Abstract
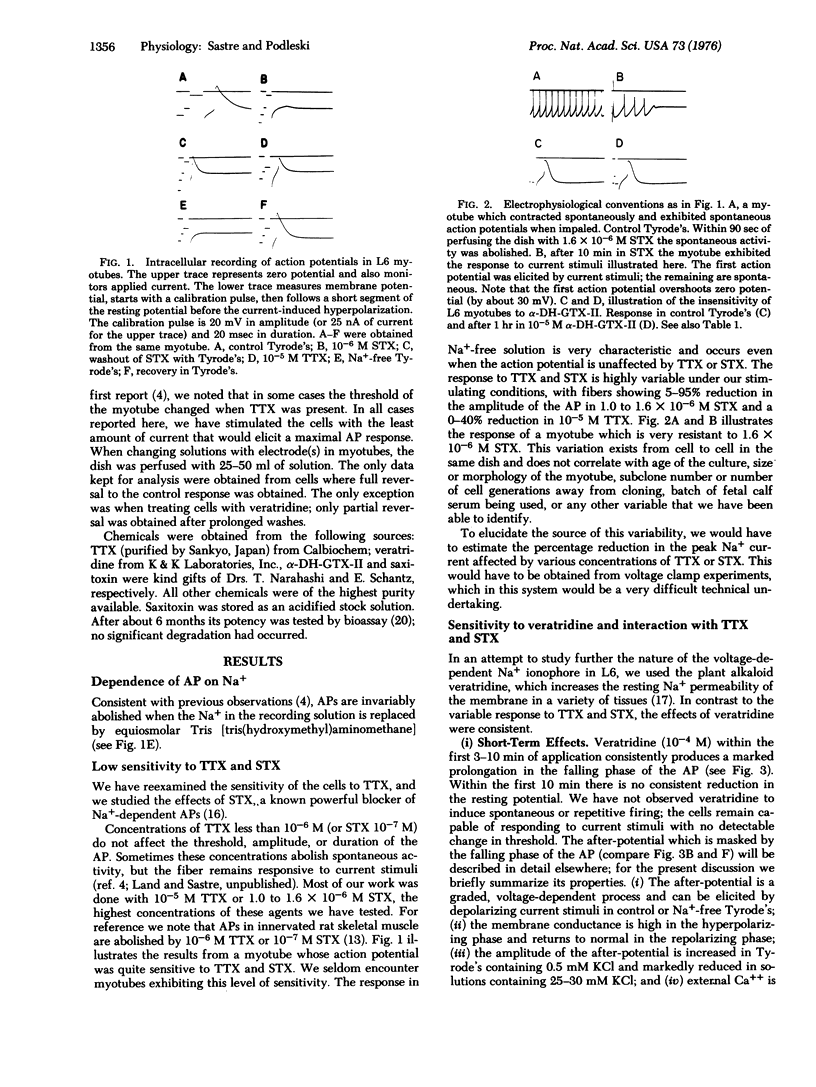
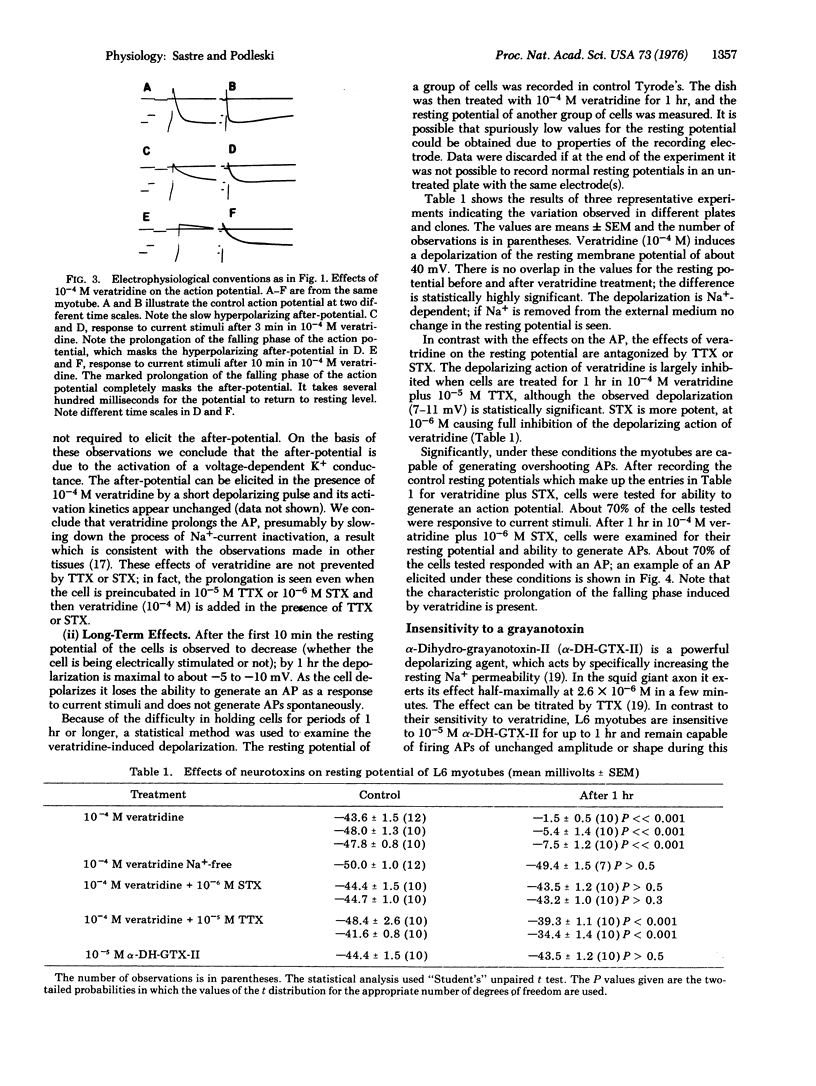
We present a pharmacologic characterization of the Na+ ionophores present in L6 myotubes in vitro. Action potentials are abolished by replacement of the external Na+ by Tris. The amplitude of the action potential is generally resistant to high concentrations of tetrodotoxin (10(-5) M) and saxitoxin (10(-6 M), but the effect of these agents is highly variable. Veratridine (10(-4 M) consistently induces, as a short-term effect, a marked prolongation of the falling phase of the action potential. As a long-term effect, veratridine consistently induces a Na+-dependent reduction in the resting potential of the cell. The effects of veratridine on the action potential are not antagonized by tetrodotoxin or saxitoxin. However, the effects of veratridine on the resting potential are strongly antagonized by tetrodotoxin (10(-5) M) and fully inhibited by saxitoxin (10(-6) M). Significantly, under conditions where saxitoxin has fully inhibited the effects of veratridine on the resting potential, the myotubes are capable of generating overshooting action potentials. In contrast to their sensitivity to veratridine, L6 myotubes are insensitive to 10(-5) M alpha-dihydro-grayanotoxin-II. These results are discussed in the contexts of developmental significance and current views about Na+ ionophores.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E. The pharmacology of batrachotoxin. IV. Interaction with tetrodotoxin on innervated and chronically denervated rat skeletal muscle. J Pharmacol Exp Ther. 1972 Mar;180(3):683–697. [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Nirenberg M. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3759–3763. doi: 10.1073/pnas.70.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. R. Electrophysiological activity of tetrodotoxin on the resting membrane of the squid giant axon. Comp Biochem Physiol A Comp Physiol. 1971 Sep 1;40(1):71–82. doi: 10.1016/0300-9629(71)90148-4. [DOI] [PubMed] [Google Scholar]
- Grundfest H. Heterogeneity of excitable membrane: electrophysiological and pharmacological evidence and some consequences. Ann N Y Acad Sci. 1966 Jul 14;137(2):901–949. doi: 10.1111/j.1749-6632.1966.tb50208.x. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Marshall M. W. Tetrodotoxin-resistant action potentials in newborn rat muscle. Nat New Biol. 1973 Jun 6;243(127):191–192. doi: 10.1038/newbio243191a0. [DOI] [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Hogan P. M., Albuquerque E. X. The pharmacology of batrachotoxin. 3. Effect on the heart Purkinje fibers. J Pharmacol Exp Ther. 1971 Mar;176(3):529–537. [PubMed] [Google Scholar]
- Kidokoro Y. Development of action potentials in a clonal rat skeletal muscle cell line. Nat New Biol. 1973 Jan 31;241(109):158–159. doi: 10.1038/newbio241158a0. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y. Developmental changes of membrane electrical properties in a rat skeletal muscle cell line. J Physiol. 1975 Jan;244(1):129–143. doi: 10.1113/jphysiol.1975.sp010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Heinemann S. Synapse formation between clonal muscle cells and rat spinal cord explants. Nature. 1974 Dec 13;252(5484):593–594. doi: 10.1038/252593a0. [DOI] [PubMed] [Google Scholar]
- Land B. R., Sastre A., Podleski T. R. Tetrodotoxin-sensitive and -insensitive action potentials in myotubes. J Cell Physiol. 1973 Dec;82(3):497–510. doi: 10.1002/jcp.1040820318. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M., Renaud D., Teillet M. A., Le Douarin G. H. Cholinergic differentiation of presumptive adrenergic neuroblasts in interspecific chimeras after heterotopic transplantations. Proc Natl Acad Sci U S A. 1975 Feb;72(2):728–732. doi: 10.1073/pnas.72.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. W., Ward M. R. Anode break excitation in denervated rat skeletal muscle fibres. J Physiol. 1974 Jan;236(2):413–420. doi: 10.1113/jphysiol.1974.sp010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Albuquerque E. X., Deguchi T. Effects of batrachotoxin on membrane potential and conductance of squid giant axons. J Gen Physiol. 1971 Jul;58(1):54–70. doi: 10.1085/jgp.58.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Seyama I. Mechanism of nerve membrane depolarization caused by grayanotoxin I. J Physiol. 1974 Oct;242(2):471–487. doi: 10.1113/jphysiol.1974.sp010718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Narahashi T., Keeler R. F. Effects of veratrum alkaloids on membrane potential and conductance of squid and crayfish giant axons. J Pharmacol Exp Ther. 1973 Jan;184(1):143–154. [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. Membrane properties underlying spontaneous activity of denervated muscle fibres. J Physiol. 1974 May;239(1):125–153. doi: 10.1113/jphysiol.1974.sp010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Increase in sodium permeability of squid axon membranes by -dihydrograyanotoxin II. J Pharmacol Exp Ther. 1973 Feb;184(2):299–307. [PubMed] [Google Scholar]
- Soeda Y., O'Brien R. D., Yeh J. Z., Narahashi T. Evidence that alpha-dihydrograyanotoxin II does not bind to the sodium gate. J Membr Biol. 1975 Aug 11;23(1):91–101. doi: 10.1007/BF01870246. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H. Acetylcholine responses on clonal myogenic cells in vitro. J Physiol. 1975 May;247(2):393–405. doi: 10.1113/jphysiol.1975.sp010937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]



