SUMMARY
DNA methylation patterns are dynamically controlled by DNA methylation and active DNA demethylation, but the mechanisms of regulation of active DNA demethylation are not well understood. Through forward genetic screens for Arabidopsis mutants showing DNA hypermethylation at specific loci and increased silencing of reporter genes, we identified IDM2 (increased DNA methylation 2) as a regulator of DNA demethylation and gene silencing. IDM2 dysfunction causes DNA hypermethylation and silencing of reporter genes and some endogenous genes. These effects of idm2 mutations are similar to those of mutations in IDM1, a regulator of active DNA demethylation. IDM2 encodes an α-crystallin domain protein in the nucleus. IDM2 and IDM1 interact physically and partially colocalize at discrete subnuclear foci. IDM2 is required for the full activity of H3K18 acetylation but not H3K23 acetylation of IDM1 in planta. Our results suggest that IDM2 functions in active DNA demethylation and in antisilencing by regulating IDM1.
INTRODUCTION
Epigenetic modifications determine the status of chromatin and consequently control the transcriptional potential (Bender, 2004; He et al., 2011; Law and Jacobsen, 2010; Matzke and Birchler, 2005; Tariq and Paszkowski, 2004). DNA methylation is an important epigenetic mark conserved in many eukaryotes. In plants, DNA methylation occurs in all three cytosine contexts, namely CG, CHG, and CHH (H represents A, T, or C) (He et al., 2011; Law and Jacobsen, 2010). Genome-wide mapping of DNA methylation in Arabidopsis revealed that gene bodies are mainly associated with CG methylation, whereas transposon-and DNA-repeat-enriched heterochromatin regions are the major targets of CHG and CHH methylation (Zhang et al., 2006). Although the function of abundant CG methylation within genic regions remains unclear, DNA methylation, especially CHG and CHH methylation, is generally correlated with transcription-repressive histone modifications and confers negative regulation of transcriptional activities (Law and Jacobsen, 2010; Pikaard, 2013; Zhang and Zhu, 2012).
DNA methylation at the fifth position of the cytosine pyrimidine ring is catalyzed by DNA methyltransferases (DNMTs) that use S-adenosylmethionine as the methyl donor. Plants possess multiple DNMT proteins that cooperatively establish and maintain DNA methylation. In Arabidopsis, DRM2 catalyzes methylation in all cytosine contexts, whereas MET1 and CMT3 catalyze symmetric CG and CHG methylation, respectively, during DNA replication to maintain the epigenetic patterns (Law and Jacobsen, 2010). Because of its asymmetric nature, CHH methylation must be established de novo during each cell cycle (Law and Jacobsen, 2010). In addition to DRM2, CMT2 was recently demonstrated to be a methyltransferase that catalyzes CHH methylation (Zemach et al., 2013).
DNA methylation patterns in plants and other eukaryotes depend not only on DNA methylation activities but also on active DNA demethylation (Zhang and Zhu, 2012; Zhu, 2009). In contrast to passive demethylation, in which DNA methylation is lost because of a lack of maintenance methylation, active demethylation refers to the enzymatic process in which 5-methylcytosine is replaced with cytosine independently of DNA replication. In plants, active DNA demethylation is catalyzed by a subfamily of bifunctional DNA glycosylases/lyases represented by Repressor of Silencing (ROS)1 and DME (Agius et al., 2006; Gehring et al., 2006; Gong et al., 2002; Ortega-Galisteo et al., 2008; Penterman et al., 2007). These DNA demethylases directly excise the 5-methylcytosine base and then cleave the DNA backbone at the abasic site. The resultant single-nucleotide gap is subsequently filled with an unmodified cytosine through the DNA base excision repair pathway (Zhang and Zhu, 2012; Zhu, 2009). In Arabidopsis, DME confers global demethylation during gametogenesis (Gehring et al., 2009; Hsieh et al., 2009; Huh et al., 2008), whereas ROS1 catalyzes active DNA demethylation at discrete genetic loci across the genome during vegetative growth (Gong et al., 2002; Lister et al., 2008; Penterman et al., 2007; Qian et al., 2012; Zhu et al., 2007).
DNA methyltransferases can be targeted to specific loci through mechanisms such as the RNA-directed DNA methylation (RdDM) pathway, in which complementary pairing between 24 nt small interfering RNAs and nascent scaffold RNAs guides the methylation complex to its target loci with the aid of protein-protein interactions within the silencing complex (Gao et al., 2010; Haag and Pikaard, 2011; He et al., 2009; Law and Jacobsen, 2010; Matzke et al., 2009; Pikaard, 2013; Wierzbicki, 2012; Zhang and Zhu, 2012). In contrast to the establishment of DNA methylation, little is known about the locus-specific guidance of active DNA demethylation. Recruitment of ROS1 to its target loci has been suggested to require assistance by the RNA-binding protein ROS3, because ros3 mutation disrupts the subnuclear localization patterns of ROS1 and causes DNA hypermethylation of some ROS1 target loci (Zheng et al., 2008). Increased DNA methylation (IDM)1, a histone acetyltransferase, was recently identified as another important regulator of active DNA demethylation (Qian et al., 2012). IDM1 recognizes chromatin that contains CG methylation and low histone 3 lysine 4 (H3K4) and arginine 2 (H3R2) methylations and catalyzes H3K18 and H3K23 acetylation, which then somehow facilitates ROS1-mediated demethylation (Qian et al., 2012).
The small heat shock protein (HSP) family of proteins is widely distributed in both prokaryotes and eukaryotes and is defined by a conserved 100- to 110-amino acid motif known as the α-crystallin domain (ACD), which is flanked by a variable N-terminal domain and a short C-terminal extension (Basha et al., 2012; MacRae, 2000). Small HSPs are known as the paramedics of cells (Hilton et al., 2013) by functioning as ATP-independent molecular chaperones that, under stress conditions, bind and stabilize denaturing proteins so that these proteins can later be renatured by ATP-dependent chaperones (Basha et al., 2012). In mammals, small HSPs not only play critical roles in modulating vital physiological processes including smooth muscle relaxation and cardiac contractility but also function as an innate protector against debilitating pathological conditions such as cardiac hypertrophy and Alzheimer’s disease (Edwards et al., 2011; Sun and MacRae, 2005). In plants, small HSPs have been shown in vitro to prevent irreversible protein aggregation and insolubilization (Basha et al., 2012; Scharf et al., 2001). Whereas most plant small HSPs are heat shock inducible, proteins in the ACD family can be involved in other cellular processes unrelated to heat stress. For instance, the ACD protein AtRTM2 is not required for heat stress tolerance, but prevents systemic spreading of tobacco etch virus in Arabidopsis (Whitham et al., 2000). Although ACD proteins have been shown to be important for diverse cellular processes, it is unclear whether any ACD proteins can modulate epigenetic regulation.
In this study, we found that an atypical small HSP, IDM2, functions in association with IDM1 to regulate active DNA demethylation in Arabidopsis. Identified through forward genetic screens, the idm2 mutants exhibit DNA hypermethylation at thousands of genetic loci and show increased transcriptional silencing of reporter genes and some endogenous genes. Regulation of DNA methylation by IDM2 requires the conserved ACD. Unlike that of typical small HSPs, the IDM2 transcript level does not respond to heat stress. IDM2 interacts and partially colocalizes with IDM1 in vivo. In addition to having DNA methylation and gene-silencing phenotypes similar to those of idm1, the idm2 mutants are similar to idm1 in displaying reduced levels of H3K18ac at loci where DNA methylation is affected. Our results suggest that plants have evolved an ACD protein that specifically functions in epigenetic regulation.
RESULTS
Isolation of idm2 Mutants
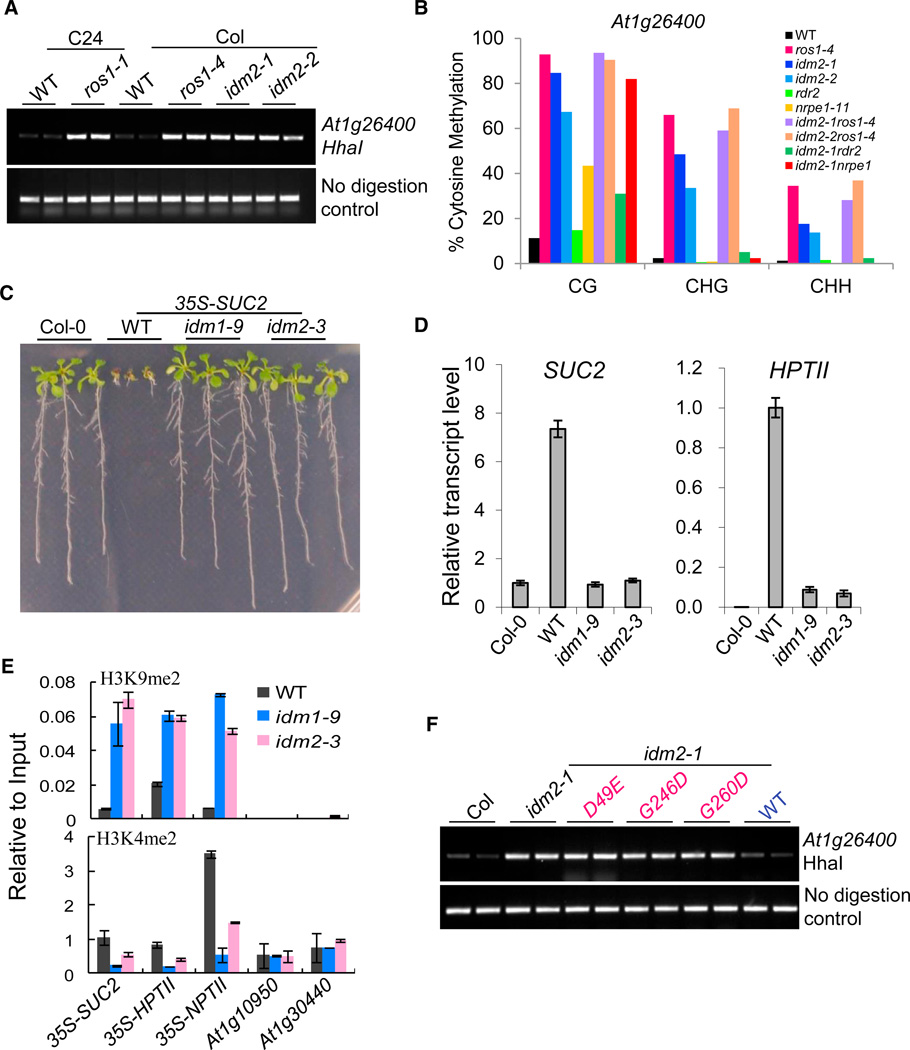
To better understand the mechanism of active DNA demethylation initiated by ROS1, we carried out a genetic screen for increased DNA methylation mutants using a Chop PCR marker, which was designed based on the whole-genome DNA methylation analysis of ros1-1 (Qian et al., 2012). One T-DNA mutant, referred to here as idm2-1, which shows almost the same DNA methylation level as the ros1 mutant at the 3′ region of At1g26400 (Figure 1A), was identified by screening a library of homozygous T-DNA insertion mutants from the Arabidopsis Biological Resource Center. This mutant has a T-DNA insertion in the gene At1g54840 (Figure S1A-S1C available online). To confirm that this mutation results in DNA hypermethylation, we tested another T-DNA mutant allele (i.e., idm2-2). Chop PCR results revealed a similar hypermethylation phenotype in the idm2-2 allele (Figure 1A). Bisulfite sequencing data showed that the DNA methylation level at the At1g26400 site was increased in idm2-1, idm2-2, and ros1-4 in all sequence contexts compared to the wild-type control (Figure 1B). To further confirm that At1g54840 is indeed the IDM2 gene, we transformed a 2.7 kb wild-type genomic fragment containing the At1g54840 promoter and its coding sequence into idm2 mutant plants. As shown in Figure S1D, the transgene was able to rescue the DNA methylation defect of idm2 mutant plants.
Figure 1. Identification of idm2 Mutants, Their Genetic Interactions with ros1, rdr2, and nrpe1 Mutations, and Their Effects on DNA Methylation, Histone Modification, and Transcriptional Silencing.
(A) Analysis of DNA methylation level at the At1g26400 locus by Chop PCR. Because HhaI digestion is sensitive to CG DNA methylation, DNA hypermethylation results in increased levels of the PCR product. Undigested DNA is shown as a control.
(B) Bisulfite sequencing data showing the effects of idm2 mutations on At1g26400 DNA methylation in different sequence contexts and genetic interactions with ros1, rdr2, or nrpe1.
(C) Effect of idm1 and idm2 mutations on 35S-SUC2 transgene-mediated root growth suppression. Seedlings were grown on plates with MS medium plus 2% sucrose for 3 weeks before they were photographed.
(D) Real-time PCR analysis of the expression levels of SUC2 and HPTII transgenes in the different genotypes. TUB8 was used as an internal control. Error bars represent standard error (n = 3).
(E) ChIP assay of H3K4me2 and K3H9me2 in the wild-type, idm1-9, and idm2-3. Ten-day-old seedlings were used for ChIP assay with antibodies against H3K9me2 or H3K4me2. The ChIP signal was quantified relative to input DNA. The no-antibody precipitates served as negative control. Two biological replicates were performed, and very similar results were obtained. Standard errors were calculated from three technical repeats.
(F) The DNA hypermethylation phenotype of idm2-1 plants is rescued by transgenic expression of wild-type IDM2 but not by expression of the D49E, G246D, or G260D mutant. Chop PCR results for At1g26400 are shown for two representative transgenic lines generated from each construct.
See also Figures S1 and S4 and Tables S4 and S5.
IDM2 Prevents the Transcriptional Silencing of Transgenes
Previous studies showed that ROS1 is required to prevent the transcriptional silencing of RD29A-LUC and 35S-NPTII trans-genes (Gong et al., 2002). Mutations in IDM1 cause the silencing of 35S-NPTII but not of the RD29A-LUC transgene (Li et al., 2012; Qian et al., 2012). An independent genetic screen for mutants impaired in the prevention of silencing of the 35S-SUC2 transgene led to the isolation of mutant alleles of IDM1 as well as ROS1 (Wang et al., 2013). This later genetic screen also led to the isolation of idm2-3(Figure 1C). Wild-type 35S-SUC2 trans-genic plants showed an inhibition of root growth on Murashige-Skoog (MS) medium with 2% sucrose due to high levels of SUC2 (sucrose transporter 2) expression (Lei et al., 2011). The root length in both idm1-9 and idm2-3 plants was similar to that in the Col-0 control that did not have the 35S-SUC2 transgene. Both the 35S-SUC2 and 35S-HPTII transgenes were silenced in idm1-9 and idm2-3 mutant plants (Figure 1D). Bisulfite sequencing analysis showed that, compared to the wild-type control, the DNA methylation level of the 35S promoter region was increased in idm1-9 and idm2-3 plants (Figure S1E). The At1g26400 region was also hypermethylated in idm1-9 and idm2-3 according to the Chop PCR results (Figure S1F). Chromatin immunoprecipitation (ChIP) assays showed an increased H3K9me2 level and a decreased H3K4me2 level in the 35S promoter region in the mutant plants (Figure 1E). In idm2-3, a single-nucleotide-substitution mutation (from GGG to GAG) in At1g54840 changed amino acid glycine 276 to glutamic acid. Consistent with the genetic mapping result (Figure S1G) suggesting that the idm2-3 mutation was responsible for the long-root phenotype, all of the F1 plants from genetic crosses between idm2-3 and idm2-1 (SALK_130656) had a long-root phenotype like that of idm2-3 (Figure S1H). We also transferred the wild-type IDM2 genomic fragment into idm2-3 mutant plants and found that it can complement the root phenotype (Figure S1H). These results demonstrated that, like IDM1 and ROS1 (Wang et al., 2013), IDM2 is required for prevention of transcriptional silencing of the 35-SUC2 and 35S-HPTII transgenes.
IDM2 Prevents DNA Hypermethylation at Thousands of Loci
To test the effect of the idm2 mutation on genomic DNA methylation status, we performed Southern blot analysis on 5S rDNA and 180 bp centromeric repeat regions. The analysis found that the idm2 mutation does not affect the DNA methylation at 5S rDNA or at the 180 bp centromeric repeat (Figure S2A). The results suggest that, like ROS1 and IDM1 mutations, the IDM2 mutation affects the methylation status of specific loci rather than the overall methylation status of the genome.
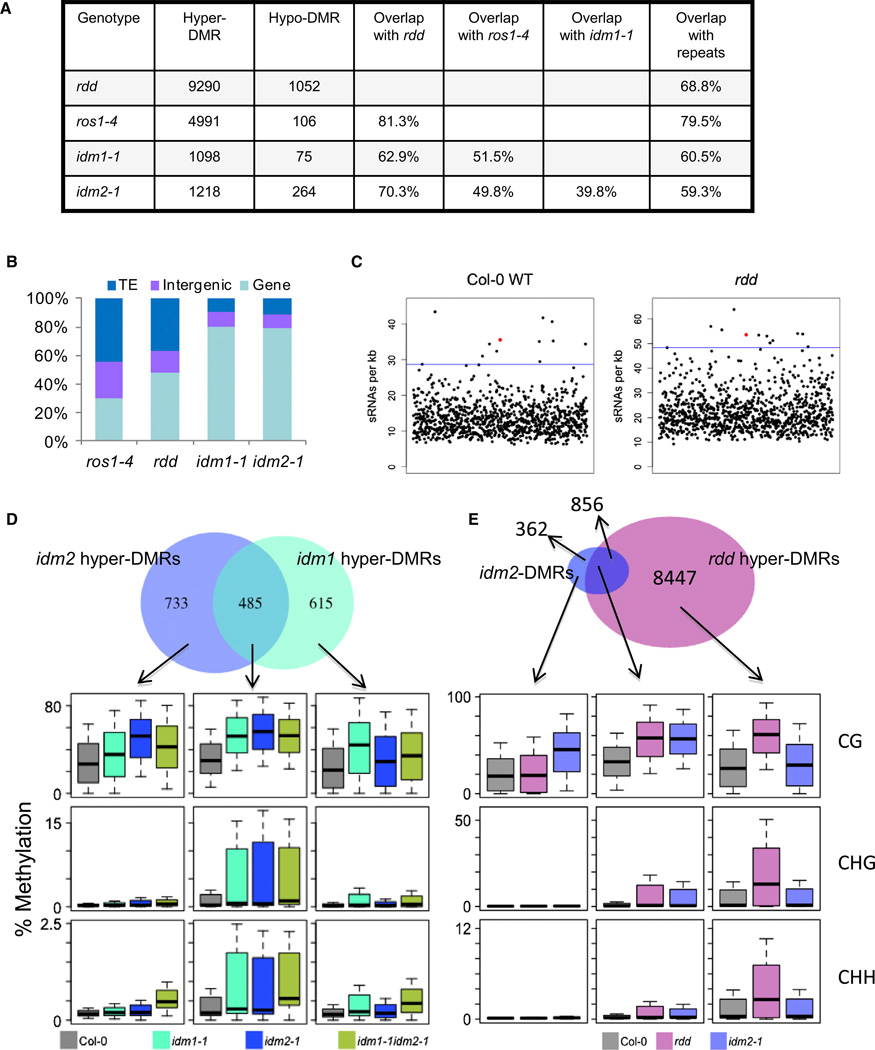
To identify the specific loci where DNA methylation is increased in idm2 mutant plants, we compared the genome-wide DNA methylation profiles of idm2-1 and wild-type Col-0 plants by next-generation sequencing after bisulfite conversion (Lister et al., 2008; Qian et al., 2012). There was no significant difference between idm2-1 and wild-type plants in their overall genome methylation patterns (data not shown). In total, we identified 1,482 differentially methylated regions (DMRs) in idm2-1. Among these, 1,218 are hyper-DMRs showing a significantly increased DNA methylation, and only 264 are hypo-DMRs, which show a significantly reduced methylation (Figure 2A; Tables S1 and S2). About 60% of the hyper-DMRs overlap with repeats as defined by RepeatMasker (http://www.repeatmasker.org) (Table S1). Interestingly, genic loci account for approximately 80% of the hyper-DMRs in idm2-1, which is the same as in idm1-1, whereas genic loci account for only about 30% and 48% of the hyper-DMRs in ros1-4 and rdd (ros1dml2dml3), respectively (Figure 2B).
Figure 2. Analysis of DMRs Identified in idm2 Mutant.
(A) The number of DMRs identified in idm2 and the overlap of hyper-DMRs between different mutant plants.
(B) Composition of the hypermethylated loci in idm2-1, idm1-1, ros1-4, and rdd mutants. TE, transposable element.
(C) Enrichment of small RNAs (sRNAs) in the 1,218 hypermethylated regions identified in idm2-1. Small RNA reads were generated from the wild-type and rdd mutant plants by Lister et al. (2008). Black dots indicate the sRNA densities from randomly selected regions in the genome, and red dots indicate the sRNA densities in the hypermethylated regions in idm2-1. Horizontal lines indicate the 99th percentile of sRNA density from 1,000 simulated runs.
(D and E) Overlap of hyper-DMRs between idm2 and idm1 (D) and between idm2 and rdd (E). Boxplots represent methylation levels of each class of DMRs.
We calculated the level of small RNAs at the hyper-DMRs according to published data sets (Lister et al., 2008) and found that small RNAs are enriched at the hyper-DMRs compared to randomly selected regions in the genome (Figure 2C). Of the 1,218 hyper-DMRs in idm2-1, six (DMR-72, DMR-232, DMR-265, DMR-333, DMR-828, and DMR-946) were selected for confirmation by individual locus bisulfite sequencing, and all were confirmed to be hypermethylated in idm2-1 (Figure S2B and S2C), as in ros1-4 or rdd (Qian et al., 2012). Many of the hypermethylated genes in idm2-1 belong to multigene families in which the member genes are highly homologous (Table S1). The Chop PCR marker locus At1g26400, which was also identified from the whole-genome bisulfite sequencing data as being hypermethylated, belongs to a highly homologous and tandemly arranged flavin adenine dinucleotide-binding berberine gene family (Figure S2D). According to publicly available microarray data, the expression of most members from this gene family is tissue specific (data not shown). As was the case for the hyper-DMRs in the idm1 mutant, hyper-DMRs in idm2 correspond to sequences with low H3K4 mono-, di-, and trimethylation relative to a comparison group of CG methylated and expressed genes (Figure S2E-S2K) (Qian et al., 2012; Zhang et al., 2009).
IDM2 Functions in the Same Genetic Pathway with IDM1 in Counteracting RNA-Directed DNA Methylation at Some Loci
Double mutant analysis indicated that the CHG and CHH hyper-methylation phenotypes of ros1-4 and idm2-1 or idm2-2 are not additive at At1g26400 (Figure 1B) or at another two loci (DMR-72 and At5g38550) that we examined (Figure S2C). An examination of the At5g38550 locus shows that the CG hypermethylation phenotypes of the two mutants are also not additive (Figure S2C). Furthermore, analysis of the idm1idm2 double mutant found no additive effects between the idm1 and idm2 mutants (Figure 2D). These results, together with previous results showing a lack of additive effects between idm1 and ros1 (Qian et al., 2012), suggest that IDM2 functions with IDM1 and ROS1 in the same genetic pathway to prevent DNA hypermethylation at some loci. The hypermethylation in CHG and CHH sequence contexts in idm2-1 is suppressed by the rdr2 and nrpe1 mutations (Figure 1B), suggesting that the methylation at the At1g26400 region is mediated through the RdDM pathway.
According to the whole-genome bisulfite sequencing data, approximately 40%, 50%, and 70% of the 1,218 hyper-DMRs in idm2-1 are also hypermethylated in the idm1, ros1-4, and rdd mutants, respectively (Figure 2A; Table S1). The DNA methylation level was increased in all sequence contexts for the overlapped loci in these mutants (Figures 2D and 2E). The DNA methylation level at the idm2-specific loci also appears increased in idm1-1 (Figure 2D), although the increases in idm1-1 were not enough to make the loci count as hyper-DMRs according to the parameters defined in this study. Similarly, the DNA methylation level at the idm1-specific loci also appears increased in idm2 (Figure 2D). These results show that a large percentage of hyper-DMRs in idm2 also have increased DNA methylation in the idm1, ros1, and rdd mutants, further supporting that IDM2 functions in active DNA demethylation at these loci.
IDM2 Prevents the Silencing of Some Endogenous Genes
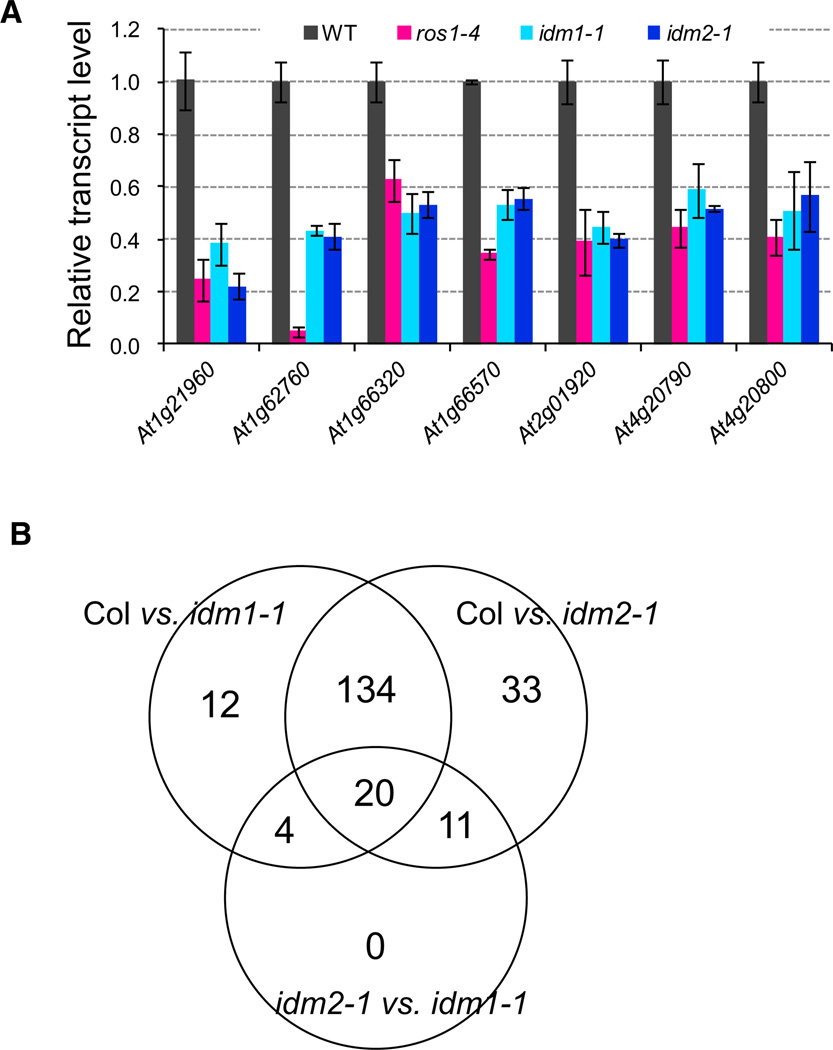
Changes in the DNA methylation level in or near a gene can affect the expression level of that gene. To determine whether DNA hypermethylation affects the expression of nearby genes in idm2 mutant plants, we examined the expression levels of seven genes that have transcript levels detectable by quantitative (q) PCR and show increased DNA methylation near or within the gene in idm2, idm1, and ros1 (Figure S2F-S2J). We found that all of the tested genes showed a substantial reduction in their transcript levels in these mutants (Figure 3A). Our results suggest that, like ROS1 and IDM1, IDM2 is critical for preventing the transcriptional silencing of some endogenous plant genes. The ROS1 transcript level is increased in the idm2 and idm1idm2 double mutants but not in the idm1 mutant, and treatment with the DNA methylation inhibitor 5′-aza-2′-deoxycytidine (5′-aza-dC) blocks ROS1 expression (Figure S3A). Unlike ROS1, the IDM1 transcript level does not show a substantial difference between idm2 mutants and the wild-type (Figure S3B). The transcript level of IDM2 does not show dramatic differences in the active DNA demethylation pathway mutants and RdDM pathway mutants (Figure S3C and S3D).
Figure 3. Gene Expression Level in idm1 and idm2 Mutants.
(A) Expression of the hypermethylated genes or genes near DMRs in 2-week-old idm1-1, idm2-1, and ros1-4 seedlings. Error bars represent standard error (n = 3).
(B) Overlap of differentially expressed genes between the different comparison groups.
To test the effect of the idm2 mutation on genome-wide transcript levels, we performed a tiling array assay in wild-type, idm1-1, and idm2-1 plants. Similar numbers of genes are down- or upregulated in idm1 and idm2 (Figure S3E; Table S3), and most of the affected genes overlapped between idm1 and idm2 (Figure 3B). Quantitative PCR assays confirmed that the genes identified by tiling array are down- or upregulated in idm1 and idm2 mutant plants (Figure S3F and S3G). The tiling array assay was not sensitive enough to detect the expression of the seven genes that have increased methylation in idm2, idm1, and ros1, although the expression could be detected by qPCR (Figure 3A). Four (At2g16280, At4g08470, At4g13340, and At5g24030) of the genes found by the tiling array assay to have an increased expression in idm1 and idm2 are hypomethylated in the mutants. However, none of the genes found by the tiling array assay to have decreased expression in idm1 and idm2 is hypermethylated in the mutants, suggesting that these genes are not regulated by IDM1 and IDM2 directly through DNA methylation.
Our previous data showed that IDM1 is important for the expression of some transposable element genes when these silent genes are released by treatment with the DNA methylation inhibitor 5′-aza-dC or by the ddm1 mutation (Li et al., 2012). We wondered whether idm2 also affects the expression level of this type of genes. The qPCR results demonstrated that the expression level of At3g18250 is lower in idm2 than in the wild-type control after 5′-aza-dC treatment and that there is no additive effect in the idm1idm2 double mutant (Figure S3H). These data suggest that IDM2 and IDM1 regulate the expression of the same groups of genes and, like IDM1, IDM2 is required for preventing transcriptional silencing of some endogenous genes.
IDM2 Is an Atypical sHsp20 Family Member
IDM2 is predicted to have an ACD in the C-terminal region (Figure S4A and S4B). IDM2 is highly conserved in plants, because homologous sequences can be found in monocots as well as dicots (Figure S4A). In Arabidopsis, IDM2 belongs to a small gene family, which has a conserved ACD at the C terminus but a variable N-terminal region (Figure S4B). The sequence of the ACD of IDM2 has high similarity with that of sHsp20s, which belong to a class of proteins that occur in all plants and that vary in size from approximately 16 to 42 kDa (Scharf et al., 2001). Most of the sHsps are induced by heat shock treatment (Scharf et al., 2001). Using qPCR, we measured the expression levels of IDM2 and two closely related genes in Arabidopsis after heat stress treatment. Interestingly, heat stress did not induce IDM2 or the two closely related genes to express at high levels, whereas the treatment induced a very high level of expression of AtHsp17.4 (Figure S4C). Unlike most of the sHsp20s, which are localized in chloroplasts or mitochondria (Basha et al., 2012), IDM2 is localized in the nucleus, according to the fluorescence signal of GFP-IDM2 in the transgenic Arabidopsis plants (Figure S4D).
The ACD Is Critical for the Function of IDM2
The ACD is highly conserved among different ACD protein family members (Scharf et al., 2001). Residues G246 and G260 of IDM2 (Figure S4A) correspond to critical positions in the known structures of ACDs (Scharf et al., 2001). We constructed wild-type and mutant versions of IDM2 containing the G246D or G260D mutation and expressed them in idm2-1 mutant plants under control of the double 35S promoter. The wild-type construct but not the G246D or G260D mutant complemented the DNA hypermethylation phenotype of the idm2-1 mutant (Figure 1F), even though the mutated proteins were expressed as well as the wild-type protein (Figure S4E). Additionally, idm2-3 also contains an amino acid change in the ACD and shows a DNA hypermethylation phenotype (Figures S1F and S4A). The results show that the ACD is important for IDM2 function in vivo. In addition to the conserved ACD at the C-terminal region, the N-terminal region of IDM2 protein is also conserved across different plant species (Figure S4A). Expression of the D49E mutant form of IDM2 driven by the double 35S promoter failed to complement the DNA hypermethylation phenotype of the At1g26400 region in idm2-1 mutant plants (Figure 1F), even though the mutant form of IDM2 was expressed at a similar level as the wild-type protein (Figure S4E). These results suggest that the conserved N-terminal region is also important for the in vivo function of IDM2 in prevention of DNA hypermethylation.
IDM2 Interacts with IDM1 In Vivo
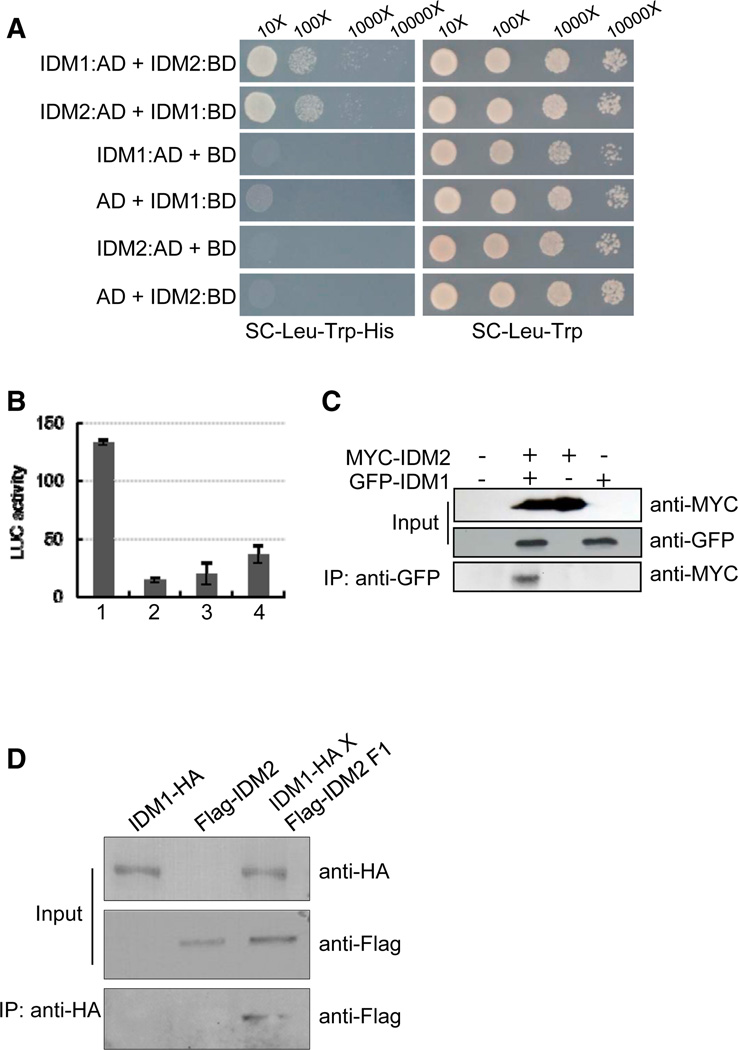
The effects of the idm2 mutation on DNA methylation and gene expression resemble those of idm1, and our genetic analysis indicated that IDM2 and IDM1 function in the same genetic pathway (Figures 1, 2, and 3; Tables S1, S2, and S3). These results suggest that IDM2 may function together with IDM1. To investigate whether IDM1 and IDM2 may interact physically, we conducted three kinds of assays. First, yeast two-hybrid assays showed that IDM2 can interact with IDM1 (Figure 4A). Second, a luciferase complementation imaging (LCI) assay (Chen et al., 2008) showed that nLUC-IDM1 can interact with cLUC-IDM2 (Figure 4B). Third, in coimmunoprecipitation (co-IP) experiments using GFP-tagged IDM1 and MYC-tagged IDM2 that were transiently expressed in Nicotiana benthamiana leaves, IDM2 was detected in the immunoprecipitate from GFP-IDM1-expressing leaves but not from leaves of control plants (Figure 4C). The interaction between IDM2 and IDM1 was also detected by co-IP in plants harboring both the Flag-IDM2 and IDM1-hemagglutinin (HA) transgenes under their native promoters (Figure 4D). These results suggest that IDM2 associates with IDM1 in vivo.
Figure 4. Protein-Protein Interactions between IDM2 and IDM1.
(A) Yeast two-hybrid analysis of IDM1 and IDM2 interaction. AD, activating domain; BD, binding domain.
(B) LCI assays showing that IDM1 can interact with IDM2 in Arabidopsis protoplasts. 1, IDM1-nLUC+IDM2-cLUC; 2, IDM1-nLUC+cLUC; 3, nLUC+IDM2-cLUC; 4, nLUC+cLUC. Three biological replicates were performed, and very similar results were obtained. Standard errors were calculated from three technical repeats.
(C) Co-IP of IDM1 and IDM2 in tobacco leaves. MYC-tagged IDM2 and GFP-tagged IDM1 were transiently expressed in N. benthamiana leaves. Anti-GFP was used for immunoprecipitation (IP); anti-MYC and anti-GFP were used for immunoblotting. Input, total protein before immunoprecipitation.
(D) The interaction between Flag-IDM2 and IDM1-HA as determined by co-IP. Transgenic plants expressing Flag-IDM2 or IDM1-HA under their native promoters and their F1 offspring were used for co-IP.
IDM2 Partially Colocalizes with IDM1 in Subnuclear Foci
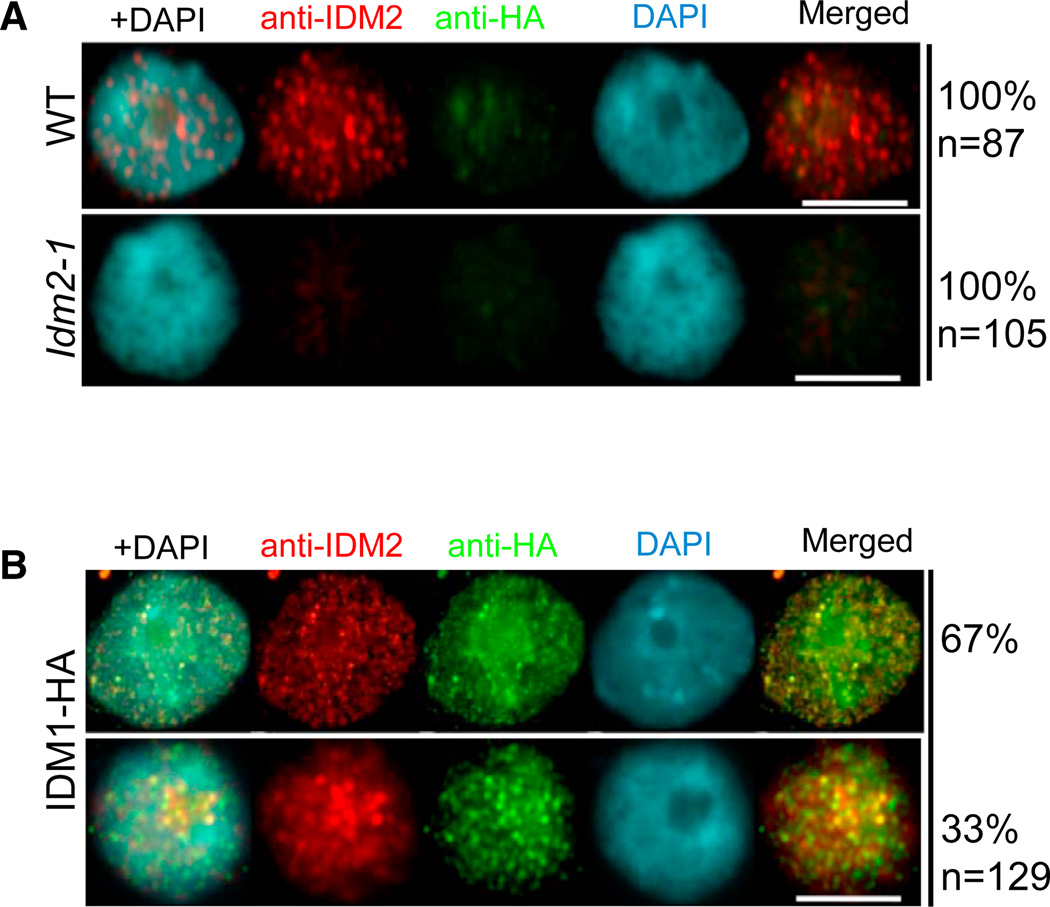
To determine the subnuclear localization pattern of IDM2 protein, we generated antibodies specific to IDM2 and used the antibodies for immunolocalization of IDM2 in Arabidopsis leaf nuclei. Immunostaining of IDM2 yielded fluorescence signals dispersed throughout the nucleoplasm without any preferential accumulation near the 4′,6-diamidino-2-phenylindole (DAPI)-intensive chromocenters (Figure 5A; Figure S5A). No signals were observed when the antibodies were applied to nuclei isolated from idm2-1 mutant plants, indicating that the observed immunolocalization signals are specific to IDM2 (Figure 5A; Figure S5A). To determine whether IDM2 may be colocalized with other components of the active DNA demethylation pathway, we performed double immunolocalization in interphase nuclei from Arabidopsis transgenic lines expressing HA-tagged IDM1 or FLAG-tagged ROS1 under their native promoters. We found that IDM2 partially colocalized with IDM1 within nucleoplasmic foci (67%) and nucleolar foci (33%), as shown by the yellow signals, which resulted from an overlap of the green and red signals (Figure 5B). A small fraction of nuclei (12%) also showed an overlap between some of the IDM2 and ROS1 signals (Figure S5B).
Figure 5. Subnuclear Localization of IDM2 and Its Colocalization with IDM1.
(A) Detection of IDM2 (red) in the wild-type and idm2-1 mutant nuclei by immunostaining using anti-iDM2. Scale bars represent 10 µm.
(B) Dual immunolocalization of IDM2 (red) and IDM1-HA (green). DNA was stained with DAPI (blue). The frequency of nuclei displaying each interphase pattern is shown on the right. Scale bars represent 10 µm. See also Figure S5.
IDM2 Is Important for the In Vivo Function of IDM1
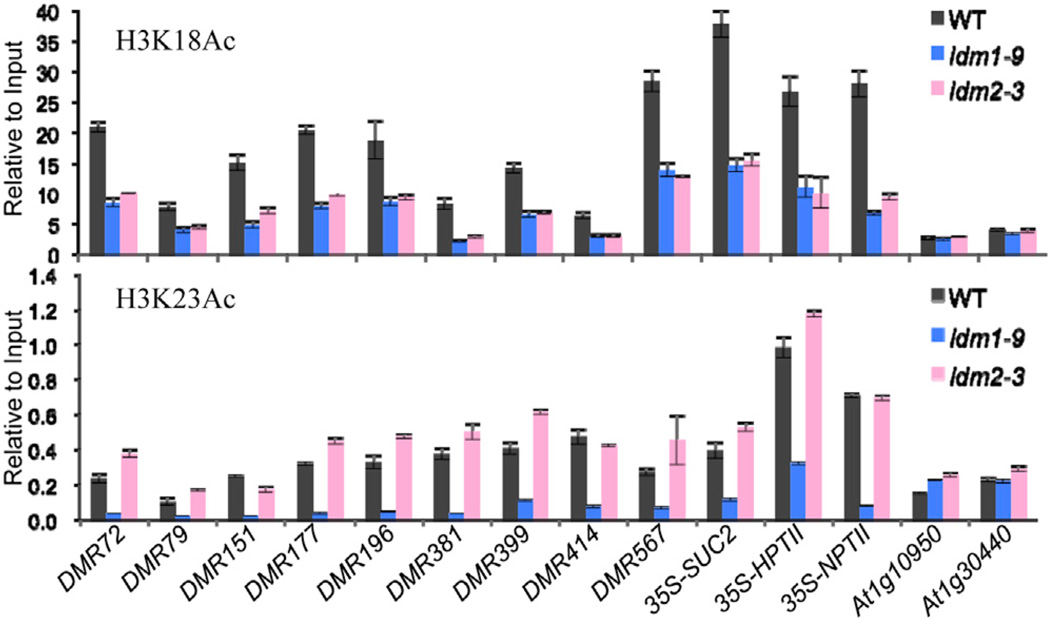
The presence of an ACD at the C-terminal region of IDM2 and the interaction between IDM2 and IDM1 suggest that IDM2 may function as a molecular chaperone of IDM1 in plants. Previous data showed that IDM1 is a histone acetyltransferase critical for H3K18 and H3K23 acetylation in vivo (Qian et al., 2012). We carried out ChIP assays and found that the H3K18ac marker was reduced substantially in not only idm1-9 but also idm2-3 mutant plants at the tested hyper-DMRs and the 35S promoter region (Figure 6; Figure S6). Surprisingly, the H3K23ac marker was reduced in idm1-9 but not in idm2-3 mutant plants (Figure 6). These results show that IDM2 is important for the full function of IDM1 in planta.
Figure 6. The Effect of idm2 Mutation on Histone Modification Marks.
H3K18ac and H3K23ac levels at the DMRs and 35S promoter and control regions were determined by ChIP on wild-type, idm1-9, and idm2-3 plants with anti-H3K18ac or anti-H3K23ac antibodies. The ChIP signal was quantified relative to input DNA. The no-antibody precipitates served as negative control. Two biological replicates were performed, and very similar results were obtained. Standard errors were calculated from three technical repeats. See also Figure S6 and Table S4.
DISCUSSION
Active DNA demethylation regulates many biological processes, including early development and locus-specific gene expression in plants and animals (Zhu, 2009). Although the enzymatic removal of methylated cytosine has been studied extensively, the mechanisms by which the enzymatic machinery is recruited to specific target sites and regulated are poorly understood. IDM1 regulates active DNA demethylation by recognizing the chromatin marks at ROS1 target sites and then creating histone acetylation marks such as H3K18ac and H3K23ac that are necessary for recruiting ROS1 (Qian et al., 2012). By identifying IDM2, a factor that functions with IDM1 and regulates the H3K18 acetylation activity of IDM1, this study provides insights into the regulation of active DNA demethylation.
Our conclusion that IDM2 functions with IDM1 in regulating active DNA demethylation is supported by the following observations. First, IDM2 functions in the same genetic pathway with IDM1 and ROS1 in preventing DNA methylation at some genomic loci, although it is clear that ROS1 also requires other regulatory factors in addition to IDM1 and IDM2, because the majority of hyper-DMRs in ros1 are not hyper-DMRs in idm1 (Qian et al., 2012) or idm2. Many of the hyper-DMRs in idm2 and idm1 mutant plants overlap. Possible functional redundancy with other proteins in the IDM2 and IDM1 families may explain the existence of idm1- or idm2-specific hyper-DMRs. Second, yeast two-hybrid assays, luciferase complementation assays, and co-IP experiments showed that IDM2 physically interacts with IDM1. In addition, immunostaining revealed a partial colocalization of IDM2 and IDM1 within subnuclear foci. Unlike IDM1, which is a histone acetyl-transferase, IDM2 does not contain any domain for enzymatic activity. Yet the levels of H3K18 acetylation are reduced in both idm2-3 and idm1-9. The result indicates that IDM1 requires IDM2 to efficiently catalyze H3K18 acetylation in planta, although we cannot exclude the possibility that IDM2 is required directly or indirectly for the function of histone acetyltransferases other than IDM1. Interestingly, H3K23 acetylation levels are decreased in idm1-9 but not in idm2-3, suggesting that IDM2 is not required for IDM1 function in H3K23 acetylation in vivo. It is possible that the H3K18 acetylation activity of IDM1 is more labile in vivo and needs the protective action of the partner protein IDM2. IDM2 also appears to partially localize with ROS1 in some cell nuclei (Figure S5B). This indicates that IDM2 may also protect ROS1 for its function in active DNA demethylation and antisilencing at some genomic loci.
IDM2 belongs to the highly conserved family of ACD proteins. Our results show that the ACD is critical for IDM2 function in vivo. ACD proteins have been extensively studied in mammals, where they play critical roles in diverse cellular processes and have important protective functions against stress and diseases such as cataracts and neurodegenerative disorders (Basha et al., 2012; Hilton et al., 2013; Welsh and Gaestel, 1998). In plants, most ACD proteins are heat shock-inducible proteins known as small HSPs (sHSPs) (Scharf et al., 2001). The vast majority of sHSPs are localized in the cell cytoplasm, chloroplasts, and mitochondria (Scharf et al., 2001). Many studies have shown that ACD proteins are molecular chaperones, although few studies have demonstrated the function of ACD proteins using knockout mutants (Basha et al., 2012; Horwitz, 1992). Structural studies have suggested that sHSPs form oligomers (Kim et al., 1998; van Montfort et al., 2001). IDM2 is a nuclear protein and its transcript levels are not dramatically induced by heat shock. It is possible that IDM2 may function as a molecular chaperone for IDM1 and other proteins that are important for active DNA demethylation and cellular antisilencing.
Our results suggest that plants have evolved α-crystallin domain proteins that function in epigenetic regulation in the cell nucleus. It is possible that certain ACD proteins in mammals also function in epigenetic regulation.
EXPERIMENTAL PROCEDURES
Plant Materials and Mutant Screening by Chop PCR
Arabidopsis homozygous T-DNA insertion lines were obtained from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org). Arabidopsis seedlings were grown on MS nutrient agar plates at 22°C with 16 hr of light and 8 hr of darkness for 2 weeks before DNA or RNA analysis. Genetic screening was performed as described (Qian et al., 2012).
Map-Based Cloning of IDM2
Based on the root-length phenotype, an ethyl methanesulfonate-mutagenized wild-type (containing the 35S-SUC2 transgene) population was generated and screened for mutants with long roots on MS plates with 2% sucrose (Wang et al., 2013). The idm2-3 mutant was obtained from this screening. The idm2-3 mutant was crossed to Ler for molecular mapping (Wang et al., 2013).
Plasmid Construction
For complementation of mutants, a 2.7 kb genomic DNA fragment containing the IDM2 gene was amplified from wild-type Col-0 genomic DNA by PCR and cloned into the pCAMBIA1305 vector for plant transformation. All mutation sites were introduced into 35S::6MYC-IDM2 (pCAMBIA1307 vector) through site-directed mutagenesis with the QuikChange Kit according to the manufacturer’s instructions (Stratagene). Agrobacterium tumefaciens strain GV3101 carrying various IDM2 constructs was used to transform mutant plants via the standard floral dipping method. Primary transformants were selected on MS plates containing hygromycin.
Individual Locus Bisulfite Sequencing
Genomic DNA was extracted using the DNeasy Plant Mini Kit (QIAGEN). About 200 ng of genomic DNA was treated with sodium bisulfite using the BisulFlash DNA Modification Kit (Epigentek) following the manufacturer’s protocol. For each PCR reaction, a 2 ml aliquot of bisulfite-treated DNA was used in a 20 ml reaction. PCR products were purified using the Wizard SV Gel and PCR Clean-Up System Kit (Promega) and then subcloned into the T-easy vector (Promega) following the supplier’s instructions. For each DMR locus from each genetic background, at least 20 independent clones were sequenced (Table S5).
Real-Time RT-PCR
Total RNA was extracted from 2-week-old seedlings using the RNeasy Plant Mini Kit (QIAGEN), and contaminating DNA was removed with the DNA-free Kit (Ambion). mRNA (2 µg) was used for first-strand cDNA synthesis with the SuperScript III First-Strand Synthesis System (Invitrogen) for RT-PCR following the manufacturer’s instructions. The cDNA synthesis reaction was then diluted five times, and 1 µl was used as template in a 20 µl PCR reaction with iQ SYBR Green Supermix (Bio-Rad). All reactions were carried out on the iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). The comparative threshold cycle method was used for determining relative transcript levels (Bulletin 5279, Real-Time PCR Applications Guide; Bio-Rad), with TUB8 as an internal control.
DNA Methylation Assay by Southern Hybridization
The Southern hybridization assay was performed as described (Gong et al., 2002). Briefly, 5 mg of genomic DNA was digested with MspI, HpaII, or HaeIII. The digested DNA was loaded onto a 1.2% agarose gel and transferred to Hybond-N+ membranes. The 180 bp centromeric repeat and 5S rDNA repeat were labeled with digoxin by PCR (Roche). Southern hybridization was done following the manufacturer’s instructions.
Whole-Genome Bisulfite Sequencing and DMR Analysis
Bisulfite conversion, Illumina library construction, sequencing, and bioinformatic analysis were performed as described in Qian et al. (2012).
Tiling Array Analysis
To identify genes that are differentially expressed in idm1-1, idm2-1, and the wild-type, we performed pairwise comparisons of the tiling array data from the three genotypes (two replicates from each genotype). We first remapped the tiling array probes to the Arabidopsis genome (TAIR9) using SOAP2 (Li et al., 2009) and retained only probes that were perfectly mapped to a unique position in the genome. We created a custom chip definition file based on the probe mapping result and the TAIR9 gene annotation, and used the aroma.affymetrix framework (Bengtsson et al., 2008) for quantile normalization of the tiling array data. Only genes with three or more probes were considered for subsequent analysis. We used the genefilter package in Bioconductor (http://www.bioconductor.org) to remove genes expressed at low levels (normalized signal intensity <100 in all samples) and genes whose expression showed little change across samples (interquartile range of log2 intensities <0.5). We applied the linear model method implemented in the limma package in Bioconductor to identify genes that showed differential expression. The Benjamini and Hochberg method (Hochberg and Benjamini, 1990) was used for adjustment for multiple comparisons.
Coimmunoprecipitation
A 2 g quantity of leaves from N. benthamiana or transgenic Arabidopsis plants was harvested and ground to a fine power in liquid N2. Total protein was extracted with protein extraction buffer (20 mM Tris•HCl [pH 8.0], 150 mM NaCl, 1 mM EDTA, 10% glycerol, 5 mM dithiothreitol, 1 mM phenylmethane-sulfonylfluoride, and 1 × protease inhibitor cocktail [Roche]). The samples were centrifuged at 12,000 × g at 4°C for 30 min. Immunoprecipitation was then performed by incubating approximately 10 mg of total protein with an excess amount of anti-GFP antibody (A-11122; Invitrogen) or anti-HA (H3663; Sigma) at 4°C for 3 hr before 50 ml protein A agarose beads (Millipore) was added for an additional 2 hr. The beads were washed three times with extraction buffer. The immunoprecipitates were analyzed by western blotting with an anti-MYC (M4439; Sigma) or anti-Flag (F3165; Sigma) antibody.
Immunolocalization
Immunofluorescence localization was performed in 2- to 3-week-old leaves as described by Pontes et al., 2006). Nuclei preparations were incubated overnight at room temperature with rabbit anti-iDM2 (anti-IDM2 peptide antibody was custom made by YenZym Antibodies) and mouse anti-HA (H3663) or anti-Flag (F3165; Sigma). Primary antibodies were visualized using mouse Alexa 488-conjugated and rabbit Alexa 594 secondary antibody at 1:200 dilution (Molecular Probes) for 2 hr at 37°C. DNA was counterstained using DAPI in ProLong Gold (Invitrogen). Nuclei were examined with a Nikon Eclipse E800i epifluorescence microscope equipped with a Photometrics CoolSNAP ES.
Luciferase Complementation Imaging Assay
LCI assays were performed as described (Chen et al., 2008).
Yeast Two-Hybrid Assay
Yeast two-hybrid assays were conducted as described previously (Xia et al., 2006).
ChIP Assays
Chromatin immunoprecipitation assays were performed according to a published protocol (Saleh et al., 2008). The following antibodies were used for ChIP assays: anti-H3K4me2 (07-030; Millipore), anti-H3K9me2 (308-32361; Wako), anti-H3K18ac (ab1191; Abcam), anti-H3K23ac (07-355; Millipore), and anti-HA (H9658; Sigma). ChIP products were eluted into 50 µl of Tris-EDTA buffer, and a 2 µl aliquot was used for each qPCR reaction.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rebecca A. Stevenson for technical assistance. This work was supported by NIH grants R01GM070795 and R01GM059138 (to J.-K.Z.) and by the Chinese Academy of Sciences.
Footnotes
ACCESSION NUMBERS
We used whole-genome bisulfite sequencing data to analyze the genome-wide methylation status in idm2-1 and idm1-1idm2-1 mutant plants. The data set has been deposited in the Gene Expression Omnibus at the National Center for Biotechnology Information (accession number GSE49421).
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, six figures, and five tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2014.06.008.
AUTHOR CONTRIBUTIONS
W.Q. and J.-K.Z. designed the study and wrote the manuscript together with H.Z.; W.Q. isolated the idm2-1 and idm2-2 alleles and characterized the mutants and performed the co-IP and transgenic experiments; D.M. performed the bisulfite sequencing and ChIP assays; M.L. performed the antisilencing mutant screen and isolated the idm2-3 allele; X.Z. performed the immunolocalization experiments; H.Z. did the split luciferase assays; Y. Liu, Y. Li, Z.L., and J.W. assisted with the experiments on mutant characterization and protein interactions; and K.T. and R.L. analyzed the whole-genome bisulfite sequencing and tiling array data.
REFERENCES
- Agius F, Kapoor A, Zhu JK. Role of the Arabidopsis DNA glycosylase/lyase ROS1 in active DNA demethylation. Proc. Natl. Acad. Sci. USA. 2006;103:11796–11801. doi: 10.1073/pnas.0603563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem. Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J. DNA methylation and epigenetics. Annu. Rev. Plant Biol. 2004;55:41–68. doi: 10.1146/annurev.arplant.55.031903.141641. [DOI] [PubMed] [Google Scholar]
- Bengtsson H, Simpson K, Bullard J, Hansen K. aroma.affyme-trix: a generic framework in R for analyzing small to very large Affymetrix data sets in bounded memory. Technical Report 745, Department of Statistics, University of California, Berkeley. 2008 http://statistics.berkeley.edu/sites/default/files/tech-reports/745.pdf.
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards HV, Cameron RT, Baillie GS. The emerging role of HSP20 as a multifunctional protective agent. Cell. Signal. 2011;23:1447–1454. doi: 10.1016/j.cellsig.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Gao Z, Liu HL, Daxinger L, Pontes O, He X, Qian W, Lin H, Xie M, Lorkovic ZJ, Zhang S, et al. An RNA polymerase II- and AGO4-associated protein acts in RNA-directed DNA methylation. Nature. 2010;465:106–109. doi: 10.1038/nature09025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL. DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell. 2006;124:495–506. doi: 10.1016/j.cell.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Bubb KL, Henikoff S. Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science. 2009;324:1447–1451. doi: 10.1126/science.1171609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Morales-Ruiz T, Ariza RR, Roldán-Arjona T, David L, Zhu JK. ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell. 2002;111:803–814. doi: 10.1016/s0092-8674(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Haag JR, Pikaard CS. Multisubunit RNA polymerases IV and V: purveyors of non-coding RNA for plant gene silencing. Nat. Rev. Mol. Cell Biol. 2011;12:483–492. doi: 10.1038/nrm3152. [DOI] [PubMed] [Google Scholar]
- He XJ, Hsu YF, Zhu S, Wierzbicki AT, Pontes O, Pikaard CS, Liu HL, Wang CS, Jin H, Zhu JK. An effector of RNA-directed DNA methylation in Arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell. 2009;137:498–508. doi: 10.1016/j.cell.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XJ, Chen T, Zhu JK. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011;21:442–465. doi: 10.1038/cr.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GR, Lioe H, Stengel F, Baldwin AJ, Benesch JL. Small heat-shock proteins: paramedics of the cell. Top. Curr. Chem. 2013;328:69–98. doi: 10.1007/128_2012_324. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat. Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Horwitz J. α-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TF, Ibarra CA, Silva P, Zemach A, Eshed-Williams L, Fischer RL, Zilberman D. Genome-wide demethylation of Arabidopsis endosperm. Science. 2009;324:1451–1454. doi: 10.1126/science.1172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JH, Bauer MJ, Hsieh TF, Fischer RL. Cellular programming of plant gene imprinting. Cell. 2008;132:735–744. doi: 10.1016/j.cell.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Liu Y, Zhang B, Zhao Y, Wang X, Zhou Y, Raghothama KG, Liu D. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis . Plant Physiol. 2011;156:1116–1130. doi: 10.1104/pp.110.171736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- Li X, Qian W, Zhao Y, Wang C, Shen J, Zhu JK, Gong Z. Antisilencing role of the RNA-directed DNA methylation pathway and a histone acetyltransferase in Arabidopsis . Proc. Natl. Acad. Sci. USA. 2012;109:11425–11430. doi: 10.1073/pnas.1208557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, O’Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR. Highly integrated single-base resolution maps of the epigenome in Arabidopsis . Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell. Mol. Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat. Rev. Genet. 2005;6:24–35. doi: 10.1038/nrg1500. [DOI] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJ. RNA-mediated chromatin-based silencing in plants. Curr. Opin. Cell Biol. 2009;21:367–376. doi: 10.1016/j.ceb.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Ortega-Galisteo AP, Morales-Ruiz T, Ariza RR, Roldán-Arjona T. Arabidopsis DEMETER-LIKE proteins DML2 and DML3 are required for appropriate distribution of DNA methylation marks. Plant Mol. Biol. 2008;67:671–681. doi: 10.1007/s11103-008-9346-0. [DOI] [PubMed] [Google Scholar]
- Penterman J, Zilberman D, Huh JH, Ballinger T, Henikoff S, Fischer RL. DNA demethylation in the Arabidopsis genome. Proc. Natl. Acad. Sci. USA. 2007;104:6752–6757. doi: 10.1073/pnas.0701861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaard CS. Methylating the DNA of the most repressed: special access required. Mol. Cell. 2013;49:1021–1022. doi: 10.1016/j.molcel.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes O, Li CF, Costa Nunes P, Haag J, Ream T, Vitins A, Jacobsen SE, Pikaard CS. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell. 2006;126:79–92. doi: 10.1016/j.cell.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Qian W, Miki D, Zhang H, Liu Y, Zhang X, Tang K, Kan Y, La H, Li X, Li S, et al. A histone acetyltransferase regulates active DNA demethylation in Arabidopsis. Science. 2012;336:1445–1448. doi: 10.1126/science.1219416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Alvarez-Venegas R, Avramova Z. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 2008;3:1018–1025. doi: 10.1038/nprot.2008.66. [DOI] [PubMed] [Google Scholar]
- Scharf KD, Siddique M, Vierling E. The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins) Cell Stress Chaperones. 2001;6:225–237. doi: 10.1379/1466-1268(2001)006<0225:tefoat>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, MacRae TH. The small heat shock proteins and their role in human disease. FEBS J. 2005;272:2613–2627. doi: 10.1111/j.1742-4658.2005.04708.x. [DOI] [PubMed] [Google Scholar]
- Tariq M, Paszkowski J. DNA and histone methylation in plants. Trends Genet. 2004;20:244–251. doi: 10.1016/j.tig.2004.04.005. [DOI] [PubMed] [Google Scholar]
- van Montfort RL, Basha E, Friedrich KL, Slingsby C, Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- Wang X, Duan CG, Tang K, Wang B, Zhang H, Lei M, Lu K, Mangrauthia SK, Wang P, Zhu G, et al. RNA-binding protein regulates plant DNA methylation by controlling mRNA processing at the intronic heterochromatin-containing gene IBM1. Proc. Natl. Acad. Sci. USA. 2013;110:15467–15472. doi: 10.1073/pnas.1315399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Gaestel M. Small heat-shock protein family: function in health and disease. Ann. N Y Acad. Sci. 1998;851:28–35. doi: 10.1111/j.1749-6632.1998.tb08973.x. [DOI] [PubMed] [Google Scholar]
- Whitham SA, Anderberg RJ, Chisholm ST, Carrington JC. Arabidopsis RTM2 gene is necessary for specific restriction of tobacco etch virus and encodes an unusual small heat shock-like protein. Plant Cell. 2000;12:569–582. doi: 10.1105/tpc.12.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AT. The role of long non-coding RNA in transcriptional gene silencing. Curr. Opin. Plant Biol. 2012;15:517–522. doi: 10.1016/j.pbi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Xia R, Wang J, Liu C, Wang Y, Wang Y, Zhai J, Liu J, Hong X, Cao X, Zhu JK, Gong Z. ROR1/RPA2A,aputative replication protein A2, functions in epigenetic gene silencing and in regulation of meristem development in Arabidopsis . Plant Cell. 2006;18:85–103. doi: 10.1105/tpc.105.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, Kim MY, Hsieh PH, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer SL, Zilberman D. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153:193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhu JK. Active DNA demethylation in plants and animals. Cold Spring Harb. Symp. Quant. Biol. 2012;77:161–173. doi: 10.1101/sqb.2012.77.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis . Cell. 2006;126:1189–1201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana . Genome Biol. 2009;10:R62. doi: 10.1186/gb-2009-10-6-r62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Pontes O, Zhu J, Miki D, Zhang F, Li WX, Iida K, Kapoor A, Pikaard CS, Zhu JK. ROS3 is an RNA-binding protein required for DNA demethylation in Arabidopsis . Nature. 2008;455:1259–1262. doi: 10.1038/nature07305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Active DNA demethylation mediated by DNA glycosylases. Annu. Rev. Genet. 2009;43:143–166. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Kapoor A, Sridhar VV, Agius F, Zhu JK. The DNA glycosylase/lyase ROS1 functions in pruning DNA methylation patterns in Arabidopsis . Curr. Biol. 2007;17:54–59. doi: 10.1016/j.cub.2006.10.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.