Abstract
Objective
Deficiency in complement factor 5 (C5) protects against arthritis development. However, there is currently no approach translating these findings successfully into arthritis therapy, as by targeting the key component, C5a.
Methods
We generated an anti-C5a vaccine by incorporating the unnatural amino acid p-nitrophenylalanine (4NPA) at selected sites into murine C5a. C5a-4NPA variants were screened for their immunogenicity on different arthritis susceptiple MHCII backgrounds. A vaccine candidate was tested for its impact on disease in the collagen induced arthritis (CIA) mouse model. Immunity towards endogenous C5a as well as type II collagen was monitored and characterized.
Results
The replacement of a single tyrosine residue in position 35 (Y35) by 4NPA generated an anti-C5a vaccine, which partly protected mice from developing CIA while strongly ameliorating severity of clinical disease. Although differing in just three atoms from wtC5a, C5aY354NPA induced loss of T and B cell tolerance towards the endogenous protein in mice expressing MHCII H2q molecules. Despite differential B cell epitope recognition, antibodies induced by either wtC5a or C5aY354NPA both neutralized C5a. Thus, anti-wtC5a IgG titers during arthritis priming were potentially critical for disease protection as high titers of C5a-neutralizing antibodies post disease onset were unable to reverse the arthritis course.
Conclusion
Our results suggest the most effective anti-C5a treatment in arthritis to be accomplished by a preventive vaccination strategy, while conventional biological or small molecule treatments targeting the C5a:C5aR axis risk missing the optimal therapeutic window for intervention during the subclinical priming phase of the disease.
The complement system constitutes an important defense line of innate immunity in combating infections but its inappropriate activation can result in tissue injury and damage. Upon activation of either complement pathway, complement component 5 (C5) is cleaved by C5 convertases to generate complement factor 5a (C5a) and complement factor 5b (C5b). C5b is an essential component of the terminal membrane attack complex (MAC) which combats infections, whereas C5a, a glycoprotein of 74 amino acids (≈11 kDa), acts as potent anaphylatoxin and powerful leukocyte chemoattractant capable of stimulating and modulating inflammatory responses (1). Leukocyte activation by C5a requires binding to its high affinity canonical G protein-coupled transmembrane receptor C5aR (CD88) (2). Based on the cell type, C5a binding to CD88 triggers recruitment of neutrophils, monocytes and macrophages, release of granular enzymes, enhanced cell adhesion molecule expression, delayed or enhanced apoptosis, phagocytosis, oxidative burst, histamine secretion and release, cytokine and chemokine production, vasodilatation and chemotaxis (3).
C5a is also implicated in the pathogenesis of rheumatoid arthritis (RA).RA affects about 0.5–1% of the world population and is a chronic systemic autoimmune disease involving autoimmunity to citrullinated proteins, IgG and articular type II collagen (CII). In the clinic RA presents by joint swelling, erythema and pain, resulting in immobility and stiffness. Further progression of the disease causes joint destruction as well as loss of bone density and architecture. Susceptibility to RA is associated with human leucocyte antigen (HLA)-DRB1 allels, in particular HLA-DRB1*0401 and HLA-DRB1*0101, as well as, among others, the TRAF1-C5 locus (4, 5).TRAF1 encodes an intracellular protein that mediates signal transduction through tumor necrosis factor (TNF) receptors 1 and 2. TNF is a critical cytokine in RA pathogenesis and treatment with TNF antagonists proofed effective (6–8). Biological data for C5 are equally compelling. C5a can be found in patients’ inflamed synovium in chemotactic concentrations (9) and mice with deficiency in C5 are resistant to antibody-mediated inflammatory arthritis models (10, 11). Nevertheless, only the anti-C5 antibody eculizumab has made it to clinical medicine to date but its effect on RA is weak, while another approach blocking C5aR in arthritis patients failed completely (12). Although these studies prospect low to no efficacy of C5a:C5aR axis targeting in established RA, it is yet possible, that antibody and complement mediated mechanisms are important during disease initiation. Recently, C5aR engagement has been highlighted as particularily essential process in early neutrophil recruitment into the joint (13). Albeit a role for C5a is still evident in later disease stages (14–17), therapeutic targeting of C5a just then may not translate into a clinical effect, as other pathways in the now complex inflammation process possibly overide this. Further, we believe it to be important to retain the possibility, that certain subtypes of RA might be more complement dependent than others. Given the well documented importance in experimental arthritis models and its possible role in early stages of RA we believe the design of a proper C5a:C5aR axis targeting therapy to require increased focus.
Previously we reported a vaccination strategy for the neutralization of endogenous C5a without affecting C5 cleavage and subsequent C5b activity (18). This vaccine required fusion with a large bacterial protein leading to protein instability and suboptimal immune responses. In the present study, we incorporated the unnatural amino acid pNO2Phe (4NPA), a phenylalanine derivative, which shares high structural similarity with tyrosine and phenylalanine (19) into C5a in a site-specific manner. The site-specific incorporation of pNO2Phe, 3NO2Tyr and SO3 Tyr into the self-proteins TNFα, retinol binding protein (RBP4) and EGF has previously been shown to induce long-lived autoantibody responses to their respective endogenous counterparts (19–21). Herein we apply this strategy to the generation of an anti-inflammatory vaccine, which might ultimately be translatable to RA susceptible individuals.
MATERIALS AND METHODS
Mice
Male B10.Q, B10R.III, B10.DR4 and (BALB/c × B10.Q)F1 mice at the age of 8–12 weeks were used in the experiments. All animals were bred and kept in our animal facility (http://www.inflam.mbb.ki.se). B10.DR4 mice express human DRB*0401 and human CD4 but no murine MHCII molecules (22). All animal experiments were approved by the local laboratory animal ethics committee and were run age-matched, blinded and randomly grouped.
Incorporation of 4NPA into mC5a
Mouse C5a was expressed either with an N-terminal methionine and a C-terminal hexahistidine tag, or with a N-terminal histidine tag followed by a cleavable factor Xa sequence. Briefly, plasmids harboring the C5a gene were modified to have an amber stop codon at the residue intended for unnatural amino acid incorporation (e.g. pNX-6His-Xa-mC5a-Y35TAG). These plasmids were separately transformed into E. coli with a plasmid containing an Methanococcus jannaschii derived aminoacyl-tRNA synthetase/tRNA pair specific for 4-nitrophenylalanine (pEVOL-4NPA) (23). A 1L GMML culture containing 50 µg/mL kanamycin, 50 µg/mL chloramphenicol and 2-NPA (4 mM) was grown at 37°C to an OD600 of 0.5. The culture was then induced by adding arabinose to a final concentration of 0.05% and incubated with shaking at 30°C for 18 hrs. The His-tagged protein was purified by Ni-NTA chromoatography under denaturing conditions according to the manufactures protocol (QIAexpressionist, 2003).
The protein was refolded while still attached to the Ni-NTA resin and eluted using 300mM imidazole. Eluted protein with N-terminal histidine was cleaved with factor Xa to generate the native C5a sequence. Endotoxin was removed with Detoxi-Gel (Thermo Scientific). Protein samples were tested for endotoxin (Pierce LAL Chromogenic Endotoxin Quantitation Kit) and confirmed to have less than 0.2 EU/mL endotoxin.
The sequence of the expressed and cleaved murine C5a wild type protein is: NLHLLRQKIEEQAAKYKHSVPKKCCYDGARVNFYETCEERVARVTIGPLCIRAFNECCTIANKIRKESPHKPVQLGR. The mass of the wildtype protein was calculated for 8895 and 8924 for C5aY354NPA. Respective masses were certified by ESI mass spectrometry.
Detection of anti-C5a antibodies in mouse serum
ELISA plates (MaxiSorp®, Nunc, Roskilde, Denmark) were coated with recombinant C5a protein (5 mg/ml, wt or 4-NPA mutants). Serum samples were titrated by logarithmic dilution and incubated 1h at RT. After washing bound IgG was detected by addition of HRPO-conjugated goat anti-mouse IgG Fcc-specific antibody (Jackson ImmunoResearch, PA) and ABTS substrate (Roche Diagnostics, Mannheim, Germany). The absorbance was read at 405 nm (Synergy-2; Bio-Tek, Winooski, VT, USA). Anti-wtC5a serum IgG in arthritis experiments was quantified using an anti-C5a IgG standard constituted from pooled serum of previous vaccination experiments. Based on the standard curve titers were calculated in arbitrary units (AU).
T cell restimulation
Spleens were collected at indicated time points. Splenocytes were seeded in microtiter plates at 1×106/well in triplicates and treated with respective antigens at indicated concentrations. All cells were cultured in DMEM+Glutamax-I (Gibco) supplemented with 5% heat inactivated calf serum and penicillin/streptomycin. For detection of IFN-γ 50 µl of culture supernatant was removed after 96hrs and transferred to capture-antibody (R46A2, 5 µg/ml, our collection) coated ELISA plates for 1h at RT. After washing AN18.17.24-biotin (0.6 µg/ml, Mabtech) followed by europium conjugated Streptavidin (DELFIA Eu-N1, Perkin-Elmer Life Sciences, Turku, Finland) in DELFIA assay buffer (Perkin-Elmer) was added for detection. After addition of DELFIA enhancement solution (Perkin-Elmer) plates were read in a fluorometer (Victor 1420, Perkin-Elmer). Cytokine concentrations were calculated based on the respective standard curves.
Collagen arthritis induction and preventive C5a-vaccination
(BALB/c × B10.Q mice) F1 mice were vaccinated with 50µg/mouse of C5aY354NPA, wtC5a (+/− 6xHIS) or PBS emulsified 1:1 in CFA (Difco, Detroit, MI), s.c. on day 0 and were boosted (s.c.) on days 14 and 46 with 25 µg/mouse emulsified 1:1 in IFA. Arthritis was induced on d18 by immunization with 100 µg/mouse of rat CII (rCII), emulsified 1:1 in CFA (Difco). Animals were boosted with 50 µg/mouse rCII emulsified 1:1 in IFA on day 39. Arthritis development was followed by visual scoring in a blinded manner using an extended scoring protocol (24).
Anti-CII antibody response
The amounts of total anti-CII IgG or CIIC1 and U1 epitope specific responses were determined using quantitative ELISA (25). ELISA plates (MaxiSorp®) were coated with rCII (10 mg/ml) or synthetic triple helical peptides (GL Biochem, Shanghai, China) covering the CIIC1 (GARGLTGRPGDA) and CIIU1 (GLVGPRGERGFP) epitope. Serum samples were titrated in logarithmic dilutions and incubated over night at 4°C. After washing bound IgG was detected using biotinylated goat anti-mouse IgG Fcγ-specific antibody (Jackson ImmunoResearch), followed by addition of europium-conjugated Streptavidin (Perkin-Elmer). Plates were developed and read using the DELFIA-system as described above. Total Anti-CII IgG concentration was calculated based on an in-house anti-CII IgG serum standard. Epitope-specific IgG concentrations were calculated based on respective CIIC1 and CIIU1 mAb standard curves.
C5a neutralization assay
Experiments were performed as described elsewhere (18). Briefly, antibody mediated C5a neutralization was assayed on rat basophilic leukemia (RBL) cells transfected with human C5aR, which release N-acetyl-µ-D-glucosaminidase (NAG) upon binding of C5a to the receptor. This can be quantified by addition of its substrate.
C5a-peptide ELISA
Peptides were immobilized on avidin (1 mg/ml) coated ELISA plates (MaxiSorp®) at a concentration of 1 mg/ml. Individual serum samples were applied at 1:1000 dilution and bound IgG was detected by HRPO-conjugated rabbit anti-mouse IgG antibody (Dako, Glostrup, Denmark), followed by addition of TMB reagent (BD Biosciences, San Diego, CA, USA). Development was terminated by addition of 0.5M H2SO4 and absorbance at 450nm was read on a Biochrom Asys Expert 96 microplate reader (Biochrom Ltd., Cambridge, UK).
Statistics
Data were analyzed by GraphPad prism software for Gaussian distribution by D’Agostino and Pearson omnibus normality test. Accordingly, data sets were subjected to Mann-Whitney U-test (non-parametric data) or Student’s t-test (parametric data). Arthritis incidence was analyzed using Fisher's exact test. p<0.05 is indicated in figures with one, p<0.01 with double and p<0.001 with triple asterisks.
RESULTS
Incorporation of 4NPA into murine C5a
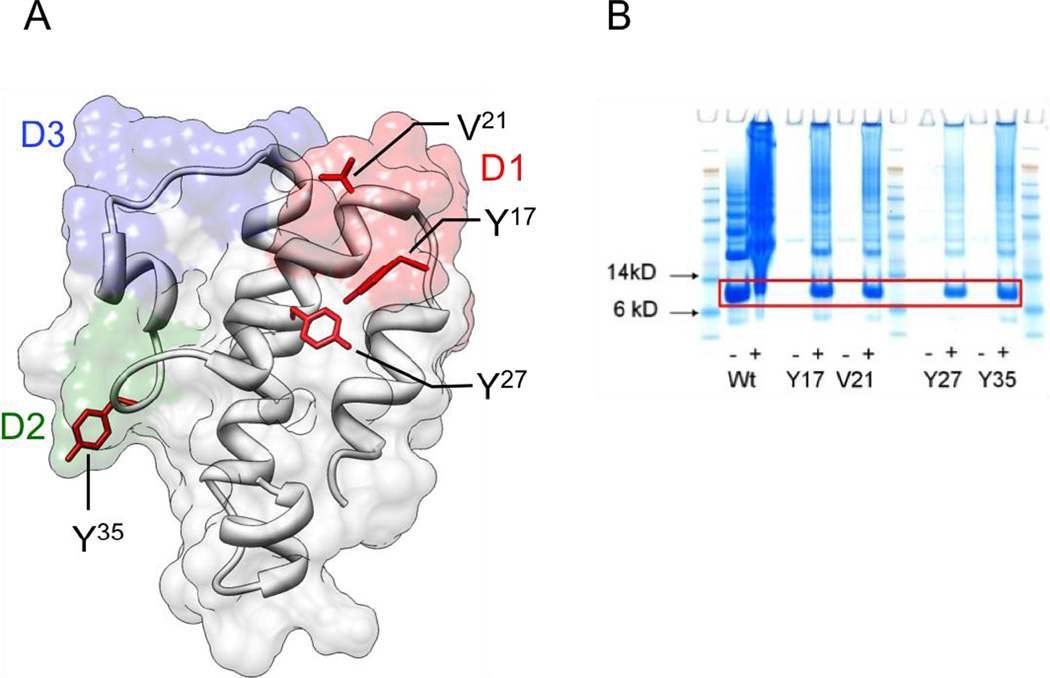
4NPA was incorporated at different positions in mC5a by recombinant protein expression in E. coli, as previously described (19–21, 23). The decision of which mC5a residues to modify was based on two criteria. Firstly, the residue should be solvent exposed in order to maintain protein stability (Figure 1A). Secondly, the residue should be within a region of the protein that is efficiently processed and potentially presented by MHCII molecules. Additionally, in the case of human C5a, it is known that peptides generated from the D1 and D2 loops are able to inhibit C5a:C5aR binding, whereas those in the D3 region do not (26). We therefore biased our selection towards the D1 and D2 regions in the mouse protein.
Figure 1. Immunization with 4NPAmC5a mutants.
(A) Model of mC5a based on the crystal structure data of human C5a (3hqa.pdb). Amino acid side chains chosen for 4NPA incorporation are highlighted. (B) SimlyBlue stained SDS PAGE of Ni-NTA purified C5a-4NPA variants (9.9 kDa). Site-specific incorporation of 4NPA was accomplished by mutating the codons for Y17, V21, Y27 or Y35 to the TAG amber codon. The different variants of mC5a were expressed in E. coli [+/− IPTG for wt and +/− 4NPA for mutants (+IPTG)] in presence of the corresponding tRNA/aminoacyl-tRNA-synthetase pair.
Several residues were selected for unnatural amino acid incorporation (K16, Y17, V21, K23, Y27, N33, Y35, H71, and K72). Wild type and mutant C5a proteins were expressed with either a C-terminal hexahistidine tag, or with a factor Xa cleavable N-terminal hexahistidine tag, which was removed following purification. Of the mutants expressed, Y17, V21, Y27, and Y35 afforded the highest expression levels and were used for subsequent vaccination experiments (Figure 1B). Mutant proteins were expressed as inclusion bodies, and so were subsequently refolded, cleaved with factor Xa (where applicable), and purified to homogenous monomeric solutions for immunization. Highly specific incorporation of the unnatural amino acid was confirmed by polyacylarmide gel electrophorsesis and ESI-mass spectrometry (data not shown).
Induction of anti-wtC5a IgG on arthritis susceptible MHCII
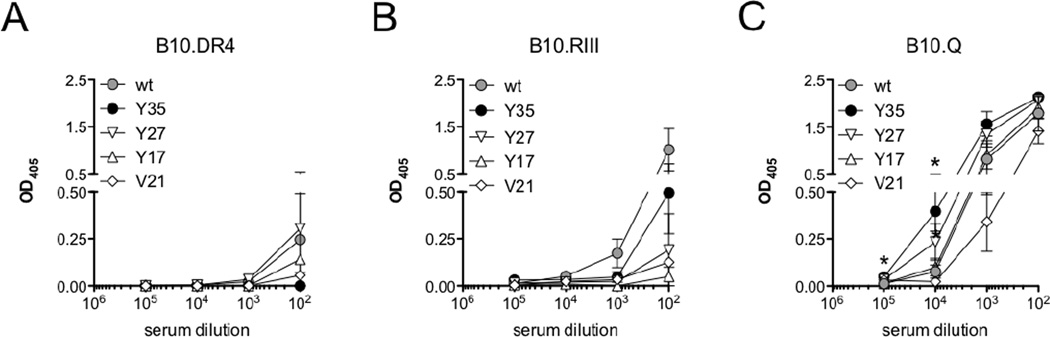
The development of arthritis upon immunization with type II collagen (CII) is MHCII dependent. Therefore, we screened the C5a-4NPA mutants in the arthritis susceptible mouse strains B10.RIII, B10.DR4 and B10.Q for the ability to induce an IgG response that cross-reacts with wtC5a and able to neutralize the endogenous antigen. These strains all have a B10 genetic background but differ in MHC molecules. Three mice per group, per strain, were independently immunized with wtC5a, C5aY354NPA, C5aY274NPA, C5aY174NPA or C5aV214NPA. Individual sera were analyzed for IgG responses towards the respective immunized proteins (data not shown) as well as anti-mC5a (wtC5a) responses on d14. Neither wtC5a nor 4NPA-modified C5a induced anti-wtC5a autoantibody responses in human DR4 mice(Figure 2A). Likewise, no C5a-mutant induced a strongly wtC5a-crossreactive response in mice with H2r background (Figure 2B). In B10.Q mice wtC5a cross-reactive IgG in sera from C5aY354NPA immunized animals revealed titers, which were significantly above those of C5aY174NPA, C5aV214NPA and wtC5a-induced antibodies at 104 and 105 serum dilutions (Figure 2C).
Figure 2. Arthritis-susceptible MHCII dependent anti-mC5a IgG induction.
(A–C) Collagen induced arthritis susceptible mouse strains expressing the human MHCII DR4 allele (hDR4, A) or murine H-2r (B) and H-2q (C) haplotypes were injected with the different C5a-4NPA variants (3 mice/group). Sera at d14 were screened for an IgG response crossreacting with murine wtC5a. Data show means with SD.
C5aY354NPA induces a wtC5a cross-reactive T cell response
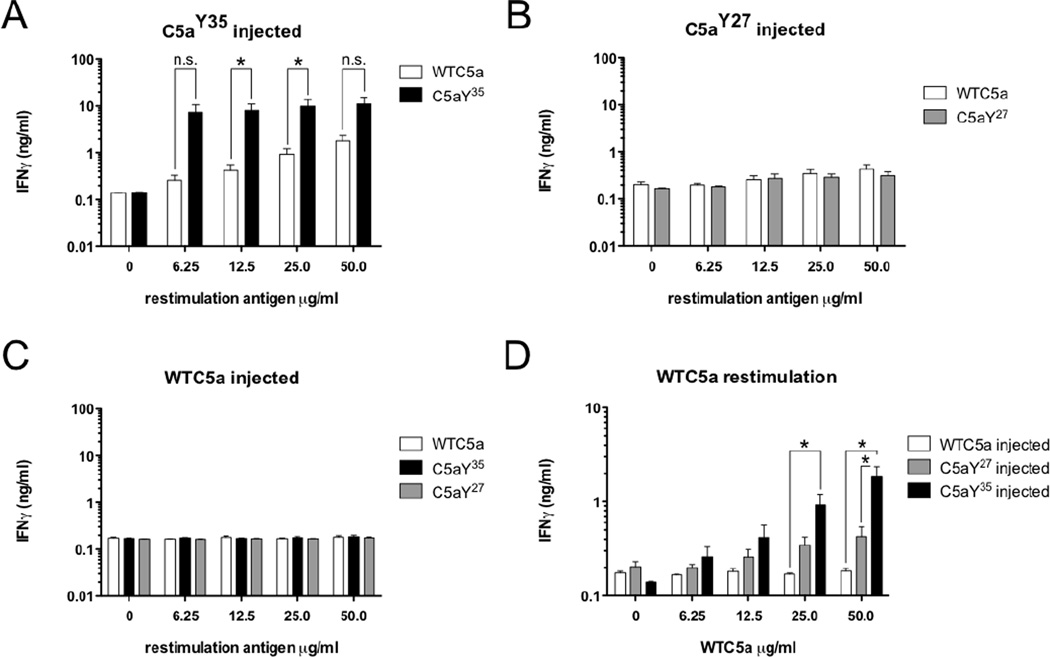
To determine whether breaking of tolerance towards mC5a on H2q-background by C5aY354NPA, C5aY274NPA or wtC5a itself is restricted to the B cell level or also involves T cells, splenocytes of C5aY354NPA, C5aY274NPA and wtC5a immunized animals were prepared at d30 and restimulated in vitro with the respective antigens at indicated concentrations. Secreted IFN-γ(Figure 3A–D) was measured 96h after restimulation. Splenocyte restimulation of C5aY274NPA and wtC5a immunized animals revealed no cytokine production above background (Figure 3B, C). In contrast, immunization of B10.Q animals with C5aY354NPA gave rise to a strongly C5aY354NPA-reactive T cell population (Figure 3A). Though they produced significantly less IFN-γ, splenic T cells obtained from C5aY354NPA-injected mice also responded to restimulation with wtC5a. This IFN-γ response was significantly higher compared to titers induced by wtC5a restimulation of splenocytes obtained from C5aY274NPA or wtC5a-immunized animals (Figure 3D). Based on these results, C5aY354NPA was chosen as candidate for preventive vaccination studies using the classical collagen induced arthritis (CIA) model.
Figure 3. B10.Q splenic T cell restimulation.
(A–C) Spleen cells obtained from individual C5aY354NPA (A), C5aY274NPA (B) and wtC5a (C) immunized B10.Q mice (n=3/group) were re-stimulated with increasing concentrations of the respective antigen as well as wtC5a for 96h. IFNγ production was quantified by ELISA. Data show means with SEM, significance was calculated by Mann-Whitney U-test. (D) Summarization of IFN-γ data for wtC5a re-stimulated spleen cells obtained from wtC5a, C5aY354NPA and, C5aY274NPA immunized B10.Q mice. Data show means with SEM, significance was calculated by Mann-Whitney U-test.
Preventive vaccination with C5aY354NPA ameliorates CIA
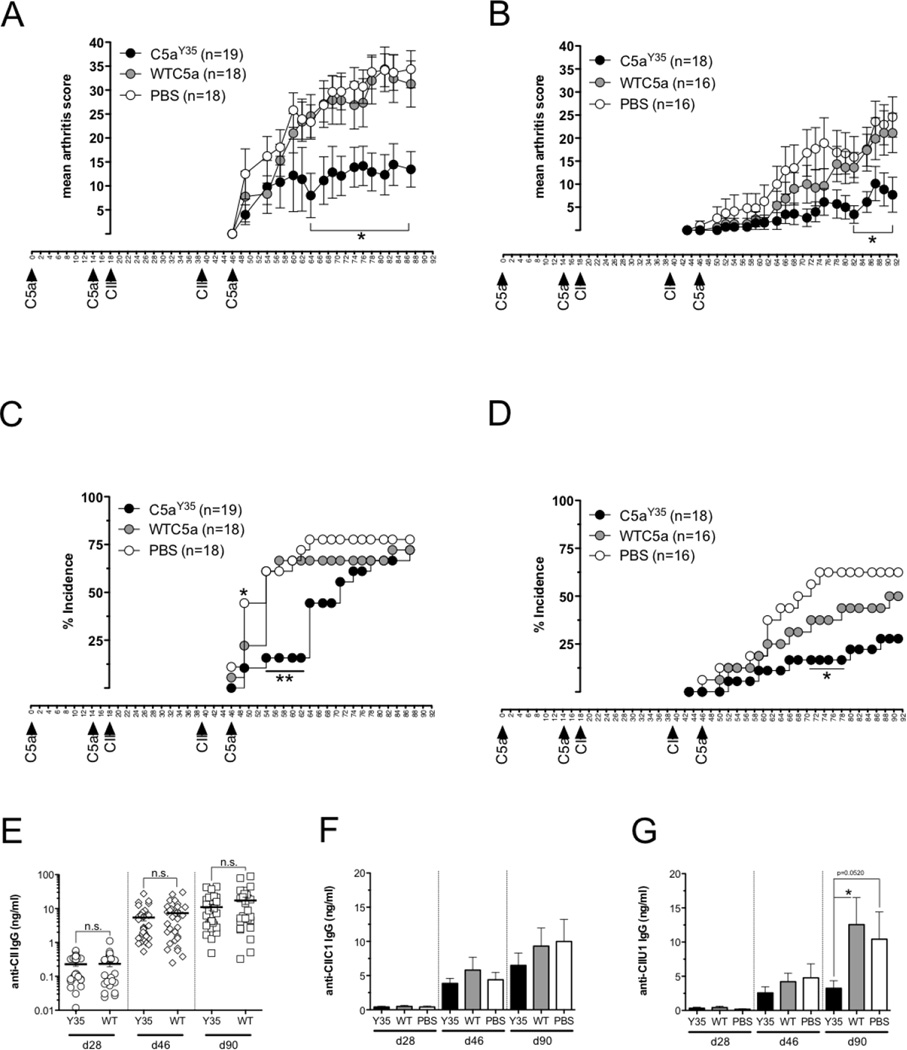
Collagen induced arthritis (CIA) shares several clinical, histopathological and immunological features with human RA. Upon immunization with heterologous type II collagen (CII) mice develop polyarthritis triggered and in part driven by CII autoimmunity, which can be reflected by anti-CII autoantibodies. The impact of preventive vaccination using C5aY354NPA on arthritis was investigated. Highly CIA susceptible (BALB/c × B10.Q) F1 male mice were vaccinated in two separate experiments with 6xHIS-tagged (Figure 4A, C) or tag-free wtC5a and C5aY354NPA (Figure 4B, D); PBS was used as the control. Mice were boosted with protein or PBS on d14 and d46. On d18 CIA was induced by injection of collagen II (CII) and mice were CII-boosted on d39. In the first experiment involving 6xHIS C-terminally tagged proteins, there was no difference in either arthritis incidence or clinical score between wtC5a-vaccinated mice and animals in the PBS control group. Disease onset in C5aY354NPA-vaccinated animals was attenuated, and disease prevalence and score was significantly reduced compared to wtC5a and PBS-injected mice (Figure 4A, C). In a second experiment using untagged proteins, vaccination with wtC5a did not significantly reduce arthritis scores compared to PBS-injected animals. However, arthritis incidence in wtC5a-vaccinated animals was reduced. As seen with 6xHIS-C5aY354NPA, the tag-free protein significantly reduced disease scores and gave partial protection from arthritis (Figure 4B, D).
Figure 4. Preventive C5a vaccination in collagen induced arthritis.
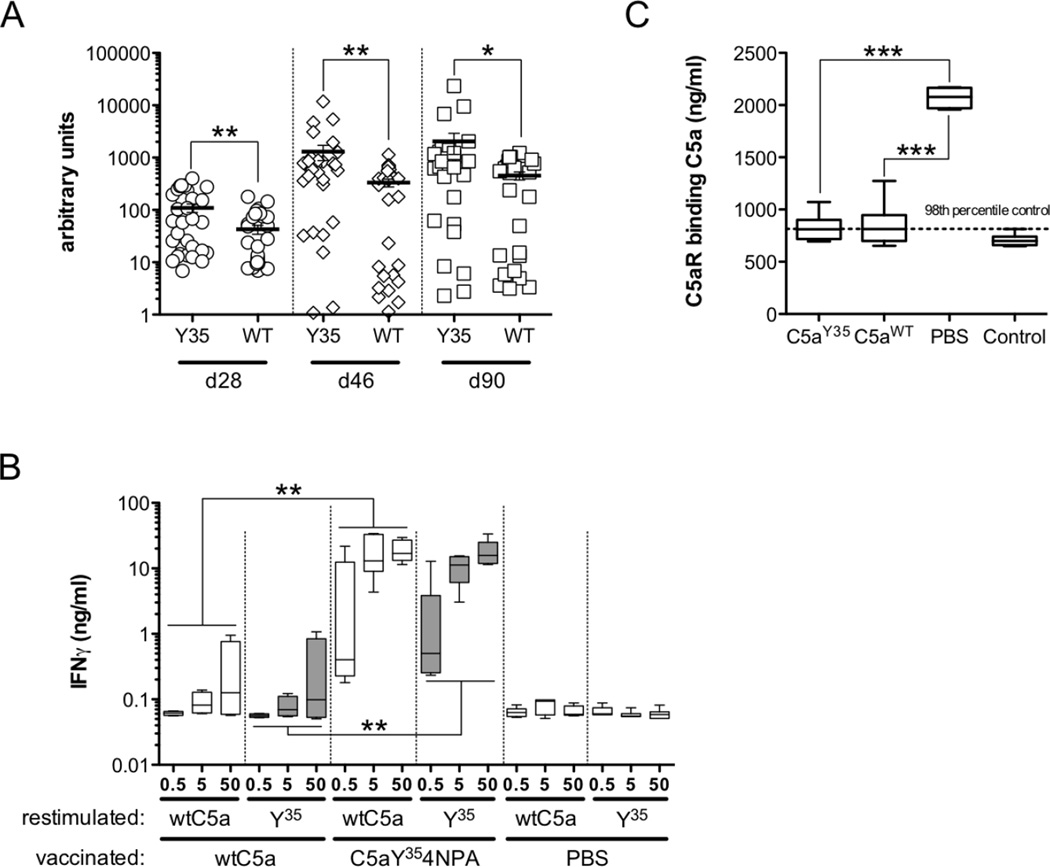
(A–D) Highly CIA susceptible (BALB/c × B10.Q) F1 male mice () were vaccinated s.c. with 50 µg 6xHIS tagged (A, C; C5aY354NPA n=19, wtC5a n=18, PBS n=18) or non-tagged (B, D; C5aY354NPA n=18, wtC5a n=16, PBS n=16) proteinor PBS on d0 and boosted with 25 µg of protein or PBS on d14 and 46. CIA was induced on d18 with one booster injection on d39. Serum samples were taken on d28, d46 and d90. Mean arthritis scores (with SEM) of sick animals (A, B) and arthritis incidence are shown (C, D). Significance in arthritis scores was calculated by Mann-Whitney U-test. (e) Anti-type II collagen antibody titers in sera of all animals at d28, d46 and d90 (C5aY354NPA n=37, wtC5a n=34, PBS n=34) were measured by quantitative ELISA. Data show means with SEM, significance was calculated by Mann-Whitney U-test. (F, G) Anti-CIIC1 (E) and anti-CIIU1 IgG titers (G) in individual sera of all animals at d28, d46 and d90 (C5aY354NPA n=37, wtC5a n=34, PBS n=34) were measured by quantitative ELISA. Data show means with SEM, significance was calculated by Mann-Whitney U-test.
Nevertheless there was a significant amelioration of clinical disease by C5aY354NPA vaccinations, none of them manifested in a significant drop in CII-specific IgG titers (Figure 4E). Although no differences in anti-CII autoantibody titers could be detected between C5aY354NPA and wtC5a vaccinations, the recognition of individual B cell epitopes that positively correlate with arthritis can differ. Therefore we analyzed IgG titers specific for the CIIC1 (Figure 4F) and CIIU1 epitopes (Figure 4G). Comparable to total anti-CII IgG, anti-CIIC1 and CIIU1 IgG titers increased in all animals over time. C5aY354NPA vaccinated mice revealed a non-significant tendency towards lower anti-CIIC1 antibody titers. Antibody levels recognizing the CIIU1 epitope were strongly decreased in animals having received C5aY354NPA vaccinations compared to wtC5a-vaccinated mice or PBS control groups.
Vaccination with C5aY354NPA results in increased anti-wtC5a antibody titers and sustained loss of CD4 T cell tolerance towards wtC5a
Throughout all preventive vaccination experiments animals were bled at days 28, 46 and 90 and sera were analyzed for anti-wtC5a antibody titers (Figure 5A). The analysis of all individual animals in vaccination experiments revealed, that anti-wtC5a IgG titers induced by C5aY354NPA vaccinations were about double to quadruple elevated (AU means: 109.7 (d28), 1296 (46), 2037 (d90)) compared to wtC5a-induced titers (AU means: 42.72 (d28), 332.4 (d46), 453.1 (d90).
Figure 5. B and T cell responses to wtC5a in vaccinated animals.
(A) Anti-wtC5a IgG titers in serum samples obtained from vaccinated animals at d28, d46 and d90 (C5aY354NPA n=37, wtC5a n=34) were quantified by ELISA and calculated in arbitrary units (AU). Data show means with SEM, significance was calculated by Mann-Whitney U-test.(B) Splenic T cells obtained from C5aY354NPA and wtC5a vaccinated animals (n=3/group) at d90 were re-stimulated with indicated concentrations of protein and IFN-γ secretion was quantified after 96h. Data show means with SEM, significance was calculated by Mann-Whitney U-test.(C) To analyze C5a neutralization by anti-wtC5a IgG, a rat basophilic leukemia (RBL) cell line transfected with human C5aR was incubated with a pre-mix of mC5a and sera (n=8/group) with identical anti-C5a IgG titers obtained from individual C5aY354NPA, wtC5a and PBS vaccinated mice at d90. N-acetyl-β-D-glucosaminidase (NAG) release upon binding of C5a to the receptor was quantified. Significance was calculated by unpaired Student’s t-test.
At the end of preventive vaccination experiments we re-investigated the antigen specific reactivity of splenic T cells. Splenocytes from C5aY354NPA, wtC5a or PBS vaccinated animals, were isolated at d92 and restimulated with either wtC5a or C5aY354NPA. IFN-γ production was quantified after 96h. C5aY354NPA vaccinated animals responded to both wtC5a and C5aY354NPA-restimulation (Figure 5B). Splenic T cells isolated post arthritis experiments and pulsed with wtC5a or C5aY354NPA released IFN-γ titers comparable to those detected in d30 restimulation experiments. Cells obtained from wtC5a-vaccinated animals also revealed a concentration dependent response to wtC5a and C5aY354NPA-restimulation. However, as seen with wtC5a-splenocyte stimulations on d30, IFN-γ titers were significantly reduced compared to those from C5aY354NPA-vaccinated mice.
C5aY354NPA and wtC5a-induced antibodies both block the C5a:C5aR interaction but recognize different epitopes
Vaccination with both C5aY354NPA and wtC5a induced wtC5a-reactive IgGs. However, only mice vaccinated with C5aY354NPA revealed significant relief of clinical symptoms and were partially protected from disease. To analyze whether this was due to higher anti-wtC5a titers in C5aY354NPA-vaccinated animals or additional functional differences between C5aY354NPA and wtC5a-induced antibodies, we analyzed the ability of the antibodies to block C5a-binding to its receptor. Eight sera with identical anti-wtC5a IgG titers obtained from C5aY354NPA, wtC5a and PBS-vaccinated animals at d90 were preincubated with recombinant mC5a before addition to the C5aR-expressing RBL cells. Sera from the PBS-vaccinated control group did not block C5a:C5aR binding (Figure5C). Both sera from C5aY354NPA and wtC5a-vaccinated mice significantly inhibited binding of C5a to its receptor. No differences in C5a-neutralization by anti-wtC5a antibodies induced by the different vaccinations could be detected between these two groups.
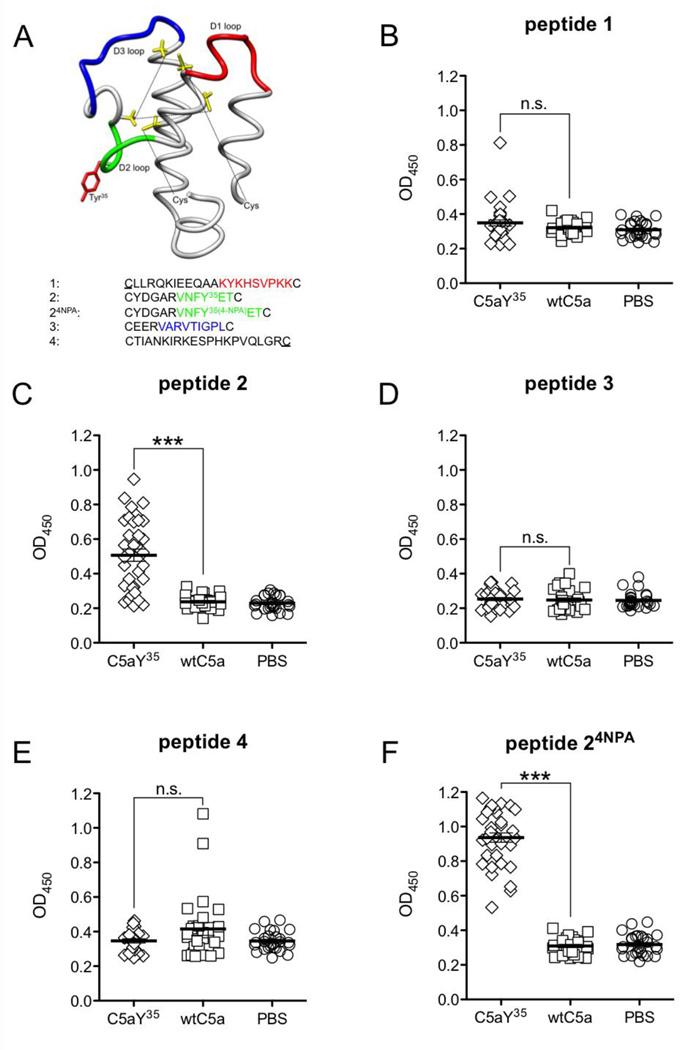
To further probe potential differences between the C5aY354NPA and wtC5a-induced antibodies, we performed epitope-mapping studies. Mimicking the loop structure of C5a, five cyclic peptides were designed from the mC5a structure by using internal cysteine residues along with two additionally introduced cysteine residues at the very N- and C-terminus (Figure 6A). Synthetic, biotinylated peptides were immobilized on avidin-coated ELISA plates and antibodies in pooled sera obtained at d90 from either C5aY354NPA, wtC5a or PBS-vaccinated animals as well as naive (BALB/c × B10.Q)F1 male mice were analyzed for binding. A differential pattern of C5aY354NPA and wtC5a-induced antibody reactivities was observed. Replacement of Y35 in peptide 2 by 4NPA resulted in a strong and exclusive binding of antibodies in sera obtained from C5aY354NPA-vaccinated animals (Figure 6F). Antibodies also bound the unmodified peptide 2 (Figure 6C). In addition to the observed IgG-binding to peptide 24NPA and peptide 2, sera of C5aY354NPA-vaccinated animals revealed a yet none-significant tendency to bind peptide 1 (Figure 6B). In contrast, IgG in sera from wtC5a-immunized mice recognized peptide 4, albeit not by statistical significance(Figure 6E). No antibody binding above naive background was observed for peptide 3 when tested for IgG-binding in sera from both groups (Figure 6D).
Figure 6. Epitope mapping of C5aY354NPA and wtC5a induced IgG.
(A) The mC5a peptide was dissected into 4 cyclic synthetic peptides. Peptide 2 was synthesized with and without 4NPA modification. The underlined cysteines were additionally introduced at the mC5a N- and C-terminus. (B–F) Sera obtained at d90 from individual animals from arthritis experiments with both 6xHIS-tagged and non-tagged C5a (C5aY35 n=37, wtC5a n=34, PBS n=34) were tested for their reactivities with the individual peptides by ELISA. Data depict means and SEM, significance was calculated by unpaired Student’s t-test.
DISCUSSION
Although current biological treatments have dramatically improved the therapeutic possibilities in RA, they are used in established disease rather than addressing the question of arthritis prevention. In the light of recent and emerging improvement in auto-antibody tests, genetic and known environmental factors (27, 28) it is quite likely that RA will become a more predictable disease. Thus, a window for preventive treatment is within reach. We believe such therapies to require increased focus.
In the present study we report the generation of a new anti-inflammatory vaccine by incorporating the unnatural amino acid 4NPA at selected, potentially immunogenic sites in the murine C5a molecule. Replacement of a tyrosine residue at position 35 (Y35) by 4NPA resulted in the MHCII dependent production of anti-wtC5a antibodies. Preventive vaccination with C5aY354NPA compared to wtC5a significantly ameliorated arthritis. Besides showing the utility of an unnatural amino acid modified self-protein in a complex arthritis disease model, our study adds additional insights into impact of minor amino acid changes such as PTMs on immunotolerance (19–21, 29).
In line with previous studies (19–21) antibodies towards the 4NPA modified protein could be readily induced. However, with regard to C5a this remained rather restricted to animals expressing H-2q MHCII molecules. In addition, immunization with wtC5a itself gave C5a-specific IgG titers in mice expressing either H-2q or H-2r haplotype, which is likely due to the aggressive immunization protocol. However none of the 4NPA modified or wtC5a proteins induced antibodies in animals expressing human DR4. Human and murine C5a share about 56% homology and thus at least the human DR4 molecule can likely not present peptides originating from murine C5a.
In important contrast to previous studies (19–21) we observed loss of T cell tolerance towards endogenous antigen. Splenic T cells from B10.Q animals that received a single injection of C5aY354NPA emulsified in complete Freund’s adjuvant (CFA) strongly responded to restimulation with C5aY354NPA. While splenic T cells from wtC5a-immunized animals could not be restimulated with either antigen, splenocytes obtained from C5aY354NPA-immunized mice also produced significant IFN-γ titers upon wtC5a restimulation. These data suggest the generation of a T cell neo-epitope upon replacement of Y35 by 4NPA. It further points to crossreactivity of a C5aY354NPA-specific T cell population generated upon C5aY354NPA-immunization to wtC5a. Splenic T cells obtained from animals post arthritis experiments, revealed similar IFNγ titers upon restimulation with either C5aY354NPA or wtC5a. These results suggest repetitive C5aY354NPA vaccinations to completely break tolerance towards wtC5a at the T cell level.
Vaccination of mice with C5aY354NPA but not wtC5a gave significant protection from collagen-induced arthritis, albeit anti-C5a antibodies were produced upon vaccination with either protein. Although, according to epitope-mapping and cross-reactivity studies (data not shown),different vaccinations did induce distinct antibody populations recognizing different antigenic determinants of mC5a, both wildtype and C5aY354NPA induced anti-mC5a IgG blocking the binding of mC5a to its receptor.
Apart from these similar functional properties, C5aY354NPA induced higher anti-C5a IgG titers compared to wtC5a throughout vaccination experiments. Consequently, the protective effect of C5aY354NPA vaccination in the CIA model can likely be attributed to the ability of the unnatural amino acid to activate T cells helping B cells to produce significantly higher IgG titers to C5a neo-epitopes. This likely results in more rapid and effective removal of C5a in vivo.
Decreased intra-articular and circulating levels of C5a will result in less chemoattraction to the site of inflammation and thus less immune cell infiltration. Recently, C5aR-engagement has been highlighted as particularily essential process in early neutrophil recruitment into the joint (13). Neutrophils are important for arthritis development in CIA (30, 31). Neutrophils can assist in elastase-mediated cartilage and thus type II collagen epitope exposure (32). Although we did not see differences in overall anti-CII IgG titers between C5aY354NPA, wtC5a and PBS vaccinated animals, antibodies with specificity to the CIIU1 epitope, which positively correlates with arthritis in mice and men (33, 34), were significantly decreased in C5aY354NPA vaccinated mice.
Together with previous data (18), our results suggest that C5a-neutralization in experimental arthritis is a very effective therapeutic strategy. However, the timing of targeting the C5a:C5aR axis may significantly affect the treatment outcome. In established disease, therapeutic anti-C5a/C5aR treatment is less promising, regardless whether accomplished by biologicals, small molecules or vaccination. In a preclinical study involving a small molecule blocker for C5aR, the drug was administered prior to disease initiation in an antigen induced arthritis model and could thus protect the animals from disease (35). However, a first clinical trial in a small cohort of RA patients involving the same molecule failed, likely due to the fact that the included patients already presented with established disease and were diagnosed with RA at least 6 month prior to inclusion in the study (12). In line with this result, our data suggest that an anti-C5a treatment post the disease’s priming phase may be far less effective. Anti-wtC5a antibodies induced by wtC5a vaccination at day 46 are threefold increase compared to those induced by C5aY354NPA at day 28. Although arthritis is still subclinical at this point, these C5a-neutralizing antibodies cannot reverse the disease course. This suggests that the most effective anti-C5a treatment of a complex inflammatory condition like autoimmune arthritis can be best accomplished by a preventive vaccination strategy, while conventional biological or small molecule treatments targeting the C5a:C5aR axis risk missing the window for intervention during the disease’s subclinical priming phase, which, even with state-of-the-art diagnostics, is impossible to determine.
Beyond the practical application of unnatural amino acid incorporation to vaccine generation, this work also bears light on the mechanisms by which naturally occurring PTMs may trigger loss of self-tolerance (19–21). It has been postulated that PTMs may play an important role in the development of inflammatory diseases and autoimmune disorders (36–38). Among the PTMs that have been observed to occur naturally due to cellular stress, inflammation, trauma or infectious agents, citrullination is one of the most studied to date (39, 40). This PTM has been shown to generate both B and T-cell neo-epitopes on various protein antigens such as CII, fibrinogen, vimentin, or MOG (41–44). One major hypothesis regarding the underlying mechanism that triggers and drives autoimmunity is the involvement of citrullination and other PTMs in the breakdown of T cell tolerance towards the native antigen, which is followed by a B cell response and epitope-spreading to the same or to other immunogens. However, this link remains to be clearly demonstrated. Here we show that a modification of one single amino acid, which substitutes a hydroxyl with a nitro-group (a change of three atoms) can break T cell tolerance towards the endogenous unmodified antigen and trigger a B cell response towards the modified epitope which a) crossreacts to the unmodified wtC5a peptide and b) also spreads to otherwise non-immunogenic amino acid residues of the native antigen. Thus, this work underscores the notion that minimal changes to native amino acid structures, such as those caused by PTM can result in the breakdown of tolerance in autoimmunity and likely play a role in the development and/or progression of autoimmune disease.
ACKNOWLEDGEMENTS
The authors thank Carlos and Kristina Palestro for excellent animal care.
This study was supported by grants from the Swedish Research Council, the Swedish Science Strategic Foundation (SSF), Knut and Alice Wallenberg foundation, the EU BeTheCure and MASTERSWITCH projects (R.H., K.S.N.) as well as by a grant from the National Institute of Health NIH GM062159 (P.G.S). This is manuscript #22068 of The Scripps Research Institute.
Footnotes
The authors have no conflicting financial interests to declare.
AUTHOR CONTRIBUTIONS
All author a have been onvolved in drafting or critically revising the manuscript. All authors approved the publication of the final version.
Study conception and design: CK, PGS, RH
Data aquisition: CK, KSN, FBP, VG
Data analysis and interpretation: CK, KSN, FBP, VG, PGS, RH
REFERENCES
- 1.Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. British journal of pharmacology. 2007;152(4):429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a) The international journal of biochemistry & cell biology. 2009;41(11):2114–2117. doi: 10.1016/j.biocel.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annual review of immunology. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 4.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis--a genomewide study. The New England journal of medicine. 2007;357(12):1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurreeman FA, Padyukov L, Marques RB, Schrodi SJ, Seddighzadeh M, Stoeken-Rijsbergen G, et al. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS medicine. 2007;4(9):e278. doi: 10.1371/journal.pmed.0040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344(8930):1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 7.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 8.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. The New England journal of medicine. 1999;340(4):253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 9.Jose PJ, Moss IK, Maini RN, Williams TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Annals of the rheumatic diseases. 1990;49(10):747–752. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Kristan J, Hao L, Lenkoski CS, Shen Y, Matis LA. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J Immunol. 2000;164(8):4340–4347. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(19):8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJ, et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 2007;46(12):1773–1778. doi: 10.1093/rheumatology/kem222. [DOI] [PubMed] [Google Scholar]
- 13.Sadik CD, Kim ND, Iwakura Y, Luster AD. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):E3177–E3185. doi: 10.1073/pnas.1213797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banda NK, Hyatt S, Antonioli AH, White JT, Glogowska M, Takahashi K, et al. Role of C3a receptors, C5a receptors, and complement protein C6 deficiency in collagen antibody-induced arthritis in mice. J Immunol. 2012;188(3):1469–1478. doi: 10.4049/jimmunol.1102310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banda NK, Levitt B, Wood AK, Takahashi K, Stahl GL, Holers VM, et al. Complement activation pathways in murine immune complex-induced arthritis and in C3a and C5a generation in vitro. Clinical and experimental immunology. 2010;159(1):100–108. doi: 10.1111/j.1365-2249.2009.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, et al. Genetic influences on the end-stage effector phase of arthritis. The Journal of experimental medicine. 2001;194(3):321–330. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monach PA, Nigrovic PA, Chen M, Hock H, Lee DM, Benoist C, et al. Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis and rheumatism. 2010;62(3):753–764. doi: 10.1002/art.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandakumar KS, Jansson A, Xu B, Rydell N, Ahooghalandari P, Hellman L, et al. A recombinant vaccine effectively induces c5a-specific neutralizing antibodies and prevents arthritis. PloS one. 2010;5(10):e13511. doi: 10.1371/journal.pone.0013511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunewald J, Tsao ML, Perera R, Dong L, Niessen F, Wen BG, et al. Immunochemical termination of self-tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11276–11280. doi: 10.1073/pnas.0804157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauba V, Grunewald J, Gorney V, Deaton LM, Kang M, Bursulaya B, et al. Loss of CD4 T-cell-dependent tolerance to proteins with modified amino acids. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12821–12826. doi: 10.1073/pnas.1110042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunewald J, Hunt GS, Dong L, Niessen F, Wen BG, Tsao ML, et al. Mechanistic studies of the immunochemical termination of self-tolerance with unnatural amino acids. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4337–4342. doi: 10.1073/pnas.0900507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fugger L, Michie SA, Rulifson I, Lock CB, McDevitt GS. Expression of HLA-DR4 and human CD4 transgenes in mice determines the variable region beta-chain T-cell repertoire and mediates an HLA-DR-restricted immune response. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(13):6151–6155. doi: 10.1073/pnas.91.13.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young TS, Ahmad I, Yin JA, Schultz PG. An enhanced system for unnatural amino acid mutagenesis in E. coli. Journal of molecular biology. 2010;395(2):361–374. doi: 10.1016/j.jmb.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Holmdahl R, Carlsen S, Mikulowska A, Vestberg M, Brunsberg U, et al. Genetic analysis of mouse models for rheumatoid arthritis. In: A KW, editor. Human Genome methods. New York: CRC Press; 1998. pp. 215–238. [Google Scholar]
- 25.Holmdahl R, Klareskog L, Andersson M, Hansen C. High antibody response to autologous type II collagen is restricted to H-2q. Immunogenetics. 1986;24(2):84–89. doi: 10.1007/BF00373114. [DOI] [PubMed] [Google Scholar]
- 26.Huber-Lang MS, Sarma JV, McGuire SR, Lu KT, Padgaonkar VA, Younkin EM, et al. Structure-function relationships of human C5a and C5aR. J Immunol. 2003;170(12):6115–6124. doi: 10.4049/jimmunol.170.12.6115. [DOI] [PubMed] [Google Scholar]
- 27.Lundstrom E, Kallberg H, Alfredsson L, Klareskog L, Padyukov L. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: all alleles are important. Arthritis and rheumatism. 2009;60(6):1597–1603. doi: 10.1002/art.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nature genetics. 2009;41(12):1319–1324. doi: 10.1038/ng.480. [DOI] [PubMed] [Google Scholar]
- 29.Backlund J, Carlsen S, Hoger T, Holm B, Fugger L, Kihlberg J, et al. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263–270) in humanized transgenic mice and in rheumatoid arthritis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(15):9960–9965. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, et al. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112(13):5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 31.Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171(2):1009–1015. doi: 10.4049/jimmunol.171.2.1009. [DOI] [PubMed] [Google Scholar]
- 32.Jasin HE, Taurog JD. Mechanisms of disruption of the articular cartilage surface in inflammation. Neutrophil elastase increases availability of collagen type II epitopes for binding with antibody on the surface of articular cartilage. The Journal of clinical investigation. 1991;87(5):1531–1536. doi: 10.1172/JCI115164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraetsch HG, Unger C, Wernhoff P, Schneider C, Kalden JR, Holmdahl R, et al. Cartilage-specific autoimmunity in rheumatoid arthritis: characterization of a triple helical B cell epitope in the integrin-binding-domain of collagen type II. European journal of immunology. 2001;31(6):1666–1673. doi: 10.1002/1521-4141(200106)31:6<1666::aid-immu1666>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Nandakumar KS, Bajtner E, Hill L, Bohm B, Rowley MJ, Burkhardt H, et al. Arthritogenic antibodies specific for a major type II collagen triple-helical epitope bind and destabilize cartilage independent of inflammation. Arthritis and rheumatism. 2008;58(1):184–196. doi: 10.1002/art.23049. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff TM, Strachan AJ, Dryburgh N, Shiels IA, Reid RC, Fairlie DP, et al. Antiarthritic activity of an orally active C5a receptor antagonist against antigen-induced monarticular arthritis in the rat. Arthritis and rheumatism. 2002;46(9):2476–2485. doi: 10.1002/art.10449. [DOI] [PubMed] [Google Scholar]
- 36.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Current opinion in immunology. 2004;16(6):753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Cloos PA, Christgau S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology. 2004;5(3):139–158. doi: 10.1023/B:BGEN.0000031152.31352.8b. [DOI] [PubMed] [Google Scholar]
- 38.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends in immunology. 2001;22(8):443–449. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 39.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annual review of immunology. 2008;26:651–675. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 40.Uysal H, Nandakumar KS, Kessel C, Haag S, Carlsen S, Burkhardt H, et al. Antibodies to citrullinated proteins: molecular interactions and arthritogenicity. Immunological reviews. 2010;233(1):9–33. doi: 10.1111/j.0105-2896.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 41.Carrillo-Vico A, Leech MD, Anderton SM. Contribution of myelin autoantigen citrullination to T cell autoaggression in the central nervous system. J Immunol. 2010;184(6):2839–2846. doi: 10.4049/jimmunol.0903639. [DOI] [PubMed] [Google Scholar]
- 42.Feitsma AL, van der Voort EI, Franken KL, el Bannoudi H, Elferink BG, Drijfhout JW, et al. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis and rheumatism. 2010;62(1):117–125. doi: 10.1002/art.25059. [DOI] [PubMed] [Google Scholar]
- 43.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis research & therapy. 2012;14(3):R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. The Journal of experimental medicine. 2008;205(4):967–979. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]