMed] [Google Scholar]
- Feinberg I, Maloney T, Campbell IG. Effects of hypnotics on the sleep EEG of healthy young adults: New data and psychopharmacologic implications. Journal of Psychiatric Research. 2000;34:423–438. doi: 10.1016/s0022-3956(00)00038-8. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Koresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. Journal of Psychiatric Research. 1967;5:107–144. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- Fenn KM, Nusbaum HC, Margoliash D. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- Fenn KM, Gallo DA, Margoliash D, Roediger HL, Nusbaum HC. Reduced false memory after sleep. Learning & Memory. 2009;16:509–513. doi: 10.1101/lm.1500808. [DOI] [PubMed] [Google Scholar]
- Fernández A, Maestú F, Amo C, Gil P, Fehr T, Wienbruch C, Ortiz T. Focal temporoparietal slow activity in Alzheimer’s disease revealed by magnetoencephalography. Biological Psychiatry. 2002;52:764–770. doi: 10.1016/s0006-3223(02)01366-5. [DOI] [PubMed] [Google Scholar]
- Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A. Change in sleep duration and cognitive function: Findings from the Whitehall II Study. Sleep. 2011;34:565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficca G, Axelsson J, Mollicone DJ, Muto V, Vitiello MV. Naps, cognition and performance. Sleep Medicine Reviews. 2010;14:249–258. doi: 10.1016/j.smrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Filtness AJ, Reyner LA, Horne JA. Driver sleepiness—comparisons between young and older men during a monotonous afternoon simulated drive. Biological Psychology. 2012;89:580–583. doi: 10.1016/j.biopsycho.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Finnigan S, Robertson IH. Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology. 2011;48:1083–1087. doi: 10.1111/j.1469-8986.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the National Academy of Sciences. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein W. Disruptive effects of rapid eye movement sleep deprivation on long-term memory. Physiology & Behavior. 1971;6:279–282. doi: 10.1016/0031-9384(71)90155-7. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, Doyon J. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Human Brain Mapping. 2014 doi: 10.1002/hbm.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Carrier J. NREM sleep oscillations and brain plasticity in aging. Frontiers in Neurology. 2012;3:176. doi: 10.3389/fneur.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Smith CT. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience & Biobehavioral Reviews. 2011;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. Journal of Psychosomatic Research. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26:596–599. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Masaki KH, Enright PL, Quan SF, White LR. Associations of symptoms of sleep apnea with cardiovascular disease, cognitive impairment, and mortality among older Japanese-American men. Journal of the American Geriatrics Society. 1999;47:524–528. doi: 10.1111/j.1532-5415.1999.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. Journal of the American Geriatrics Society. 2001;49:1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Reviews Neuroscience. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Aβ oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiology of Aging. 2011;32:2211–2218. doi: 10.1016/j.neurobiolaging.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L, Miró J, Ripollés P, Vilá-Balló A, Juncadella M, Castañer S, Rodríguez-Fornells A. Hippocampus-dependent strengthening of targeted memories via reactivation during sleep in humans. Current Biology. 2013;23:1769–1775. doi: 10.1016/j.cub.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Fröberg JE, Karlsson CG, Levi L, Lidberg L. Circadian rhythms of catecholamine excretion, shooting range performance and self-ratings of fatigue during sleep deprivation. Biological Psychology. 1975;2:175–188. doi: 10.1016/0301-0511(75)90018-6. [DOI] [PubMed] [Google Scholar]
- Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learning & Memory. 2006;13:259–262. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaldo AA, Allaire JC, Whitfield KE. The relationship between reported problems falling asleep and cognition among African American elderly. Research on Aging. 2008;30:752–767. [Google Scholar]
- Gamaldo AA, Allaire JC, Whitfield KE. Exploring the within-person coupling of sleep and cognition in older African Americans. Psychology and Aging. 2010;25:851–857. doi: 10.1037/a0021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Dresler M, Cornu M, Jäger E, Konrad B, Adamczyk M, Goya-Maldonado R. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.06.004. DOI: http://dx.doi.org/10.1016/j.biopsych.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. Journal of Neuroscience. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra LM, Arenberg D. Adult age differences in forgetting sentences. Psychology and Aging. 1993;8:451–462. doi: 10.1037//0882-7974.8.3.451. [DOI] [PubMed] [Google Scholar]
- Gildner TE, Liebert MA, Kowal P, Chatterji S, Snodgrass JJ. Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: Results from the Study on Global Ageing and Adult Health (SAGE) Journal of Clinical Sleep Medicine. 2014 doi: 10.5664/jcsm.3782. http://dx.doi.org/10.5664/jcsm.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göder R, Born J. Can sleep heal memory? Sleep Medicine Reviews. 2013;17:89–90. doi: 10.1016/j.smrv.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Goerke M, Cohrs S, Rodenbeck A, Kunz D. Differential effect of an anticholinergic antidepressant on sleep-dependent memory consolidation. Sleep. 2014;37:977–985. doi: 10.5665/sleep.3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychological Science. 2006;17:670–674. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Weaver TE, Cater JR, Pack FM, Arner HM, Greenberg AS, Pack AI. Functional outcomes of excessive daytime sleepiness in older adults. Journal of the American Geriatrics Society. 2003;51:642–649. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature Reviews Neuroscience. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Drummond S. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Medicine Reviews. 2007;11:341–360. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guazzelli M, Feinberg I, Aminoff M, Fein G, Floyd TC, Maggini C. Sleep spindles in normal elderly: Comparison with young adult patterns and relation to nocturnal awakening, cognitive function and brain atrophy. Electroencephalography and Clinical Neurophysiology. 1986;63:526–539. doi: 10.1016/0013-4694(86)90140-9. [DOI] [PubMed] [Google Scholar]
- Habte-Gabr E, Wallace RB, Colsher PL, Hulbert JR, White LR, Smith IM. Sleep patterns in rural elders: Demographic, health, and psychobehavioral correlates. Journal of Clinical Epidemiology. 1991;44:5–13. doi: 10.1016/0895-4356(91)90195-f. [DOI] [PubMed] [Google Scholar]
- Hahn EA, Wang HX, Andel R, Fratiglioni L. A change in sleep pattern may predict Alzheimer disease. American Journal of Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2013.04.015. http://dx.doi.org/10.1016/j.jagp.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Haimov I, Shatil E. Cognitive training improves sleep quality and cognitive function among older adults with insomnia. PLoS ONE. 2013;8:e61390. doi: 10.1371/journal.pone.0061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Porter JM, Schweitzer PK, Eisenstein RD, Ahmed HA, Walsh JK. The effect of two benzodiazepine receptor agonist hypnotics on sleep-dependent memory consolidation. Journal of Clinical Sleep Medicine. 2013;10:27–34. doi: 10.5664/jcsm.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harand C, Bertran F, Doidy F, Guénolé F, Desgranges B, Eustache F, Rauchs G. How aging affects sleep-dependent memory consolidation? Frontiers in Neurology. 2012;3:8. doi: 10.3389/fneur.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults—A model for healthy aging? Sleep. 2000;23:1067–1073. [PubMed] [Google Scholar]
- Hayward LB, Mant A, Eyland EA, Hewitt H, Pond CD, Saunders NA. Neuropsychological functioning and sleep patterns in the elderly. Medical Journal of Australia. 1992;157:51–52. doi: 10.5694/j.1326-5377.1992.tb121609.x. [DOI] [PubMed] [Google Scholar]
- Hemmeter U, Müller M, Bischof R, Annen B, Holsboer-Trachsler E. Effect of zopiclone and temazepam on sleep EEG parameters, psychomotor and memory functions in healthy elderly volunteers. Psychopharmacology. 2000;147:384–396. doi: 10.1007/s002130050007. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Huetz C, Edeline JM. Neural representations during sleep: From sensory processing to memory traces. Neurobiology of Learning and Memory. 2007;87:416–440. doi: 10.1016/j.nlm.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J. A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. British Journal of Clinical Pharmacology. 2006;62:538–545. doi: 10.1111/j.1365-2125.2006.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: The role of memory decline and ApoE ε4 genotype. Current Alzheimer Research. 2012;9:290–297. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Cantero JL. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep. 2013;36:1327–1334. doi: 10.5665/sleep.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch CC, Dew MA, Reynolds CF, Monk TH. A longitudinal study of laboratory-and diary-based sleep measures in healthy" old old" and" young old" volunteers. Sleep. 1994;17:489–496. doi: 10.1093/sleep/17.6.489. [DOI] [PubMed] [Google Scholar]
- Horne J. Exercise benefits for the aging brain depend on the accompanying cognitive load: Insights from sleep electroencephalogram. Sleep Medicine. 2013;14:1208–1213. doi: 10.1016/j.sleep.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Danker-Hopfe H, Heuser I. Age-related changes in sleep and memory: Commonalities and interrelationships. Experimental Gerontology. 2005;40:279–285. doi: 10.1016/j.exger.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Regen F, Danker-Hopfe H, Schredl M, Heuser I. The relationship between REM sleep and memory consolidation in old age and effects of cholinergic medication. Biological Psychiatry. 2007;61:750–757. doi: 10.1016/j.biopsych.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Hornung OP, Regen F, Dorn H, Anghelescu I, Kathmann N, Schredl M, Heuser I. The effects of donepezil on postlearning sleep EEG of healthy older adults. Pharmacopsychiatry. 2009;42:9–13. doi: 10.1055/s-0028-1083820. [DOI] [PubMed] [Google Scholar]
- Hot P, Rauchs G, Bertran F, Denise P, Desgranges B, Clochon P, Eustache F. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer's disease. Biological Psychology. 2011;87:334–339. doi: 10.1016/j.biopsycho.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychological Science. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huxter JR, Miranda JA, Dias R. The hippocampal physiology of approaching middle-age: Early indicators of change. Hippocampus. 2012;22:1923–1940. doi: 10.1002/hipo.22027. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jaussent I, Bouyer J, Ancelin ML, Berr C, Foubert-Samier A, Ritchie K, Dauvilliers Y. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–1207. doi: 10.5665/sleep.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) International Journal of Geriatric Psychiatry. 2002;17:73–77. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- Jenkins JG, Dallenbach KM. Oblivescence during sleep and waking. American Journal Psychology. 1924;35:605–612. [Google Scholar]
- Jennum P, Sjøl A. Self-assessed cognitive function in snorers and sleep apneics. An epidemiological study of 1,504 females and males aged 30–60 years: The Dan-Monica II study. European Neurology. 1994;34:204–208. doi: 10.1159/000117039. [DOI] [PubMed] [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurology. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado JL, Luna Villegas G, Buela–Casal G. Normal human subjects with slow reaction times and larger time estimations after waking have diminished delta sleep. Electroencephalography and Clinical Neurophysiology. 1989;73:124–128. doi: 10.1016/0013-4694(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Kahn E, Fisher C. Some correlates of rapid eye movement sleep in the normal aged male. Journal of Nervous and Mental Disease. 1969;148:495–505. doi: 10.1097/00005053-196905000-00003. [DOI] [PubMed] [Google Scholar]
- Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Medicine. 2007;8:215–221. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Medicine. 2012;13:886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Balkin TJ, Wesensten NJ. Impaired decision-making following 49 hours of sleep deprivation. Journal of Sleep Research. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Killgore WDS. Effects of sleep deprivation on cognition. Progress in Brain Research. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. American Journal of Geriatric Psychiatry. 2011;19:374–381. doi: 10.1097/JGP.0b013e3181e9b976. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep—implications for insomnia. Current Biology. 2008;18:1118–1123. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloepfer C, Riemann D, Nofzinger EA, Feige B, Unterrainer J, O'Hara R, Nissen C. Memory before and after sleep in patients with moderate obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2009;5:540. [PMC free article] [PubMed] [Google Scholar]
- Kopasz M, Loessl B, Hornyak M, Riemann D, Nissen C, Piosczyk H, Voderholzer U. Sleep and memory in healthy children and adolescents–a critical review. Sleep Medicine Reviews. 2010;14:167–177. doi: 10.1016/j.smrv.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Krieg EF, Chrislip DW, Letz RE, Otto DA, Crespo CJ, Brightwell WS, Ehrenberg RL. Neurobehavioral test performance in the third National Health and Nutrition Examination Survey. Neurotoxicology and Teratology. 2001;23:569–589. doi: 10.1016/s0892-0362(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Kronholm E. Sleep in cognitive life-time trajectory. Sleep Medicine. 2012;13:777–778. doi: 10.1016/j.sleep.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. Journal of Sleep Research. 2009;18:436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Kulmala J, von Bonsdorff MB, Stenholm S, Törmäkangas T, von Bonsdorff ME, Nygård CH, Rantanen T. Perceived stress symptoms in midlife predict disability in old age: A 28-year prospective cohort study. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013 doi: 10.1093/gerona/gls339. [DOI] [PubMed] [Google Scholar]
- Kummer J, Guendel L, Linden J, Eich FX, Attali P, Coquelin JP, Kyrein HJ. Long-term polysomnographic study of the efficacy and safety of zolpidem in elderly psychiatric in-patients with insomnia. Journal of International Medical Research. 1993;21:171–184. doi: 10.1177/030006059302100402. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Mishima K, Suzuki H, Aritake S, Uchiyama M. Sleep accelerates the improvement in working memory performance. Journal of Neuroscience. 2008;28:10145–10150. doi: 10.1523/JNEUROSCI.2039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, Dement WC. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafortune M, Gagnon JF, Martin N, Latreille V, Dubé J, Bouchard M, Carrier J. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. Journal of Sleep Research. 2014;23:159–167. doi: 10.1111/jsr.12108. [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- Leufkens TR, Vermeeren A. Highway driving in the elderly the morning after bedtime use of hypnotics: A comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. Journal of Clinical Psychopharmacology. 2009;29:432–438. doi: 10.1097/JCP.0b013e3181b57b43. [DOI] [PubMed] [Google Scholar]
- Lichtenberg PA, Ross T, Millis SR, Manning CA. The relationship between depression and cognition in older adults: A cross-validation study. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1995;50:P25–P32. doi: 10.1093/geronb/50b.1.p25. [DOI] [PubMed] [Google Scholar]
- Lim AS, Yu L, Costa MD, Leurgans SE, Buchman AS, Bennett DA, Saper CB. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–640. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurology. 2013;70:1544–1551. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Loh KK, Zheng H, Sim SK, Chee MW. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37:1171–1178. doi: 10.5665/sleep.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Sim SK, Chee MW. Sleep reduces false memory in healthy older adults. Sleep. 2014;37:665–671. doi: 10.5665/sleep.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo A, López-Antón R, De-La-Cámara C, Quintanilla MÁ, Campayo A, Saz P. Non-cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, Alzheimer’s type. Neurotoxicity Research. 2008;14:263–272. doi: 10.1007/BF03033815. [DOI] [PubMed] [Google Scholar]
- Loerbroks A, Debling D, Amelang M, Stürmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. International Journal of Geriatric Psychiatry. 2010;25:100–109. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- Loewenstein RJ, Weingartner H, Gillin JC, Kaye W, Ebert M, Mendelson WB. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiology of Aging. 1982;3:371–377. doi: 10.1016/0197-4580(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Oeltermann A. Hippocampal-cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- Lovato N, Lack L, Wright H, Cant M, Humphreys J. Working memory performance of older adults with insomnia. Journal of Sleep Research. 2013;22:251–257. doi: 10.1111/jsr.12010. [DOI] [PubMed] [Google Scholar]
- Lowden A, Anund A, Kecklund G, Peters B, Åkerstedt T. Wakefulness in young and elderly subjects driving at night in a car simulator. Accident Analysis & Prevention. 2009;41:1001–1007. doi: 10.1016/j.aap.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Lucassen EA, Piaggi P, Dsurney J, de Jonge L, Zhao XC, Mattingly MS Sleep Extension Study Group. Sleep extension improves neurocognitive functions in chronically sleep-deprived obese individuals. PLoS ONE. 2014;9:e84832. doi: 10.1371/journal.pone.0084832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi S, Langlois JA, Minicuci N, Grigoletto F, Pavan M, Foley DJ, Enzi G. Sleep complaints in community-dwelling older persons: Prevalence, associated factors, and reported causes. Journal of the American Geriatrics Society. 1998;46:161–168. doi: 10.1111/j.1532-5415.1998.tb02533.x. [DOI] [PubMed] [Google Scholar]
- Maggio M, Colizzi E, Fisichella A, Valenti G, Ceresini G, Dall’Aglio E, Ceda GP. Stress hormones, sleep deprivation and cognition in older adults. Maturitas. 2013;76:22–44. doi: 10.1016/j.maturitas.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Mah CD, Mah KE, Kezirian EJ, Dement WC. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34:943–950. doi: 10.5665/SLEEP.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cerebral Cortex. 2013a doi: 10.1093/cercor/bht188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature Neuroscience. 2013b;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biological Psychiatry. 2004;56:951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. Journal of Psychiatric Research. 2010;44:112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Peters J-M, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: Behavioral and neurobiological correlates. Neurobiology of Aging. 1989;10:31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. Journal of Neuroscience. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Carrier J. Topography of age-related changes in sleep spindles. Neurobiology of Aging. 2013;34:468–476. doi: 10.1016/j.neurobiolaging.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Martin-Ordas G, Call J. Memory processing in great apes: The effect of time and sleep. Biology Letters. 2011;7:829–832. doi: 10.1098/rsbl.2011.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary A, Schreiner S, Peigneux P. Accelerated long-term forgetting in aging and intra-sleep awakenings. Frontiers in Psychology. 2013;4:750. doi: 10.3389/fpsyg.2013.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. Journal of Neuroscience. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S, Zihl J, Steiger A, Lancel M. Effect of repeated gaboxadol administration on night sleep and next-day performance in healthy elderly subjects. Neuropsychopharmacology. 2005;30:833–841. doi: 10.1038/sj.npp.1300641. [DOI] [PubMed] [Google Scholar]
- Mawdsley M, Grasby K, Talk A. The effect of sleep on item recognition and source memory recollection among shift-workers and permanent day-workers. Journal of Sleep Research. 2014 doi: 10.1111/jsr.12149. [DOI] [PubMed] [Google Scholar]
- Mazzoni G, Gori S, Formicola G, Gneri C, Massetani R, Murri L, Salzarulo P. Word recall correlates with sleep cycles in elderly subjects. Journal of Sleep Research. 1999;8:185–188. doi: 10.1046/j.1365-2869.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McCrae CS, Vatthauer KE, Dzierzewski JM, Marsiske M. Habitual sleep, reasoning, and processing speed in older adults with sleep complaints. Cognitive Therapy and Research. 2012;36:156–164. doi: 10.1007/s10608-011-9425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends in Neurosciences. 2011;34:504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SP. The critical role of sleep spindles in hippocampal-dependent memory: A pharmacology study. Journal of Neuroscience. 2013;33:4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC. Take a Nap! Change Your Life. Workman Publishing; 2006. [Google Scholar]
- Mehra R, Stone KL, Ancoli-Israel S, Litwack-Harrison S, Ensrud KE, Redline S. Interpreting wrist actigraphic indices of sleep in epidemiologic studies of the elderly: The Study of Osteoporotic Fractures. Sleep. 2008;31:1569–1576. doi: 10.1093/sleep/31.11.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens HW, Collins WE. The effects of age, sleep deprivation, and altitude on complex performance. Human Factors. 1986;28:541–551. doi: 10.1177/001872088602800504. [DOI] [PubMed] [Google Scholar]
- Miller MA, Wright H, Ji C, Cappuccio FP. Cross-sectional study of sleep quantity and quality and amnestic and non-amnestic cognitive function in an ageing population: The English Longitudinal Study of Ageing (ELSA) PLoS ONE. 2014;9:e100991. doi: 10.1371/journal.pone.0100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner CE, Cote KA. A dose-response investigation of the benefits of napping in healthy young, middle-aged and older adults. Sleep and Biological Rhythms. 2008;6:2–15. [Google Scholar]
- Milner CE, Cote KA. Benefits of napping in healthy adults: Impact of nap length, time of day, age, and experience with napping. Journal of Sleep Research. 2009;18:272–281. doi: 10.1111/j.1365-2869.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N. Poor sleep quality impairs cognitive performance in older adults. Journal of Sleep Research. 2013;22:535–541. doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon "siesta" naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24:680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep and Breathing. 2012;16:913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- Monti JM, Spence DW, Pandi-Perumal SR, Langer SZ, Hardeland R. Pharmacotherapy of insomnia: Focus on Zolpidem eextended release. Clinical Medicine: Therapeutics. 2009;1:123–140. [Google Scholar]
- Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for Insomnia: A meta-analysis of treatment efficacy. American Journal of Psychiatry. 1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hupbach A, Gomez R, Newman-Smith K. Memory formation, consolidation and transformation. Neuroscience & Biobehavioral Reviews. 2012;36:1640–1645. doi: 10.1016/j.neubiorev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nader RS, Smith CT. The relationship between stage 2 sleep spindles and intelligence. Sleep. 2001;24(suppl):A160. [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. Journal of Neuroscience. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E, Penev PD, Orbeta L, Janssen I, Ortiz R, Colecchia EF, Zee PC. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. Journal of Gerontology: Psychological Sciences. 2009;64:180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Király K, Londe Z, Németh K, Fazekas K, Csányi A. Probabilistic sequence learning in mild cognitive impairment. Frontiers in Human Neuroscience. 2013;7:318. doi: 10.3389/fnhum.2013.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Londe Z, Ullman MT, Howard DV, Howard JH., Jr Sleep has no critical role in implicit motor sequence learning in young and old adults. Experimental Brain Research. 2010;201:351–358. doi: 10.1007/s00221-009-2024-x. [DOI] [PubMed] [Google Scholar]
- Nesthus TE, Scarborough AL, Schroeder DJ. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2. Vol. 42. SAGE Publications; 1998a. Changes in the performance of a synthetic work task as a function of age, gender, and sleep deprivation; pp. 181–184. [Google Scholar]
- Nesthus TE, Scarborough AL, Schroeder DJ, Kaplan R, Loparo K, Thome D. Four-choice serial reaction time and visual search performance during 34 hr of sleep loss. Abstract: Aviation, Space, and Environmental Medicine. 1998b [Google Scholar]
- Newman AB, Enright PL, Manolio TA, Haponik EF. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: The Cardiovascular Health Study. Journal of the American Geriatrics Society. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Wulff LL, Arentsen TJ. Muscle tension induced after learning enhances long-term narrative and visual memory in healthy older adults. Neurobiology of Learning and Memory. 2014;109:144–150. doi: 10.1016/j.nlm.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Nissen C, Kloepfer C, Feige B, Piosczyk H, Spiegelhalder K, Voderholzer U, Riemann D. Sleep-related memory consolidation in primary insomnia. Journal of Sleep Research. 2011;20:129–136. doi: 10.1111/j.1365-2869.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- Nissen C, Kloepfer C, Nofzinger EA, Feige B, Voderholzer U, Riemann D. Impaired sleep-related memory consolidation in primary insomnia—a pilot study. Sleep. 2006;29:1068–1073. doi: 10.1093/sleep/29.8.1068. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Archives of Internal Medicine. 2002;162:201–208. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Vecchierini M. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–989. [PubMed] [Google Scholar]
- Olaithe M, Skinner TC, Hillman D, Eastwood PE, Bucks RS. Cognition and nocturnal disturbance in OSA: The importance of accounting for age and premorbid intelligence. Sleep and Breathing. 2014 doi: 10.1007/s11325-014-1000-2. [DOI] [PubMed] [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits on the radial maze and in fear conditioning: Hippocampal processing and consolidation. Hippocampus. 1998;8:402–415. doi: 10.1002/(SICI)1098-1063(1998)8:4<402::AID-HIPO8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. Journal of Sleep Research. 2009;18:129–135. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Pirraglia E, Agüera-Ortiz LF, During EH, Sacks H, Ayappa I, de Leon MJ. Greater risk of Alzheimer's disease in older adults with insomnia. Journal of the American Geriatrics Society. 2011;59:559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmani S, Demazieres A, Staner C, Jacob N, Nir T, Zisapel N, Staner L. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Human Psychopharmacology: Clinical and Experimental. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Constantinescu I, Leclair-Visonneau L, Vidailhet M, Schwartz S, Arnulf I. Evidence for the re-enactment of a recently learned behavior during sleepwalking. PloS ONE. 2011;6:e18056. doi: 10.1371/journal.pone.0018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends in Cognitive Sciences. 2013;17:142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC. Age-related changes in the cognitive function of sleep. Progress in Brain Research. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM. Age-related changes in consolidation of perceptual and muscle-based learning of motor skills. Frontiers in Aging Neuroscience. 2013;5:83. doi: 10.3389/fnagi.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JA, Urrestarazu E, Iriarte J. Sleep loss as risk factor for neurologic disorders: A review. Sleep Medicine. 2013;14:229–236. doi: 10.1016/j.sleep.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep. 2007;30:1362–1369. doi: 10.1093/sleep/30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey CM, Schmitter-Edgecombe M, Belenky G. Sleep and everyday functioning in older adulthood. Journal of Applied Gerontology. 2012 doi: 10.1177/0733464812458364. [DOI] [PubMed] [Google Scholar]
- Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, Tucker MA, Stickgold R. The role of sleep in false memory formation. Neurobiology of Learning and Memory. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Medicine. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: A pilot study. American Journal of Geriatric Psychiatry. 2004;12:432–436. doi: 10.1176/appi.ajgp.12.4.432. [DOI] [PubMed] [Google Scholar]
- Pedraza S, Al Snih S, Ottenbacher KJ, Markides KS, Raji MA. Sleep quality and sleep problems in Mexican Americans aged 75 and older: Sleep quality and sleep problems in Mexican-American elders. Aging Clinical and Experimental Research. 2012;24:391–397. doi: 10.3275/8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C. Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. Journal of Sleep Research. 2008;17:23–33. doi: 10.1111/j.1365-2869.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N, Bioulac B. Age, performance and sleep deprivation. Journal of Sleep Research. 2004;13:105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Platano D, Fattoretti P, Balietti M, Bertoni-Freddari C, Aicardi G. Long-term visual object recognition memory in aged rats. Rejuvenation Research. 2008;11:333–339. doi: 10.1089/rej.2008.0687. [DOI] [PubMed] [Google Scholar]
- Platt B, Drever B, Koss D, Stoppelkamp S, Jyoti A, Plano A, Riedel G. Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PloS ONE. 2011;6:e27068. doi: 10.1371/journal.pone.0027068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Potvin O, Lorrain D, Forget H, Dube M, Grenier S, Préville M, Hudon C. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–499. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz PN. Sleep patterns in the healthy aged: Relationship with intellectual function. Journal of Gerontology. 1977;32:179–186. [Google Scholar]
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiology of Aging. 1982a;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, Gerber CJ. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. Journal of the American Geriatrics Society. 1982b;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- Purnell MT, Feyer AM, Herbison GP. The impact of a nap opportunity during the night shift on the performance and alertness of 12-h shift workers. Journal of Sleep Research. 2002;11:219–227. doi: 10.1046/j.1365-2869.2002.00309.x. [DOI] [PubMed] [Google Scholar]
- Quesnot A, Alperovitch A. Snoring and risk of cognitive decline: A 4-year follow-up study in 1389 older individuals. Journal of the American Geriatrics Society. 1999;47:1159–1160. doi: 10.1111/j.1532-5415.1999.tb05252.x. [DOI] [PubMed] [Google Scholar]
- Racsmány M, Conway MA, Demeter G. Consolidation of episodic memories during sleep: Long-term effects of retrieval practice. Psychological Science. 2010;21:80–85. doi: 10.1177/0956797609354074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AR, Dong C, Elkind MS, Boden-Albala B, Sacco RL, Rundek T, Wright CB. Association between sleep duration and the mini-mental score: The Northern Manhattan study. Journal of Clinical Sleep Medicine. 2013;9:669–673. doi: 10.5664/jcsm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AR, Dong C, Rundek T, Elkind MS, Boden-Albala B, Sacco RL, Wright CB. Sleep duration is associated with white matter hyperintensity volume in older adults: The Northern Manhattan Study. Journal of Sleep Research. 2014 doi: 10.1111/jsr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep's role in memory. Physiological Reviews. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Carrier J, Peigneux P. Sleep and cognition in the elderly. Frontiers in Neurology. 2012;4:71. doi: 10.3389/fneur.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Feyers D, Landeau B, Bastin C, Luxen A, Maquet P, Collette F. Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. Journal of Neuroscience. 2011;31:2563–2568. doi: 10.1523/JNEUROSCI.3972-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Anderer P. Is there a link between sleep changes and memory in Alzheimer's disease? Neuroreport. 2008;19:1159–1162. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, D.C.: Public Health Service, U.S. Government Printing Office; 1968. [Google Scholar]
- Regestein QR, Friebely J, Shifren JL, Scharf MB, Wiita B, Carver J, Schiff I. Self-reported sleep in postmenopausal women. Menopause. 2004;11:198–207. doi: 10.1097/01.gme.0000097741.18446.3e. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Medicine. 2010;11:934–940. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, III, Kupfer DJ, Houck PR, Hoch CC, Stack JA, Berman SR, Zimmer B. Reliable discrimination of elderly depressed and demented patients by electroencephalographic sleep data. Archives of General Psychiatry. 1988;45:258–264. doi: 10.1001/archpsyc.1988.01800270076009. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, III, Kupfer DJ, Taska LS, Hoch CC, Spiker DG, Sewitch DE, Morycz R. EEG sleep in elderly depressed, demented, and healthy subjects. Biological Psychiatry. 1985;20:431–442. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC. Sleep does not enhance motor sequence learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:834–842. doi: 10.1037/0278-7393.34.4.834. [DOI] [PubMed] [Google Scholar]
- Ritter SM, Strick M, Bos MW, Van Baaren RB, Dijksterhuis A. Good morning creativity: Task reactivation during sleep enhances beneficial effect of sleep on creative performance. Journal of Sleep Research. 2012;21:643–647. doi: 10.1111/j.1365-2869.2012.01006.x. [DOI] [PubMed] [Google Scholar]
- Roane BM, Johnson L, Edwards M, Hall J, Al-Farra S, O'Bryant SE. The link between sleep disturbance and depression among Mexican Americans: A project FRONTIER study. Journal of Clinical Sleep Medicine. 2013;10:427–431. doi: 10.5664/jcsm.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck J. When does" old age begin?: The evolution of the English definition. Journal of Social History. 1979:416–428. [Google Scholar]
- Roig M, Ritterband-Rosenbaum A, Lundbye-Jensen J, Nielsen JB. Aging increases the susceptibility to motor memory interference and reduces off-line gains in motor skill learning. Neurobiology of Aging. 2014;35:1892–1900. doi: 10.1016/j.neurobiolaging.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proceedings of the National Academy of Sciences. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. Journal of Neuroscience. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22:S354–S358. [PubMed] [Google Scholar]
- Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer′ s Disease. Brain Research. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079–1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74:444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Taillard J, Amiéva H, Beck A, Rascol O, Dartigues J-F, Philip P. Influence of age, circadian and homeostatic processes on inhibitory motor control: A Go/Nogo task study. PLoS ONE. 2012;7:e39410. doi: 10.1371/journal.pone.0039410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Martin M, Sforza E, Barthelemy JC, Thomas-Anterion C, Roche F. Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep Medicine. 2012;13:1146–1152. doi: 10.1016/j.sleep.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Salas RE, Galea JM, Gamaldo AA, Gamaldo CE, Allen RP, Smith MT, Celnik PA. Increased use-dependent plasticity in chronic insomnia. Sleep. 2013;37:535–544. doi: 10.5665/sleep.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio RAC, Sampaio PYS, Yamada M, Tsuboyama T, Arai H. Self-reported quality of sleep is associated with bodily pain, vitality and cognitive impairment in Japanese older adults. Geriatrics & Gerontology International. 2013 doi: 10.1111/ggi.12149. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Mayleben DW, Kaffeman M, Krall R. Dose response effects of zolpidem in normal geriatric subjects. Journal Of Clinical Psychiatry. 1991;52:77–83. [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Cajochen C. Age-related changes in sleep and circadian rhythms: Impact on cognitive performance and underlying neuroanatomical networks. Frontiers in Neurology. 2012;3:188. doi: 10.3389/fneur.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredl M, Weber B, Leins ML, Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Experimental Gerontology. 2001;36:353–361. doi: 10.1016/s0531-5565(00)00206-0. [DOI] [PubMed] [Google Scholar]
- Schweitzer PK, Randazzo AC, Stone K, Erman M, Walsh JK. Laboratory and field studies of naps and caffeine as practical countermeasures for sleep-wake problems associated with night work. Sleep. 2006;29:39–50. doi: 10.1093/sleep/29.1.39. [DOI] [PubMed] [Google Scholar]
- Scullin MK. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychology and Aging. 2013;28:105–114. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, McDaniel MA. Remembering to execute a goal: Sleep on it! Psychological Science. 2010;21:1028–1035. doi: 10.1177/0956797610373373. [DOI] [PubMed] [Google Scholar]
- Scullin M, McDaniel M, Howard D, Kudelka C. Sleep and testing promote conceptual learning of classroom materials. Sleep. 2011;34(Suppl.):A83. [Google Scholar]
- Scullin MK, Trotti LM, Wilson AG, Greer SA, Bliwise DL. Nocturnal sleep enhances working memory training in Parkinson’s disease but not Lewy body dementia. Brain. 2012;135:2789–2797. doi: 10.1093/brain/aws192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relationship between cognitive functioning and self reported sleep complaints in nondemented older subjects: Results of the Bronx aging study. Behavioral Sleep Medicine. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier PC, Weinhold SL, Dittmar M, Heiermann S, Aldenhoff JB, Goder R. Declarative memory performance is associated with the number of sleep spindles in elderly women. American Journal of Geriatric Psychiatry. 2012;20:782–788. doi: 10.1097/JGP.0b013e31823033da. [DOI] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychological Bulletin. 2012;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siengsukon CF, Boyd LA. Sleep enhances implicit motor skill learning in individuals poststroke. Topics in Stroke Rehabilitation. 2008;15:1–12. doi: 10.1310/tsr1501-1. [DOI] [PubMed] [Google Scholar]
- Siengsukon CF, Boyd LA. Sleep to learn after stroke: Implicit and explicit off-line motor learning. Neuroscience Letters. 2009a;451:1–5. doi: 10.1016/j.neulet.2008.12.040. [DOI] [PubMed] [Google Scholar]
- Siengsukon CF, Boyd LA. Sleep enhances off-line spatial and temporal motor learning after stroke. Neurorehabilitation and Neural Repair. 2009b;23:327–335. doi: 10.1177/1545968308326631. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Omvik S, Havik OE, Pallesen S, Bjorvatn B, Nielsen GH, Nordhus IH. A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep. 2006;29:1353–1358. doi: 10.1093/sleep/29.10.1353. [DOI] [PubMed] [Google Scholar]
- Smulders FTY, Kenemans JL, Jonkman LM, Kok A. The effects of sleep loss on task performance and the electroencephalogram in young and elderly subjects. Biological Psychology. 1997;45:217–239. doi: 10.1016/s0301-0511(96)05229-5. [DOI] [PubMed] [Google Scholar]
- Snigdha S, de Rivera C, Milgram NW, Cotman CW. Exercise enhances memory consolidation in the aging brain. Frontiers in Aging Neuroscience. 2014;6 doi: 10.3389/fnagi.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learning & Memory. 2007;14:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel R. Sleep and sleeplessness in advanced age. Spectrum Publications; 1981. . [Google Scholar]
- Spiegel R, Köberle S, Allen SR. Significance of slow wave sleep: Considerations from a clinical viewpoint. Sleep. 1986;9:66–79. doi: 10.1093/sleep/9.1.66. [DOI] [PubMed] [Google Scholar]
- Spiegel R, Herzog A, Köberle S. Polygraphic sleep criteria as predictors of successful aging: An exploratory longitudinal study. Biological Psychiatry. 1999;45:435–442. doi: 10.1016/s0006-3223(98)00042-0. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Espie C, Nissen C, Riemann D. Sleep-related attentional bias in patients with primary insomnia compared with sleep experts and healthy controls. Journal of Sleep Research. 2008;17:191–196. doi: 10.1111/j.1365-2869.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors CU, Hanson LM, Oxenstierna G, Theorell T, Nilsson LG. Psychosocial working conditions and cognitive complaints among Swedish employees. PloS ONE. 2013;8:e60637. doi: 10.1371/journal.pone.0060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenuit P, Kerkhofs M. Age modulates the effects of sleep restriction in women. Sleep. 2005;28:1283–1288. doi: 10.1093/sleep/28.10.1283. [DOI] [PubMed] [Google Scholar]
- Stenuit P, Kerkhofs M. Effects of sleep restriction on cognition in women. Biological Psychology. 2008;77:81–88. doi: 10.1016/j.biopsycho.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. Journal of Neuroscience. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DA, Ballard K, Hardy JL, Katz B, Doraiswamy PM, Scanlon M. The largest human cognitive performance dataset reveals insights into the effects of lifestyle factors and aging. Frontiers in Human Neuroscience. 2013;7:292. doi: 10.3389/fnhum.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Current Alzheimer Research. 2013;10:767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. Journal of Cognitive Neuroscience. 1999;11:182–193. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory triage: Evolving generalization through selective processing. Nature Neuroscience. 2013;16:139–145. doi: 10.1038/nn.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Altman HJ, Berman RF, Caldwell DF, Kilbey MM. Association of sleep parameters and memory in intact old rats and young rats with lesions in the nucleus basalis magnocellularis. Behavioral Neuroscience. 1989;103:755–764. doi: 10.1037//0735-7044.103.4.755. [DOI] [PubMed] [Google Scholar]
- Stone WS, Rudd RJ, Parsons MW, Gold PE. Memory scores in middle-aged rats predict later deficits in memory, paradoxical sleep, and blood glucose regulation in old age. Experimental Aging Research. 1997;23:287–300. doi: 10.1080/03610739708254285. [DOI] [PubMed] [Google Scholar]
- Sun J, Kang J, Wang P, Zeng H. Self-relaxation training can improve sleep quality and cognitive functions in the older: A one-year randomised controlled trial. Journal of Clinical Nursing. 2013;22:1270–1280. doi: 10.1111/jocn.12096. [DOI] [PubMed] [Google Scholar]
- Sutter C, Zöllig J, Allemand M, Martin M. Sleep quality and cognitive function in healthy old age: The moderating role of subclinical depression. Neuropsychology. 2012;26:768–775. doi: 10.1037/a0030033. [DOI] [PubMed] [Google Scholar]
- Talamini LM, Nieuwenhuis IL, Takashima A, Jensen O. Sleep directly following learning benefits consolidation of spatial associative memory. Learning & Memory. 2008;15:233–237. doi: 10.1101/lm.771608. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Shirota AI, Tanaka H, Hayashi M, Hori T. Effects of a daytime nap in the aged. Psychiatry and Clinical Neurosciences. 1999;53:273–275. doi: 10.1046/j.1440-1819.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Shirota A, Hayashi M, Hori T. Restorative effects of a short afternoon nap (< 30 min) in the elderly on subjective mood, performance and EEG activity. Sleep Research Online. 2000;3:131–139. [PubMed] [Google Scholar]
- Tanaka H, Shirakawa S. Sleep health, lifestyle and mental health in the Japanese elderly: Ensuring sleep to promote a healthy brain and mind. Journal of Psychosomatic Research. 2004;56:465–477. doi: 10.1016/j.jpsychores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Taira K, Arakawa M, Toguti H, Urasaki C, Yamamoto Y, Shirakawa S. Effects of short nap and exercise on elderly people having difficulty in sleeping. Psychiatry and Clinical Neurosciences. 2001;55:173–174. doi: 10.1046/j.1440-1819.2001.00813.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Taira K, Arakawa M, Urasaki C, Yamamoto Y, Okuma H, Shirakawa S. Short naps and exercise improve sleep quality and mental health in the elderly. Psychiatry and Clinical Neurosciences. 2002;56:233–234. doi: 10.1046/j.1440-1819.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- Terpening Z, Naismith S, Melehan K, Gittins C, Bolitho S, Lewis SJ. The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson's disease. Journal of Sleep Research. 2013;22:398–405. doi: 10.1111/jsr.12028. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Research Bulletin. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R. Sleep optimizes motor skill in older adults. Journal of the American Geriatrics Society. 2011;59:603–609. doi: 10.1111/j.1532-5415.2011.03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic; 1972. pp. 381–403. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Disease & Associated Disorders. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- van den Heuvel CJ, Ferguson SA, Mila Macchi M, Dawson D. Melatonin as a hypnotic: Con. Sleep Medicine Reviews. 2005;9:71–80. doi: 10.1016/j.smrv.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Vanderlind WM, Beevers CG, Sherman SM, Trujillo LT, McGeary JE, Matthews MD, Schnyer DM. Sleep and sadness: Exploring the relation among sleep, cognitive control and depressive symptoms in young adults. Sleep Medicine. 2014;15:144–149. doi: 10.1016/j.sleep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJ. Sleep benefits subsequent hippocampal functioning. Nature Neuroscience. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–129. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Vance DE, Heaton K, Eaves Y, Fazeli PL. Sleep and cognition on everyday functioning in older adults: Implications for nursing practice and research. Journal of Neuroscience Nursing. 2011;43:261–271. doi: 10.1097/JNN.0b013e318227efb2. [DOI] [PubMed] [Google Scholar]
- Vermeeren A, Coenen AML. Effects of the use of hypnotics on cognition. Progress in Brain Research. 2011;190:89–103. doi: 10.1016/B978-0-444-53817-8.00005-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Memory consolidation in sleep: Dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Siegel JM. Time for the sleep community to take a critical look at the purported role of sleep in memory processing. Sleep. 2005;28:1228–1229. doi: 10.1093/sleep/28.10.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virta JJ, Heikkila K, Perola M, Koskenvuo M, Räihä I, Rinne JO, Kaprio J. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36:1533–1541. doi: 10.5665/sleep.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV, Bokan JA, Kukull WA, Muniz RL, Smallwood RG, Prinz PN. Rapid eye movement sleep measures of Alzheimer's-type dementia patients and optimally healthy aged individuals. Biological Psychiatry. 1984;19:721–734. [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. Journal of Clinical Endocrinology & Metabolism. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Bixler EO, Lin HM, Prolo P, Vela-Bueno A, Chrousos GP. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: Physiologic and therapeutic implications. Journal of Clinical Endocrinology & Metabolism. 2003;88:2087–2095. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- Vlahou EL, Thurm F, Kolassa IT, Schlee W. Resting-state slow wave power, healthy aging and cognitive performance. Scientific Reports. 2014;4:5101. doi: 10.1038/srep05101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning & Memory. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002a;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep–wake cycle: REM-sleep enhancement of anagram problem solving. Cognitive Brain Research. 2002b;14:317–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Randazzo AC, Stone K, Eisenstein R, Feren SD, Kajy S, Schweitzer PK. Tiagabine is associated with sustained attention during sleep restriction: Evidence for the value of slow-wave sleep enhancement? Sleep. 2006;29:433. [PubMed] [Google Scholar]
- Walsh JK, Hall-Porter JM, Griffin KS, Dodson ER, Forst EH, Curry DT, Schweitzer PK. Enhancing slow wave sleep with sodium oxybate reduces the behavioral and physiological impact of sleep loss. Sleep. 2010;33:1217–1225. doi: 10.1093/sleep/33.9.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker M, Payne JD, Benavides JA, Stickgold R. Dreaming of a learning task is associated with enhanced sleep-dependent memory consolidation. Current Biology. 2010;20:850–855. doi: 10.1016/j.cub.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J. Cognitive functioning in the community elderly: The role of sleep and caffeine. Dissertation presented to the University of Hong Kong; 2013. [Google Scholar]
- Ward AM, McLaren DG, Schultz AP, Chhatwal J, Boot BP, Hedden T, Sperling RA. Daytime sleepiness is associated with decreased default mode network connectivity in both young and cognitively intact elderly subjects. Sleep. 2012;36:1609–1615. doi: 10.5665/sleep.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MT, Oler JA, Markus EJ. Hippocampal dysfunction during aging I: Deficits in memory consolidation. Neurobiology of Aging. 1999;20:363–372. doi: 10.1016/s0197-4580(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Webb WB. A further analysis of age and sleep deprivation effects. Psychophysiology. 1985;22:156–161. doi: 10.1111/j.1469-8986.1985.tb01579.x. [DOI] [PubMed] [Google Scholar]
- Webb WB, Levy CM. Age, sleep deprivation, and performance. Psychophysiology. 1982;19:272–276. doi: 10.1111/j.1469-8986.1982.tb02561.x. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Lundgren EM, Florczak SM, Mesulam MM, Weintraub S, Zee PC, Paller KA. Sleep influences the severity of memory disruption in amnestic mild cognitive impairment: Results from sleep self-assessment and continuous activity monitoring. Alzheimer Disease and Associated Disorders. 2010;24:325–333. doi: 10.1097/WAD.0b013e3181e30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, Paller KA. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. Journal of the International Neuropsychological Society. 2012;18:490. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: The Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: The role of the PFC and sleep. Neural Plasticity. 2012 doi: 10.1155/2012/624795. Article ID 624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Erickson KI, Wheeler ME. Sleep continuity and total sleep time are associated with task-switching and preparation in young and older adults. Journal of Sleep Research. 2014 doi: 10.1111/jsr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HL, Gieseking CF, Lubin A. Some effects of sleep loss on memory. Perceptual and Motor Skills. 1966;23:1287–1293. doi: 10.2466/pms.1966.23.3f.1287. [DOI] [PubMed] [Google Scholar]
- Williams HL, Lubin A, Goodnow JJ. Impaired performance with acute sleep loss. Psychological Monographs. 1959;73:1–26. [Google Scholar]
- Williams SE, Horst JS. Goodnight book: Sleep consolidation improves word learning via storybooks. Frontiers in Psychology. 2014;5:184. doi: 10.3389/fpsyg.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of Aging. 2012;33:991. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wixted JT. The psychology and neuroscience of forgetting. Annual Review of Psychology. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Jiang CQ, Lam TH, Liu B, Jin YL, Zhu T, Thomas GN. Short or long sleep duration is associated with memory impairment in older Chinese: The Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–580. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. Risk factors of mild cognitive impairment in older Chinese: Guangzhou Biobank Cohort Study. Dissertation presented to the University of Hong Kong; 2012. [Google Scholar]
- Yaffe K, Blackwell T, Barnes DE, Ancoli-Israel S, Stone KL. Preclinical cognitive decline and subsequent sleep disturbance in older women. Neurology. 2007;69:237–242. doi: 10.1212/01.wnl.0000265814.69163.da. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Journal of the American Medical Association. 2011;306:613. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T. Big correlations in little studies: Inflated fMRI correlations reflect low statistical power—Commentary on Vul et al. (2009) Perspectives on Psychological Science. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Zepelin H, McDonald CS, Zammit GK. Effects of age on auditory awakening thresholds. Journal of Gerontology. 1984;39:294–300. doi: 10.1093/geronj/39.3.294. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV. Melatonin as a hypnotic: Pro. Sleep Medicine Reviews. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]