Fig. 1.
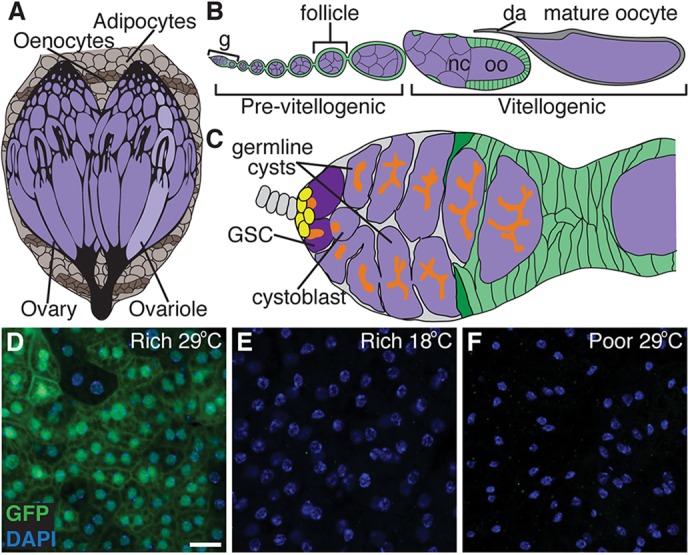
A tool to determine how genetic manipulation of nutrient-dependent pathways in adult adipocytes impacts the GSC lineage in the Drosophila ovary. (A) The Drosophila fat body is an endocrine organ awash in hemolymph and composed of sheets of adipocytes intercalated with hepatocyte-like oenocytes. The fat body underlies the cuticle and surrounds the brain, gut and ovaries in females. (B) Developing follicles arranged in chronological order make up an ovariole. Follicles, formed in an anterior germarium (g), are germline cysts (one oocyte, oo, plus 15 nurse cells, nc; purple) surrounded by follicle cells (green), and develop to form a mature oocyte containing a dorsal appendage (da). (C) Each germarium contains two or three GSCs in a well-defined niche composed primarily of cap cells (yellow), and each GSC division yields a GSC and a cystoblast that forms a 16-cell cyst. GSCs and other early germline stages are identifiable based on the position and morphology of a germline-specific organelle, the fusome (orange). Follicle cells derived from follicle stem cells (dark green) envelop the cyst, making a follicle. (D-F) In females raised at 18°C and subsequently switched to 29°C, Gal80ts; Lsp2 drives UAS transgene expression specifically in adult adipocytes (see supplementary material Figs S1 and S2). A UAS-GFP reporter (green) driven by Gal80ts; Lsp2 shows robust expression in adipocytes on a rich diet at 29°C (D), but is not expressed either at 18°C (E) or on a poor diet (F). DAPI (blue) labels nuclei. Scale bar: 50 μm.
