Abstract
Stem cells are influenced by their surrounding microenvironment, or niche. In the testis, Sertoli cells are the key niche cells directing the population size and differentiation fate of spermatogonial stem cells (SSCs). Failure to properly regulate SSCs leads to infertility or germ cell hyperplasia. Several Sertoli cell-expressed genes, such as Gdnf and Cyp26b1, have been identified as being indispensable for the proper maintenance of SSCs in their niche, but the pathways that modulate their expression have not been identified. Although we have recently found that constitutively activating NOTCH signaling in Sertoli cells leads to premature differentiation of all prospermatogonia and sterility, suggesting that there is a crucial role for this pathway in the testis stem cell niche, a true physiological function of NOTCH signaling in Sertoli cells has not been demonstrated. To this end, we conditionally ablated recombination signal binding protein for immunoglobulin kappa J region (Rbpj), a crucial mediator of NOTCH signaling, in Sertoli cells using Amh-cre. Rbpj knockout mice had: significantly increased testis sizes; increased expression of niche factors, such as Gdnf and Cyp26b1; significant increases in the number of pre- and post-meiotic germ cells, including SSCs; and, in a significant proportion of mice, testicular failure and atrophy with tubule lithiasis, possibly due to these unsustainable increases in the number of germ cells. We also identified germ cells as the NOTCH ligand-expressing cells. We conclude that NOTCH signaling in Sertoli cells is required for proper regulation of the testis stem cell niche and is a potential feedback mechanism, based on germ cell input, that governs the expression of factors that control SSC proliferation and differentiation.
Keywords: RBPJ, Spermatogenesis, Fertility, NOTCH signaling, Spermatogonial stem cell, Testicular microlithiasis, Mouse
INTRODUCTION
Long-term maintenance of tissue homeostasis depends on stem cells, whose activity is regulated by their microenvironment, or niche. The niche provides for local extrinsic factors and signals provided by nurse cells and the extracellular matrix. In mammals, niches have been identified in many tissues, such as in bone marrow, intestinal epithelium, skin epidermis, hair follicles and the brain, to name a few. However, regulation of the niche itself and how stem cells interact with their environment to direct their own fate is still poorly understood.
The testicular germ cell niche contributes to maintenance of the proper number of spermatogonial stem cells, and its dysregulation results in disruption of continuous spermatogenesis (Kanatsu-Shinohara and Shinohara, 2013). Perpetual feedback between germ cells and Sertoli cells is necessary to maintain the balance between proliferation and differentiation. Although proteins expressed by Sertoli cells, such as glial cell line-derived neurotrophic factor (GDNF) (Meng et al., 2000), GJA1 (Brehm et al., 2007), SIN3A (Payne et al., 2010), ETV5 (Chen et al., 2005) and CYP26B1 (Li et al., 2009; MacLean et al., 2007), have been identified as being indispensable for the proper maintenance of male germ cells in their niche, the signaling pathways that regulate the expression of these genes in Sertoli cells have not been identified. Pioneering studies in invertebrates (Kiger et al., 2001; Kimble and Crittenden, 2007; Kitadate and Kobayashi, 2010; Spradling et al., 2001; Xie and Spradling, 2000) have underscored the importance of the gonadal germline stem cell niche and demonstrated that NOTCH signaling is crucial for either spermatogonial maintenance and proliferation (Kimble and Crittenden, 2007) or niche determination (Kitadate and Kobayashi, 2010). Interestingly, NOTCH signaling seems disrupted or altered in testes of a number of patients who have non-obstructive azoospermia or testicular cancer (Hayashi et al., 2004), but a true link between NOTCH signaling and these conditions has not been thoroughly investigated.
We recently demonstrated that Sertoli cells, a major somatic component of the mammalian germline stem cell niche, depend on intrinsic NOTCH signaling to modulate gonocyte, or prospermatogonia, fate. In a gain-of-function mouse model that we previously generated, constitutively active NOTCH signaling in fetal Sertoli cells led to premature differentiation of all prospermatogonia and sterility by postnatal day 2 (Garcia et al., 2013). These effects were associated with downregulation of specific genes – Gdnf and Cyp26b1 – that are normally required for the maintenance of germ cells in an undifferentiated state. Therefore, in the mammalian testis stem cell niche, NOTCH signaling might regulate, through Sertoli cells, the decision of a germ cell to self-renew or differentiate.
In the present study, we sought to determine whether NOTCH signaling is physiologically required for germline stem cell homeostasis. The recombination signal binding protein for immunoglobulin kappa J region gene (Rbpj) encodes the transcriptional regulator protein RBPjκ (hereafter referred to simply as RBPJ), which controls multiple cellular processes in response to ligand-induced activation of all NOTCH receptors (Kato et al., 1996). In the absence of NOTCH receptor activation, RBPJ acts as a transcriptional repressor, but when bound to the activated and nuclear-localized intracellular domain of the NOTCH receptor (NICD), RBPJ acts as a transcriptional activator at promoters containing RBPJ-binding sites (Kato et al., 1996). Mice that are conditionally deficient in Rbpj or NOTCH signaling-related genes show a multitude of deficits in proper tissue and organ development and maintenance, which is in general due to improper control of cell proliferation and differentiation. In our previous studies using a transgenic NOTCH signaling reporter mouse (TNR-GFP), we also demonstrated that RBPJ protein is present – and that NOTCH signaling is activated – in Sertoli cells at various developmental stages before and after birth (Garcia et al., 2013).
To explore the role of RBPJ in the testis stem cell niche, we used a Cre recombinase-loxP conditional knockout strategy to disrupt Rbpj function in Sertoli cells before birth. Knockout testes exhibited a phenotype that was the opposite of that of the constitutively activated NOTCH phenotype. Loss of Rbpj in Sertoli cells resulted in increases in the number of undifferentiated and differentiated germ cells by 1 month of age. These increased germ cell numbers – although accompanied by an upregulation of Gdnf and Cyp26b1 – were not accompanied by any change in the total number of Sertoli cells. Testicular failure, presumably due to aberrant germ cell proliferation, occurred in ∼19% of knockout mice by 4-6 months of age. Furthermore, through a series of in vitro experiments, some using Sertoli cells isolated from transgenic NOTCH reporter mice (Duncan et al., 2005), we were able to demonstrate that germ cells, through JAG1, are capable of activating NOTCH signaling in Sertoli cells. Therefore, our study is the first to demonstrate that canonical NOTCH signaling in Sertoli cells is required, at least in part, for niche maintenance and the fate of male germline stem cells based on germ cell input.
RESULTS
Inactivation of Rbpj in Sertoli cells by cell-specific gene deletion
We previously reported that constitutive activation of NOTCH signaling in Sertoli cells produced a distinct phenotype with 100% penetrance: germ cells differentiated before birth during a period of gonad development normally characterized by their quiescence. This ultimately resulted in apoptosis and a complete loss of germ cells shortly after birth (Garcia et al., 2013; Garcia and Hofmann, 2013). To confirm that germ cell fate depends on the canonical NOTCH to RBPJ signaling pathway, we conditionally deleted exons 6 and 7 of the Rbpj gene (supplementary material Fig. S1A) driven by the Sertoli cell-specific Amh-cre transgene (Lecureuil et al., 2002) (Amh-cre;Rbpjfl/fl; for clarity, they are also referred to here as RbpjSCKO, where SCKO means Sertoli cell-specific knockout). Exons 6 and 7 of Rbpj encode the DNA-binding and NICD-binding domains, and loss of these exons results in the complete loss of RBPJ-mediated NOTCH signaling (Han et al., 2002). To identify and isolate pure populations of knockout and control Sertoli cells through fluorescence-activated cell sorting (FACS), we included the Cre-mediated lineage tracer allele, RosaYFP (supplementary material Fig. S1B,C; Fig. S2). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis, using primers spanning the exon 6 and 7 boundary, of total RNA obtained from Sertoli cells isolated through FACS from four independent control and RbpjSCKO mice at postnatal day 21 (P21) showed that the RbpjSCKO Sertoli cells had undetectable expression of Rbpj mRNA and, as expected, presented a sharp and significant decrease in the expression of the canonical NOTCH target genes Hes1, Hey1 and Heyl (supplementary material Fig. S1D). Likewise, neither wild-type nor floxed alleles could be detected through PCR in YFP+, FACS-sorted RbpjSCKO Sertoli cells, demonstrating effective Cre-mediated deletion of the critical floxed Rbpj exons (supplementary material Fig. S3). YFP+ cells were confirmed to be Sertoli cells (DAPI+, SOX9+ and TRA98−) through immunocytochemistry (supplementary material Fig. S4).
Ablation of RBPJ function induces increases in testicular size accompanied by testicular failure and atrophy in adult mice
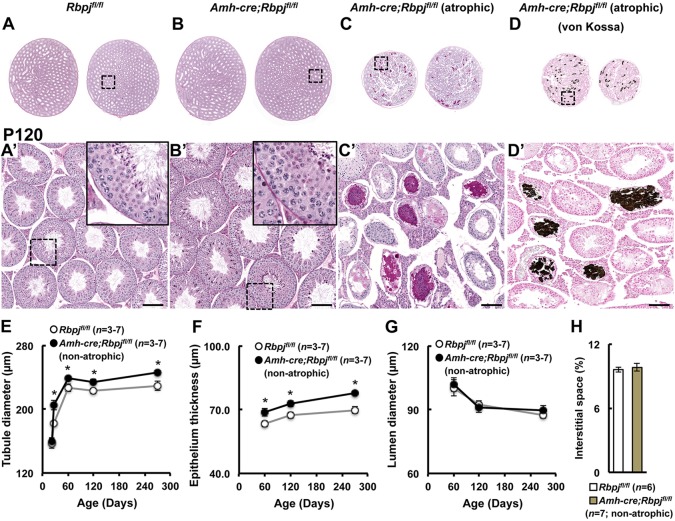
Embryonic gonads from RbpjSCKO mice were grossly normal with normal germ cell and Sertoli cell numbers in comparison to controls (supplementary material Fig. S5). Additionally, all gonocytes in immunohistochemical sections of RbpjSCKO and control mice were negative for the meiosis entry marker, stimulated by retinoic acid, gene 8 (STRA8), indicating appropriate maintenance of gonocytes in an undifferentiated state (supplementary material Fig. S6). Mature RbpjSCKO mice were grossly normal, with body weights throughout adulthood not significantly different from those of controls (Fig. 1A). However, our first observation was that sizes and weights of knockout testes were significantly greater than those of littermate control testes, a feature that became visible at 2 months of age (13.1% greater testis weight; Fig. 1B). Testis weight differences increased over time to 18.9% at P270. However, this excludes the subset of knockouts, ∼19%, which displayed severe bilateral testicular hypoplasia (atrophy) occurring between 4 and 6 months of age (Fig. 1C). Atrophic testes were approximately half the size of control testes, ranging in weight from 72.4 to 92.6 mg (79.7±3.0 mg; mean±s.e.m.; n=6). Atrophy was accompanied by high numbers of degenerated tubules, as observed by gross dissection and histology (Fig. 2C,C′). Degenerated tubules often were devoid of germ cells or contained a mix of proteinaceous and calcified material as identified by von Kossa staining (Fig. 2D,D′). None of the littermate controls (n>82; Rbpjfl/fl;RosaYFP/YFP and Amh-cre;Rbpj+/+;RosaYFP/YFP) at any time point presented with this or any form of atrophy.
Fig. 1.
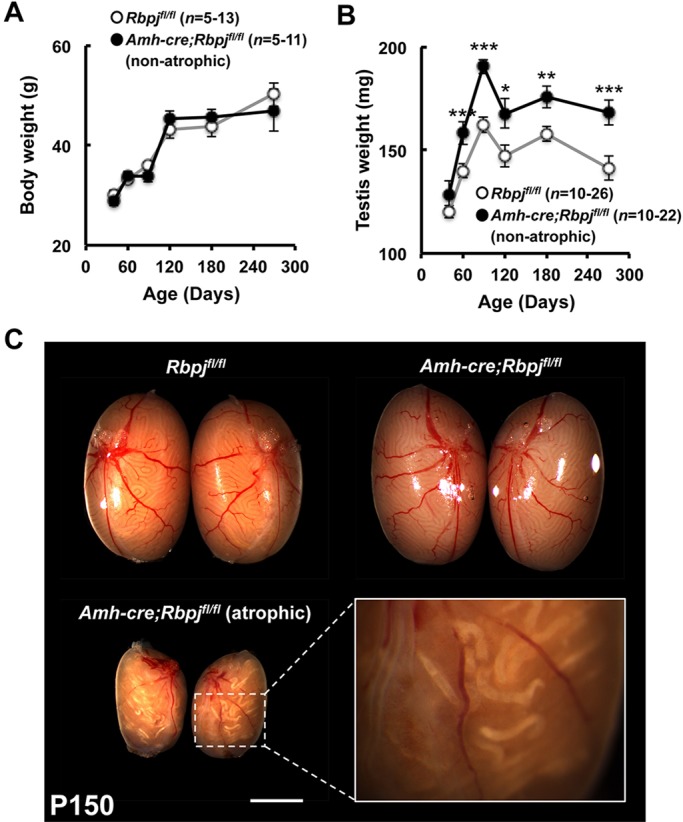
Testis weights are significantly increased in non-atrophic knockout mice. (A) Body weights are not significantly altered in control (Rbpjfl/fl) and RbpjSCKO (Amh-cre;Rbpjfl/fl) mice throughout adulthood. (B) Non-atrophic testis weights are significantly increased in knockout mice beginning at 2 months of age and persist throughout adulthood. (C) Representative images of control and non-atrophic and atrophic RbpjSCKO testes at P150. Results are given as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.005. Scale bar: 2 mm.
Fig. 2.
Tubule diameter and epithelial thickness are significantly increased in non-atrophic RbpjSCKO mice. Representative periodic acid-Schiff staining of bilateral control (Rbpjfl/fl) (A), non-atrophic (B) and atrophic (C) RbpjSCKO (Amh-cre;Rbpjfl/fl) mouse testes at P120. (D) von Kossa stain of atrophic RbpjSCKO mouse testes showing calcified tubules. (A′-D′) Higher magnification insets of the indicated region in A-D. Quantification of tubule diameter (E), epithelial thickness (F), lumen diameter (G) and percentage of interstitial space (H) in control and non-atrophic RbpjSCKO mouse testes at the indicated time points. Results are given as mean±s.e.m. *P<0.05. Scale bars: 100 µm.
To gain insight into the cause of increased testis weights in non-atrophic RbpjSCKO mice, we next performed morphometric analysis of histological sections. The volume of interstitial spaces between the testicular tubules was not significantly different between controls (n=6) and RbpjSCKO mice (n=7) (Fig. 2H). However, there was a significant increase of ∼5-6% in tubule diameters in RbpjSCKO testes at all ages investigated (P27, 60, 120 and 270) (Fig. 2A,B,E). Mean lumen diameters were not significantly different from controls at any of these time points (Fig. 2G), but seminiferous epithelium thickness was significantly increased, by 8-11%, in the RbpjSCKO testes (Fig. 2B,F). Overall, these results indicate that the increases in testis weights in RbpjSCKO mice arose from increases in the number of cells within the epithelial compartment.
Numbers of Sertoli cells in the seminiferous epithelia of knockout mice remain normal
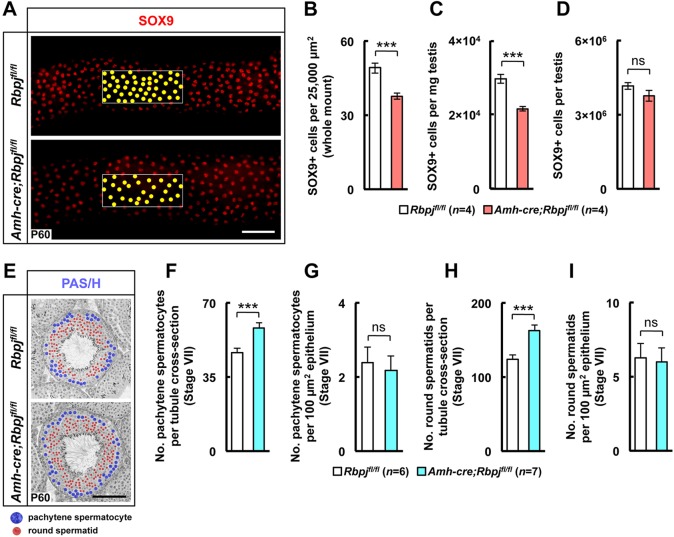
Given that testis size correlates with germ cell numbers, germ cell number depends on the number of Sertoli cells (Berndtson et al., 1987; Berndtson and Thompson, 1990; Thompson and Berndtson, 1993), and testicular enlargement can be accompanied by increased numbers of Sertoli cells (Hess et al., 1993), we next sought to determine whether Sertoli cell numbers were altered in RbpjSCKO mice. The numbers of Sertoli (SOX9+) cells per tubule area and milligram of testis were significantly decreased in the knockout mouse testes (Fig. 3A-C; supplementary material Fig. S7A-D; isotype control staining in supplementary material Fig. S8), but the overall number of Sertoli cells was not significantly different from that in the littermate control testes (Fig. 3D). Therefore, although changes in the spatial arrangement of the Sertoli cells might have been due to increases in the number of germ cells, the absolute number of Sertoli cells per testis did not change over time. This result indicates expansion of the volume of the stem cell niche independently of the number of Sertoli cells.
Fig. 3.
Increase in testis weight is associated with an increase in number of germ cells, whereas the total number of Sertoli cells is unchanged in RbpjSCKO mouse testes. (A) In a representative example of anti-SOX9 antibody whole-mount staining of control (Rbpjfl/fl) and RbpjSCKO (Amh-cre;Rbpjfl/fl) tubules at P60, yellow dots highlight individual Sertoli (SOX9+) cells within a 25,000-µm2 (100 µm×250 µm) tubule outer area. SOX9+ cells were quantified per 25,000 µm2 (B), per mg testis (C) and per whole testis (D). (E) Representative periodic acid-Schiff (PAS/H)-stained tubule at P60. Red and blue dots highlight round spermatids and pachytene spermatocytes, respectively. Quantification of round spermatids and pachytene spermatocytes per tubule cross-section (F,H) and per unit seminiferous epithelium area (µm2) (G,I), respectively. Results are given as mean±s.e.m. ***P<0.005; ns, not significant. Scale bars: 100 µm.
Germ cells accumulate in the seminiferous epithelia of knockout mice
Given that an increase in epithelium thickness without an increase in the number of Sertoli cells points to an increase in germ cell numbers in the knockout testes, we then assessed the number of pachytene spermatocytes and round spermatids at P60 (Fig. 3E). At this age there was a 24.5% greater mean number of pachytene spermatocytes and a 28.4% greater mean number of round spermatids per stage VII tubule cross-section in RbpjSCKO mice than in controls (Fig. 3F,H). When the numbers of pachytene spermatocytes and round spermatids per tubule were normalized to the cross-sectional area of the seminiferous epithelium, this difference was not significant (Fig. 3G,I), further demonstrating that epithelium thickness correlates with germ cell numbers. Despite the normal appearance of spermatogenesis, there was a significant increase in the number of large, luminal residual bodies, particularly at stages of spermatogenesis where their existence is not expected (supplementary material Figs S9, S10). This finding is consistent with the observed increase in the number of germ cells and likely due to a finite limit to the number residual bodies that Sertoli cells can engulf and reabsorb.
Therefore, knockout testes accumulate germ cells, although differentiation seems to proceed normally. This phenotype is the opposite of that of the phenotype when NOTCH signaling is constitutively activated, in which germ cells differentiate prematurely and die before birth. To the best of our knowledge, this is the first mouse model to demonstrate an increase in the number of germ cells while the number of Sertoli cells remains normal. Therefore, it appears that NOTCH signaling in Sertoli cells does not regulate Sertoli cell proliferation and/or fate determination as in other tissues.
Testicular hyperplasia does not affect fertility of RbpjSCKO mice
To assess the fertility of RbpjSCKO mice, nine control and nine RbpjSCKO males were each housed individually with a single wild-type female for 6 months, beginning at 2 months of age. Interestingly, the average number of litters and the average number of male and female pups per litter were not significantly different (supplementary material Table S1), and at the end of breeding assessment and upon gross dissection, none of these males exhibited testicular failure and atrophy. Taken together, these data suggest that spermatogenesis in mice with RBPJ-deficient Sertoli cells is largely normal and that aberrant increases in germ cell numbers might contribute to testicular failure.
Defect of NOTCH signaling in Sertoli cells leads to increases in factors crucial for the maintenance of undifferentiated germ cells
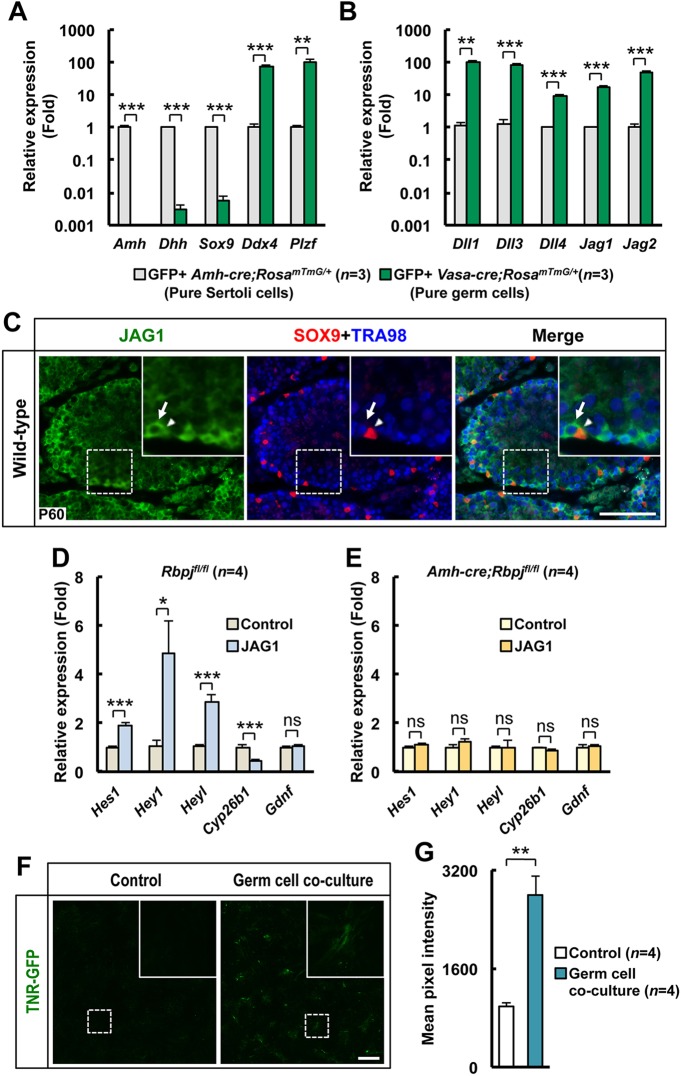
To understand the effects of NOTCH-RBPJ signaling in germ cell hyperplasia, we assessed the expression of genes that we previously measured in the Amh-cre;RosaNICD/+ constitutive activation mutant (Fig. 4). Gene expression in the knockout mice showed a pattern that was the opposite of that in the constitutionally activated NOTCH mutant, confirming a role for NOTCH signaling in Sertoli cells. Direct NOTCH target genes, such as Hes1, Hey1 and Heyl, were downregulated, indicating that the NOTCH canonical pathway is normally active in these cells. Furthermore, expression of Gdnf was significantly upregulated in the NOTCH knockout mutant. GDNF is a growth factor that is crucial for maintenance and proliferation of the stem cell pool in the testis (Kubota et al., 2004; Meng et al., 2000), and it is conceivable that NOTCH signaling regulates the numbers of stem cells and ultimately the overall output of germ cells. In the constitutively activated NOTCH mutant, Gdnf was downregulated (Fig. 4A), whereas in the knockout mice, it was upregulated (Fig. 4B), indicating that NOTCH signaling plays a role in the regulation of Gdnf expression.
Fig. 4.
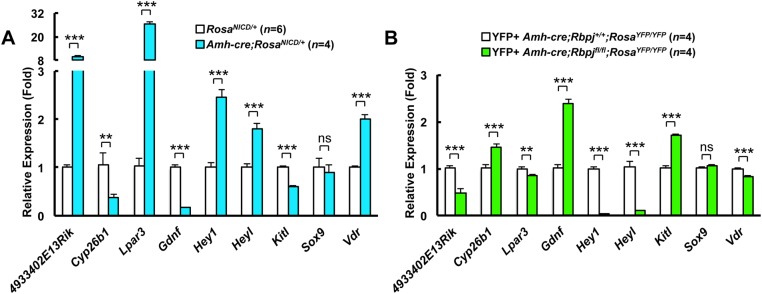
The expression of Sertoli cell-expressed genes that are required for germ cell maintenance and differentiation is regulated by NOTCH signaling. Quantitative RT-PCR analysis of whole fetal (E14.5) overexpressor (Amh-cre;RosaNICD/+) and control (RosaNICD/+) gonads (A) and pure YFP+ FACS-sorted Sertoli cells from knockout (Amh-cre;Rbpjfl/fl;RosaYFP/YFP) and control (Amh-cre;Rbpj+/+;RosaYFP/YFP) testes (B). Results are given as mean±s.e.m. **P<0.01, ***P<0.005.
The co-receptor for GDNF at the surface of germ cells is GDNF family receptor alpha 1 (GFRA1), which is expressed at the surface of undifferentiated spermatogonia, in particular Asingle, Apaired and some Aaligned spermatogonia (Hofmann et al., 2005). It is believed that the Asingle germ cell compartment contains spermatogonial stem cells (de Rooij and Russell, 2000). To understand whether increases in pachytene spermatocytes and spermatids originated from an increase of the stem cell compartment, we quantified the number of GFRA1-positive spermatogonia at P27 as well as the number of Asingle, Apaired and Aaligned GFRA1-positive spermatogonia in whole-mount testicular tubule preparations. The number of cells expressing GFRA1 was significantly increased at P27 in the RbpjSCKO (Fig. 5B,D,F; isotype control staining in supplementary material Fig. S8). As seen in Fig. 5G, the number of GFRA1-positive cells was similarly increased in knockout testes in whole-mount preparations. We also established that the number of Asingle spermatogonia and their direct progeny (Apaired and Aaligned spermatonia) were all significantly increased (Fig. 5H-J), strengthening the notion that the stem cell pool was increased in knockout testes. The results obtained with GFRA1 were confirmed by examination of PLZF (ZBTB16 – Mouse Genome Informatics), which marks all early-stage spermatogonia. The results showed that their number was also increased in RbpjSCKO testes (Fig. 5A,C,E).
Fig. 5.
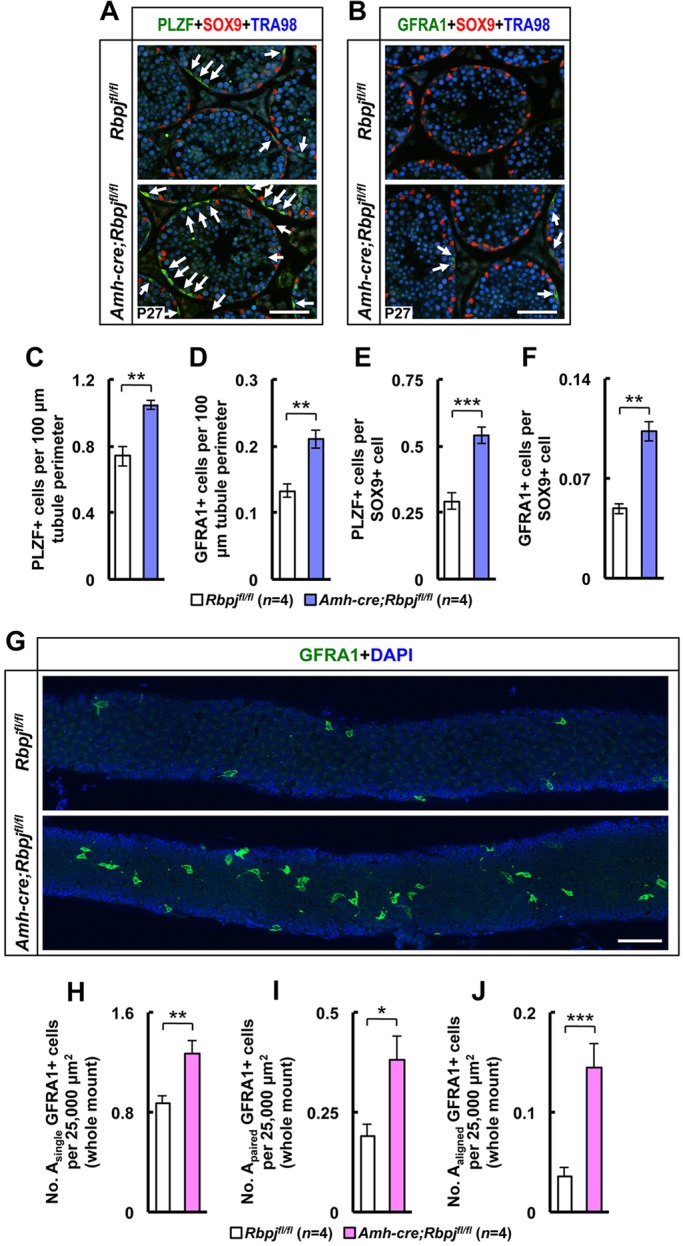
Spermatogonial stem cell numbers are increased in RbpjSCKO mouse testes. (A,B) Representative tubule cross-sections from control (Rbpjfl/fl) and knockout (Amh-cre;Rbpjfl/fl) testes at P27 stained for PLZF or GFRA1 (SSC markers), SOX9 (Sertoli cell marker) and TRA98 (germ cell marker). Arrows show representative PLZF+ or GFRA1+ cells. (C,D) Quantification of the number of PLZF+ or GFRA1+ SSCs per 100 µm tubule perimeter and (E,F) quantification of the number of PLZF+ or GFRA1+ SSCs per SOX9+ cell. (G) Representative anti-GFRA1 antibody whole-mount staining of control and knockout tubules. (H-J) Quantification of Asingle, Apaired and Aaligned GFRA1+ cells per 25,000 µm2 tubule surface. Results are given as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.005. Scale bars: 100 µm.
Kit ligand (Kitl), another growth factor that is crucial for spermatogonial differentiation, was also upregulated in the knockout (Fig. 4B). KITL binds to KIT receptor at the surface of differentiating spermatogonia and early spermatocytes. The molecular regulation of KITL, like that of GDNF, is poorly understood. It is possible that the increase in KITL further stimulates the production of differentiating cells from the increased number of SSCs in the RbpjSCKO mice.
The cytochrome P450 26B1 (CYP26B1) is encoded by the gene Cyp26b1 and is crucial for maintaining male germ cells in an undifferentiated state in the fetal stages because it mediates degradation of retinoic acid in the developing gonads. Shortly after birth, CYP26B1 expression normally decreases, allowing retinoic acid to exert its differentiating effects on germ cells, which will then express the gene Stra8. Regulation of CYP26B1 expression in the testis before and after birth is still poorly understood. Data from our constitutively activated NOTCH signaling mutant established that CYP26B1 was downregulated, which correlated with premature differentiation and apoptosis of germ cells (Garcia et al., 2013). In fact, this gain-of-function mutant exhibited a phenotype nearly identical to CYP26B1 global and Sertoli cell-specific conditional null knockout testes (Li et al., 2009; MacLean et al., 2007). In the present work, we show that Cyp26b1 was upregulated when NOTCH signaling was ablated (Fig. 4B), suggesting that this signaling pathway is likely involved, either directly or indirectly, in Cyp26b1 gene regulation. Because CYP26B1 expression seems to be tightly regulated spatially and temporally (i.e. it is present in the fetal testis but disappears shortly after birth to allow meiosis in the first wave of spermatogenesis) (Abu-Abed et al., 2002), we sought to follow differential expression of Cyp26b1 in relation to the NOTCH target genes Hes1, Hey1 and Heyl in the perinatal testis. Heyl and Cyp26b1 transcript levels showed an inverse relationship in Sertoli cells in the perinatal testis (supplementary material Fig. S11). This further supports the possibility that Cyp26b1 expression in Sertoli cells depends on the canonical NOTCH signaling pathway and might be a direct target of the HEYL transcriptional repressor in the perinatal testis.
Lastly, in our previous gene expression analyses of the gain-of-function mutant, we found several novel genes (4933402E13Rik, Lpar3 and Vdr) that were differentially regulated. We examined the expression of these same genes in the knockout Sertoli cells and found that their changes in expression were consistent with, and complementary to, those in the gain-of-function mutant (Fig. 4).
Germ cells are the ligand-presenting cells that initiate NOTCH signaling in Sertoli cells
We next sought to identify which cells provide the ligand for NOTCH signaling in Sertoli cells. The testicular seminiferous epithelium is composed of two main cell types, Sertoli cells and germ cells. We used RosamTmG transgenic mice, which allowed us to isolate pure GFP-positive Sertoli cells and germ cells when crossed with Amh-cre and Vasa-cre transgenic mice, respectively (Muzumdar et al., 2007). We established that a population of pure germ cells (Fig. 6A) expressed 10- to 100-fold more NOTCH ligands than Sertoli cells (Fig. 6B). Furthermore, we showed by immunohistochemical analysis that expression of the ligand JAG1 was specific to spermatogonia (Fig. 6C). We then stimulated pure Sertoli cells in vitro with a series of NOTCH ligands. Only JAG1 induced increases in Hes1, Hey1 and Heyl expression, with concomitantly increased expression of Cyp26b1 (Fig. 6D). Gdnf expression was not upregulated in these cells, indicating that GDNF control might be only indirectly dependent on the canonical NOTCH signaling pathway. As expected, Sertoli cells expressing a truncated and non-functional RBPJ did not respond to JAG1 stimulation (Fig. 6E). We confirmed that the culture conditions that we used only allowed growth and expansion of Sertoli cells, by demonstrating that cultured cells isolated from mice containing Amh-cre and RosaYFP emit specific cytoplasmic YFP fluorescence (supplementary material Fig. S12). To demonstrate that germ cells are the signal-presenting cells, we cultured Sertoli cells isolated from the TNR-GFP mouse model (Duncan et al., 2005; Garcia et al., 2013). In these cells, GFP expression disappeared within 24 h of isolation, indicating that there is probably no ligand-receptor interaction between Sertoli cells (Fig. 6F). After 5 days in culture, we added germ cells isolated from CD1 mice not expressing GFP. Addition of germ cells induced GFP expression in Sertoli cells within 24 h, demonstrating that germ cells are the ligand-presenting cells ultimately activating RBPJ (Fig. 6G). Taken together, these results indicate that germ cells use JAG1 and the NOTCH canonical pathway in Sertoli cells to control their own numbers, ensuring proper homeostasis and sperm output.
Fig. 6.
Germ cells are the ligand expressing cells. (A,B) Pure GFP+ germ cells (Vasa-cre;RosamTmG/+) and Sertoli cells (Vasa-cre;RosamTmG/+) were isolated by FACS and subjected to qRT-PCR gene expression analysis. (A) Markers of germ cells and Sertoli cells were differentially expressed in these cell isolates. (B) All NOTCH ligands were more highly expressed, by 10- to 100-fold, in germ cells than in Sertoli cells. (C) Representative tubule cross-sections from wild-type testes at P60 stained for JAG1 (NOTCH ligand), SOX9 (Sertoli cell marker) and TRA98 (germ cell marker) showing JAG1 staining in spermatogonia but not in Sertoli cells. A magnified view of the indicated area is shown in the inset. The arrows show a representative JAG1+ germ cell; the arrowheads show a representative Sertoli cell that is JAG1−. (D,E) Sertoli cells from control (Rbpjfl/fl, D) or knockout (Amh-cre;Rbpjfl/fl, E) testes were cultured in vitro in the presence or absence of the NOTCH ligand JAG1; the lack of a significant difference in response showed that the Notch signaling response was ablated in the knockout testes. (F) Sertoli cells isolated from TNR-GFP mice were cultured in vitro in the presence or absence of germ cells, demonstrating NOTCH activation in the presence of germ cells. A magnified view of the indicated area is shown in the inset. (G) Quantification of GFP fluorescence in the cultures represented in F. Results are given as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.005; ns, not significant. Scale bars: 100 µm.
DISCUSSION
We have presented here data demonstrating that NOTCH signaling in mouse Sertoli cells is a paramount component of the germline stem cell niche. Its activation in Sertoli cells does not appear to regulate Sertoli cell proliferation and lineage fate as it does in other cells in other tissues. Rather, NOTCH signaling in Sertoli cells influences the production of growth factors that are crucial for germ cell maintenance, self-renewal and differentiation. We attribute the increase in germ cell numbers in RbpjSCKO mice to, among other factors, an elevated production of GDNF by the mutant Sertoli cells. This finding is important because, for many years, it has been postulated that germ cells must communicate to Sertoli cells in a manner that can regulate their own fate and numbers, but evidence towards a signaling pathway fulfilling this role had yet to emerge. Here, we provide strong evidence implicating NOTCH signaling in a stem cell niche (Sertoli cells) as a negative-feedback mechanism based on germ cell input that, in turn, can control germ cell numbers.
The phenotype of RbpjSCKO mice is a near complete opposite of that of the published Amh-cre;RosaNICD/+ overexpressor, and when taken together, the data demonstrate that NOTCH signaling in Sertoli cells is a negative regulator of germ cell numbers. Recently, however, two independent groups reported that NOTCH signaling in Sertoli cells is dispensable for spermatogenesis, a conclusion that they both drew from conditional null mutants of Notch1 and Pofut1 in Sertoli cells (Batista et al., 2012; Hasegawa et al., 2011). It is important to note that the two strains of Amh-cre used in their studies were different from the strain that we used, which could contribute to differences in knockout efficiency. Indeed, Batista et al. reported low deletion efficiency of Pofut1 in Sertoli cells with the Amh-cre strain used in their study (Batista et al., 2012). With regards to the Sertoli cell-specific Notch1-null mutants, the lack of phenotype could be due to possible redundancy and compensation by the Notch2, Notch3 and Notch4 genes. With regards to the Pofut1 conditional null mutants, the conclusions of Batista et al. and Hasegawa et al. hold true because 81% of RbpjSCKO mice in our study did not exhibit testicular atrophy. Indeed, in non-atrophic RbpjSCKO knockout mice spermatogenesis was seemingly normal, except for a significant increase (30%) in the number of germ cells, which only became apparent after thorough histomorphometric analysis with larger sample sizes. However, 19% of our RbpjSCKO mice exhibited severe testicular atrophy, arguing that there is an important role for this signaling pathway in maintaining spermatogenesis.
Studies in other developmental systems have shown that cell specific deletions of Pofut1 can lead to less severe phenotypes than corresponding deletions of Rbpj (Guilmeau et al., 2008; Lin et al., 2011; van Es et al., 2005). For instance, terminal differentiation of sebaceous glands into sebocytes is close to normal in Tgfb3-cre;Pofut1fl/fl mice, whereas Tgfb3-cre;Rbpjfl/fl mice display sebaceous gland atrophy (Lin et al., 2011). Likewise, intestinal defects are less severe in Villin-cre;Pofut1fl/fl mutants than in comparable Villin-cre;Rbpjfl/fl mutants (Guilmeau et al., 2008; van Es et al., 2005). Given that the intestinal phenotypic changes in mice treated with the gamma secretase inhibitor dibenzazepine (DBZ), are reportedly indistinguishable from those observed in Rbpj mutants (van Es et al., 2005), yet Pofut1 null mutants have a comparably mild phenotype (Guilmeau et al., 2008), it has been suggested that NOTCH receptors might at least partially be able to transduce signals independent of POFUT1 in mouse intestinal and colonic epithelium (Guilmeau et al., 2008). These studies therefore demonstrate that the approaches used to turn-off the NOTCH pathway (Rbpj or Pofut1 silencing) are not equivalent to one another.
We cannot exclude the possibility that transcriptional activity of RBPJ, independent of NICD (Beres et al., 2006; MacKenzie et al., 2004; Tang and Kadesch, 2001), might contribute to the severity of phenotype we observed in our RbpjSCKO mice. However, our previous studies reporting serious deleterious effects in response to NOTCH pathway overactivation (Garcia et al., 2013; Garcia and Hofmann, 2013) suggest that there is a threshold of NOTCH activation in Sertoli cells above which spermatogenesis is permanently disrupted, supporting the concept that NOTCH plays an important role in spermatogenesis. Our findings with RbpjSCKO mice confirm these results and in most respects are complementary in phenotype to that of the overexpressor.
We were not surprised to find that proliferation and differentiation of gonocytes in the fetal gonads were normal in RbpjSCKO mice. NOTCH pathway overactivation in fetal Sertoli cells leads to decreases in factors that are crucial for gonocyte maintenance in an undifferentatied state and, subsequently, premature gonocyte differentiation during a period of gonad development characterized by gonocyte quiescence (Garcia et al., 2013; Garcia and Hofmann, 2013). Therefore, complementary inhibition of this pathway during this time should result in an increase in maintenance factors and, given that the gonocytes are already quiescent, no change in their state. Regulation of gonocyte exit from quiescence and transition to undifferentiated or differentiating spermatogonia, evidently, relies upon other pivotal factors besides NOTCH in perinatal Sertoli cells as neonatal gonad development and the initiation of spermatogenesis after birth was grossly normal (data not shown).
The incomplete incidence of testicular atrophy in RbpjSCKO mice is similar to several other notable examples – such as the incomplete incidence of teratoma formation in 129 mice (Shetty et al., 2012) or the varying levels of phenotype severity in a genetically pure mouse model of osteogenesis imperfecta (Pereira et al., 1994) – to which no proven explanations exist. The finding that none of the RbpjSCKO male mice subjected to breeding assessment experienced testicular failure and atrophy might be an indication of the underlying reason why approximately one-fifth of mice experienced atrophy and the other four-fifths did not. It is possible that aberrant increases in germ cell numbers are acceptable up to a certain threshold beyond which testicular failure can result. Minute behavioral and/or environmental differences leading to subtle changes in testicular function and usage might lead to this threshold being exceeded in some mice, but not others. Interestingly, mice that overexpress GDNF in a testis-specific manner eventually exhibit testicular atrophy with a chimeric histological pattern (Meng et al., 2000); this is similar – with the exception of tubule lithiasis – to the histological pattern that we observed in atrophic RbpjSCKO testes (Fig. 2C,D).
The large amount of tubule lithiasis present in RbpjSCKO atrophic testes (Fig. 1C; Fig. 2C,D), to the best of our knowledge, is a novel finding in a mutant mouse model and reminiscent of testicular microlithiasis in humans. Detected by testicular ultrasound, this rare condition in humans is characterized by multiple scattered echogenic foci (measuring 1-3 mm in diameter) within the testis (Bushby et al., 2002). These microliths are thought to form owing to severe seminiferous tubule damage and cell degeneration, although the etiology of this damage, and how or why they form, remains unknown. Testicular microlithiasis has been found in asymptomatic men as well as men with testicular germ cell tumor and carcinoma in situ (Husmann, 2005). The presence of lithiasis in RbpjSCKO atrophic testes might be an indication of the etiology of human testicular microlithiasis, pointing to either defects in NOTCH signaling or otherwise aberrant levels of germ cell proliferation.
In conclusion, the Sertoli cell is a key component of the germline stem cell niche, which provides spermatogonial stem cells with extrinsic growth factors and molecules crucial for self-renewal of the SSC pool and maintenance of the undifferentiated state. However, downregulating the production of these molecules is equally important to ensure germ cell homeostasis and allow differentiation. We provide here evidence that NOTCH signaling in Sertoli cells negatively regulates GDNF and CYP26B1 expression to ensure adequate amounts of these factors. Dysregulation of this essential niche component results in improper dosages that, below or above a certain threshold, will lead to sterility or might facilitate testicular cancer development. In addition, we were able to demonstrate that germ cells, through JAG1, are capable of activating NOTCH signaling in Sertoli cells, providing a potential feedback mechanism that controls their own numbers.
MATERIALS AND METHODS
Mice and generation of Sertoli cell-specific conditional null mutants of Rbpj
Heterozygous Tg(AMH-cre)1Flor (Amh-cre) (Lecureuil et al., 2002) mice were mated to homozygous floxed Rbpjtm1Hon (Rbpjfl/fl) (Han et al., 2002) mice, which were also homozygous for the lineage reporter transgene, Rosa26-LoxP-STOP-LoxP-EYFP (RosaYFP/YFP) (Srinivas et al., 2001), to generate Amh-cre;Rbpjfl/+;RosaYFP/+ offspring. These F1 animals were then interbred to obtain the breeding colony comprising male and female Rbpj+/+;RosaYFP/YFP, Rbpjfl/fl;RosaYFP/YFP, Amh-cre;Rbpj+/+;RosaYFP/YFP and Amh-cre;Rbpjfl/fl;RosaYFP/YFP. For all studies except the gene expression analyses that used YFP for the isolation of Sertoli cells, Rbpjfl/fl;RosaYFP/YFP male progeny served as controls for Amh-cre;Rbpjfl/fl;RosaYFP/YFP conditional knockout males. Heterozygous Tg(AMH-cre)1Flor (Amh-cre) (Lecureuil et al., 2002) female mice were mated to homozygous floxed Gt(Rosa)26Sortm1(Notch1)Dam/J (RosaNICD) (Murtaugh et al., 2003) male mice to generate F1 Amh-cre;RosaNICD/+ overexpressors and RosaNICD/+ controls as previously described (Garcia et al., 2013). Heterozygous Amh-cre and Vasa-cre mice were intercrossed with homozygous Gt(ROSA26)ACTB-tdTomato-EGFP (RosamTmG/mTmG) (Muzumdar et al., 2007) to generate F1 Amh-cre;RosamTmG/+ and Vasa-cre;RosamTmG/+ mice to obtain [through fluorescence-activated cell sorting (FACS)] pure populations of GFP+ Sertoli cells and germ cells, respectively. Transgenic Notch reporter GFP (TNR-GFP) mice (Duncan et al., 2005) were maintained as homozygotes, and either homozygous progeny or F1 progeny, obtained through mating with CD1 females, were used for experiments as indicated. Genotyping primers are listed in supplementary material Table S2. For timed matings to obtain fetal samples, male and female mice were paired together and the females checked for the presence of a vaginal plug. The day a vaginal plug was detected was considered E0.5. Mice were housed in accordance with National Institutes of Health guidelines, and experimental protocols were approved by the Institutional Animal Care and Use Committees at The University of Texas MD Anderson Cancer Center and the Institute for Biosciences and Technology, Texas A&M Health Science Center.
Histology, immunostaining and live-cell imaging
Histologic and immunohistochemical analyses were carried out as previously described (Garcia et al., 2013). Whole-mount staining was carried out as previously described (Hu et al., 2013). Von Kossa stain was applied to paraformaldehyde-fixed and paraffin-embedded sections, with nuclear Fast Red as a counterstain. Histological images were acquired with an Aperio AT2 slide scanner (Leica Microsystems), multi-channel fluorescent images were acquired with a Nikon A1 laser scanning confocal microscope (Nikon), and TNR-GFP Sertoli cells were imaged with an IN Cell Analyzer 6000 (GE Healthcare Bio-Sciences). All images were acquired with equivalent exposure levels between control and mutant sections at each endpoint. The primary antibodies used and their dilutions are listed in supplementary material Table S3.
Testis stereology and quantitative cell analyses from histologic and immunohistochemical sections
Tubule diameter, lumen diameter and epithelial thickness were calculated from ImageJ measurements of freehand outlines drawn along the circumference of circular tubule cross-sections (measured in μm) and their respective lumens, as follows:
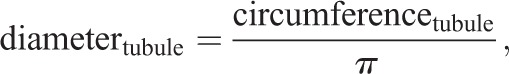 |
1 |
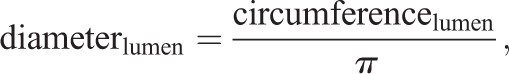 |
2 |
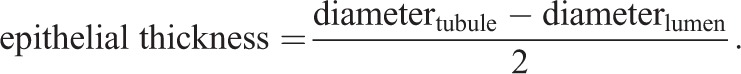 |
3 |
A minimum of 60 tubule measurements per animal from a minimum of two separate testis sections per animal was utilized to obtain a mean value per animal. Interstitial space was calculated by subtracting the total area (µm2) occupied by tubule cross-sections within a 1000 µm×1000 µm (1,000,000 µm2) box from the total area of the box, as follows:
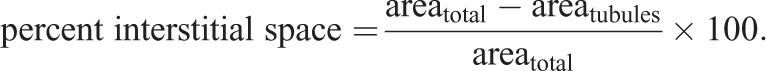 |
4 |
Three separate boxes per animal from a minimum of two separate testis sections per animal were utilized to obtain a mean value per animal.
Periodic acid and Schiff-stained testis cross-sections were examined for stages of spermatogenesis and the stages quantified by applying standard staging criteria (Meistrich and Hess, 2013). A total of 150 tubules per animal from a minimum of two separate testis sections per animal were staged and the number of large, lumenal residual bodies present in each staged tubule was recorded. The percentage total of each stage represents the number of times a particular stage was counted within the histological sections of an animal divided by the total number of tubules that were staged (×100). A mean value of the number of residual bodies found within each staged tubule was assigned to an individual mouse before the final calculation on a per mouse basis was carried out. The numbers of round spermatids and pachytene spermatocytes per round Stage VII tubule cross-section at postnatal day 60 (P60) were manually counted within the epithelium of a minimum of 20 tubules per animal from a minimum of two separate testis sections per animal. The tubule and lumen diameters determined from these tubules were then used to calculate seminiferous epithelium area (µm2) of each tubule to determine the number of each cell type per unit epithelium area. Mean values on a per mouse basis were used for final calculations.
The number of SOX9+, PLZF+ and GFRA1+ cells per 100 µm tubule cross-section perimeter was determined by counting the number of each respective cell type within a given tubule cross-section and normalizing to the respective tubule perimeter (µm), as determined through ImageJ measurements of freehand drawn outlines. This was done for a minimum of 36 tubule cross-sections per animal from a minimum of two separate testis sections per animal.
Whole-mount quantitation and determination of total SOX9+ cells per testis
Contiguous tiled and stitched images along a total minimum distance of 2 cm of whole-mount tubule per animal from the indicated number of animals was utilized for quantitation of the number of SOX9+ and GFRA1+ cells per 25,000 µm2 tubule surface. SOX9+ cells were quantified by randomly placing 20 boxes at low magnification (100 µm×250 µm in dimensions; 25,000 µm2 in area) along the length of the imaged tubule(s). Once the boxes were placed, at high magnification, the number of SOX9+ cells within each box was manually counted (yellow dots depict counted cells in Fig. 3A). Given that the density of GFRA1+ cells was lower and more variable along the length of the tubule, the entire area of tubule from entire contiguous tiled and stitched images was quantified and counted for GFRA1+ Asingle, Apaired and Aaligned spermatogonia. The total number of GFRA1+ Asingle, Apaired and Aaligned spermatogonia per animal was normalized to the total area of tubule (µm2) considered within the image.
The total number of Sertoli (SOX9+) cells per testis was determined as follows. Testis mass (mg) was converted to testis volume (mm3) by assuming the density of the testis is approximately equal to 1 mg/ml (120 mg of testis ≈120 mm3 of testis). The seminiferous compartment volume (mm3) was calculated from the mean percentage of interstitial area (µm2) previously determined through histological images. The percentage of interstitial area of 9.7%, as previously determined through analysis of histological images of 2-dimensional planes through the testis at P60, is equivalent to an interstitial area of 14.2% within the 3-dimensional spheroid of the testis. Therefore, the seminiferous compartment volume (in μm3)=testis volume (in μm3)×0.86.
Given that tubule diameters were also quantified in histological images at P60 and tubules are cylindrical in shape, the total tubule surface of the seminiferous compartment (in μm2) was calculated using the formula for the volume of a cylinder:
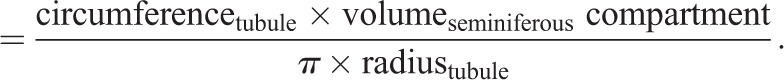 |
5 |
Therefore,
 |
6 |
Primary cell isolation and Sertoli cell cultures
Primary cells were isolated by two-step enzymatic digestion as previously described (Garcia and Hofmann, 2012). For quantitative polymerase chain reaction (qRT-PCR) analyses of pure populations of germ cells and Sertoli cells, 100,000 cells were obtained through FACS as previously described (Garcia and Hofmann, 2012) using the appropriate fluorophore-negative controls to allow for proper gating. One sample consists of one sort from one isolation, and at least three separate isolations per genotype were used for subsequent analyses.
For ligand activation experiments, cells in 96-well plates were treated with phosphate-buffered saline (PBS) or recombinant rat JAG1 (R&D Systems) diluted in PBS to a final concentration of 10 μg/ml and incubated overnight at 4°C. Under these conditions, some of the ligand adheres to the surface of the plate, allowing proper activation of the NOTCH receptors. Prior to primary cell plating, the PBS or PBS+ligand was removed from the wells, and a 1:5 dilution of Matrigel (BD Biosciences) in Dulbecco's modified Eagle's medium with Ham's F12 (DMEM/F12) was added on top of the immobilized ligand, the excess was removed and the plates were incubated at 37°C for at least 30 min to allow the thin layer of Matrigel to cure. Sertoli cells (50,000), previously isolated and expanded on Matrigel for approximately 5 days, were added to each ligand- or control-containing well and subjected to mRNA isolation and qRT-PCR analysis 18 h later. For TNR-GFP Sertoli cell co-culture with primary isolated germ cells, Sertoli cells isolated from TNR-GFP mice were cultured in wells of glass-bottomed black 96-well plates and wild-type germ cells isolated through differential plating were plated on top of the Sertoli cells.
For Sertoli cell culture, cells isolated from P2 wild-type testes, or P10 RosaNICD and Amh-cre;RosaNICD testes, were resuspended in DMEM/F-12 and plated on Matrigel-coated dishes. The medium was changed after 4 h to remove residual non-adherent cells, and the Sertoli cells were cultured for an additional 10 days before RNA isolation, with passages on the third and seventh days to ensure complete removal of residual spermatogonia. The use of DMEM/F-12 without serum as medium, and dishes pre-coated with Matrigel throughout the culture period, ensured proper growth conditions for Sertoli cells only (Simon et al., 2010).
RNA extraction and quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was carried out as previously described (Garcia et al., 2013). The TaqMan assays used for specific transcripts are listed in supplementary material Table S4.
Statistical analysis
All data were analyzed using GraphPad Prism 6 (GraphPad Software) and are presented as mean±s.e.m. Control and treated groups at one time point were compared by using a two-tailed Student’s t-test; comparisons between control and treated groups at two or more time points were conducted using analysis of variance followed by the Newman–Keuls post-hoc test. For all comparisons, statistical significance was assigned at P<0.05.
Supplementary Material
Acknowledgements
We extend our gratitude to Dr Tasuku Honjo (Kyoto University) for permission to obtain the Rbpj floxed mice; Dr Stacey S. Huppert (Cincinnati Children's Hospital) for providing the Rbpj floxed mice; Drs Yueh-Chiang Hu (Cincinnati Children's Hospital) and William H. Walker (University of Pittsburgh) for sharing expertise on whole-mount immunostaining; Dr Rex Hess (University of Illinois) for insightful discussion on testicular microlithiasis; Drs Marvin L. Meistrich and Gunapala Shetty (MD Anderson Cancer Center) for critical reading of the manuscript; Wendy Schober and Nalini Patel at the North Campus Flow Cytometry and Cellular Imaging Core Facility at MD Anderson Cancer Center for FACS; Dr Walter N. Hittelman and the Center for Targeted Therapy at MD Anderson Cancer Center for generously providing access to the Nikon confocal microscope; and Jeannie Zhong and the joint IBT-BCM Center for Advanced Imaging for generously providing access to the IN Cell Analyzer 6000.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
T.X.G. and M.-C.H. conceived the project, designed the experiments, analyzed and discussed the data, and wrote and edited the manuscript. T.X.G., J.K.F. and S.K. performed the experiments, and analyzed and discussed the data.
Funding
This work was supported by National Institutes of Health (NIH) [R01HD044543, R21HD068989, R01HD081244 to M.-C.H.]; and an NIH fellowship award [T32 ES007326 to T.X.G.]; and in part through an MD Anderson Cancer Center Support Grant [CA16672]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.113969/-/DC1
References
- Abu-Abed S., MacLean G., Fraulob V., Chambon P., Petkovich M. and Dollé P. (2002). Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech. Dev. 110, 173-177 10.1016/S0925-4773(01)00572-X [DOI] [PubMed] [Google Scholar]
- Batista F., Lu L., Williams S. A. and Stanley P. (2012). Complex N-glycans are essential, but core 1 and 2 mucin O-glycans, O-fucose glycans, and NOTCH1 are dispensable, for mammalian spermatogenesis. Biol. Reprod. 86, 179 10.1095/biolreprod.111.098103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres T. M., Masui T., Swift G. H., Shi L., Henke R. M. and MacDonald R. J. (2006). PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol. Cell. Biol. 26, 117-130 10.1128/MCB.26.1.117-130.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndtson W. E. and Thompson T. L. (1990). Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J. Androl. 11, 429-435. [PubMed] [Google Scholar]
- Berndtson W. E., Igboeli G. and Pickett B. W. (1987). Relationship of absolute numbers of Sertoli cells to testicular size and spermatogenesis in young beef bulls. J. Anim. Sci. 64, 241-246. [DOI] [PubMed] [Google Scholar]
- Brehm R., Zeiler M., Rüttinger C., Herde K., Kibschull M., Winterhager E., Willecke K., Guillou F., Lécureuil C., Steger K. et al. (2007). A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am. J. Pathol. 171, 19-31 10.2353/ajpath.2007.061171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby L. H., Miller F. N. A. C., Rosairo S., Clarke J. L. and Sidhu P. S. (2002). Scrotal calcification: ultrasound appearances, distribution and aetiology. Br. J. Radiol. 75, 283-288 10.1259/bjr.75.891.750283 [DOI] [PubMed] [Google Scholar]
- Chen C., Ouyang W., Grigura V., Zhou Q., Carnes K., Lim H., Zhao G.-Q., Arber S., Kurpios N., Murphy T. L. et al. (2005). ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 436, 1030-1034 10.1038/nature03894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij D. G. and Russell L. D. (2000). All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 21, 776-798. [PubMed] [Google Scholar]
- Duncan A. W., Rattis F. M., DiMascio L. N., Congdon K. L., Pazianos G., Zhao C., Yoon K., Cook J. M., Willert K., Gaiano N. et al. (2005). Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6, 314-322 10.1038/ni1164 [DOI] [PubMed] [Google Scholar]
- Garcia T. and Hofmann M.-C. (2012). Isolation of undifferentiated and early differentiating type A spermatogonia from Pou5f1-GFP reporter mice. Methods Mol. Biol. 825, 31-44 10.1007/978-1-61779-436-0_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia T. X. and Hofmann M.-C. (2013). NOTCH signaling in Sertoli cells regulates gonocyte fate. Cell Cycle 12, 2538-2545 10.4161/cc.25627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia T. X., DeFalco T., Capel B. and Hofmann M.-C. (2013). Constitutive activation of NOTCH1 signaling in Sertoli cells causes gonocyte exit from quiescence. Dev. Biol. 377, 188-201 10.1016/j.ydbio.2013.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilmeau S., Flandez M., Bancroft L., Sellers R. S., Tear B., Stanley P. and Augenlicht L. H. (2008). Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology 135, 849-860.e6, 860 e841-846 10.1053/j.gastro.2008.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Tanigaki K., Yamamoto N., Kuroda K., Yoshimoto M., Nakahata T., Ikuta K. and Honjo T. (2002). Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637-645 10.1093/intimm/dxf030 [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Okamura Y. and Saga Y. (2011). Notch signaling in Sertoli cells regulates cyclical gene expression of Hes1 but is dispensable for mouse spermatogenesis. Mol. Cell. Biol. 32, 206-215 10.1128/MCB.06063-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Yamada T., Kageyama Y. and Kihara K. (2004). Expression failure of the notch signaling system is associated with the pathogenesis of testicular germ cell tumor. Tumour Biol. 25, 99-105 10.1159/000079140 [DOI] [PubMed] [Google Scholar]
- Hess R. A., Cooke P. S., Bunick D. and Kirby J. D. (1993). Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology 132, 2607-2613. [DOI] [PubMed] [Google Scholar]
- Hofmann M.-C., Braydich-Stolle L. and Dym M. (2005). Isolation of male germ-line stem cells; influence of GDNF. Dev. Biol. 279, 114-124 10.1016/j.ydbio.2004.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-C., de Rooij D. G. and Page D. C. (2013). Tumor suppressor gene Rb is required for self-renewal of spermatogonial stem cells in mice. Proc. Natl. Acad. Sci. USA 110, 12685-12690 10.1073/pnas.1311548110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann D. A. (2005). Cryptorchidism and its relationship to testicular neoplasia and microlithiasis. Urology 66, 424-426 10.1016/j.urology.2004.10.020 [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M. and Shinohara T. (2013). Spermatogonial stem cell self-renewal and development. Annu. Rev. Cell Dev. Biol. 29, 163-187 10.1146/annurev-cellbio-101512-122353 [DOI] [PubMed] [Google Scholar]
- Kato H., Sakai T., Tamura K., Minoguchi S., Shirayoshi Y., Hamada Y., Tsujimoto Y. and Honjo T. (1996). Functional conservation of mouse Notch receptor family members. FEBS Lett. 395, 221-224 10.1016/0014-5793(96)01046-0 [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B. and Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542-2545 10.1126/science.1066707 [DOI] [PubMed] [Google Scholar]
- Kimble J. and Crittenden S. L. (2007). Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 23, 405-433 10.1146/annurev.cellbio.23.090506.123326 [DOI] [PubMed] [Google Scholar]
- Kitadate Y. and Kobayashi S. (2010). Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc. Natl. Acad. Sci. USA 107, 14241-14246 10.1073/pnas.1003462107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H., Avarbock M. R. and Brinster R. L. (2004). Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 101, 16489-16494 10.1073/pnas.0407063101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécureuil C., Fontaine I., Crepieux P. and Guillou F. (2002). Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 33, 114-118 10.1002/gene.10100 [DOI] [PubMed] [Google Scholar]
- Li H., MacLean G., Cameron D., Clagett-Dame M. and Petkovich M. (2009). Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS ONE 4, e7501 10.1371/journal.pone.0007501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.-Y., Kao C.-H., Lin K. M.-C., Kaartinen V. and Yang L.-T. (2011). Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS ONE 6, e15842 10.1371/journal.pone.0015842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie F., Duriez P., Wong F., Noseda M. and Karsan A. (2004). Notch4 inhibits endothelial apoptosis via RBP-Jkappa-dependent and -independent pathways. J. Biol. Chem. 279, 11657-11663 10.1074/jbc.M312102200 [DOI] [PubMed] [Google Scholar]
- MacLean G., Li H., Metzger D., Chambon P. and Petkovich M. (2007). Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 148, 4560-4567 10.1210/en.2007-0492 [DOI] [PubMed] [Google Scholar]
- Meistrich M. L. and Hess R. A. (2013). Assessment of spermatogenesis through staging of seminiferous tubules. Methods Mol. Biol. 927, 299-307 10.1007/978-1-62703-038-0_27 [DOI] [PubMed] [Google Scholar]
- Meng X., Lindahl M., Hyvönen M. E., Parvinen M., de Rooij D. G., Hess M. W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M. et al. (2000). Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489-1493 10.1126/science.287.5457.1489 [DOI] [PubMed] [Google Scholar]
- Murtaugh L. C., Stanger B. Z., Kwan K. M. and Melton D. A. (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 100, 14920-14925 10.1073/pnas.2436557100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593-605 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Payne C. J., Gallagher S. J., Foreman O., Dannenberg J. H., Depinho R. A. and Braun R. E. (2010). Sin3a is required by sertoli cells to establish a niche for undifferentiated spermatogonia, germ cell tumors, and spermatid elongation. Stem Cells 28, 1424-1434 10.1002/stem.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira R., Halford K., Sokolov B. P., Khillan J. S. and Prockop D. J. (1994). Phenotypic variability and incomplete penetrance of spontaneous fractures in an inbred strain of transgenic mice expressing a mutated collagen gene (COL1A1). J. Clin. Invest. 93, 1765-1769 10.1172/JCI117161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty G., Comish P. B., Weng C. C. Y., Matin A. and Meistrich M. L. (2012). Fetal radiation exposure induces testicular cancer in genetically susceptible mice. PLoS ONE 7, e32064 10.1371/journal.pone.0032064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L., Ekman G. C., Garcia T., Carnes K., Zhang Z., Murphy T., Murphy K. M., Hess R. A., Cooke P. S. and Hofmann M.-C. (2010). ETV5 regulates sertoli cell chemokines involved in mouse stem/progenitor spermatogonia maintenance. Stem Cells 28, 1882-1892 10.1002/stem.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Drummond-Barbosa D. and Kai T. (2001). Stem cells find their niche. Nature 414, 98-104 10.1038/35102160 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.-S., William C. M., Tanabe Y., Jessell T. M. and Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. and Kadesch T. (2001). Identification of a novel activation domain in the Notch-responsive transcription factor CSL. Nucleic Acids Res. 29, 2284-2291 10.1093/nar/29.11.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson T. L. and Berndtson W. E. (1993). Testicular weight, Sertoli cell number, daily sperm production, and sperm output of sexually mature rabbits after neonatal or prepubertal hemicastration. Biol. Reprod. 48, 952-957 10.1095/biolreprod48.5.952 [DOI] [PubMed] [Google Scholar]
- van Es J. H., van Gijn M. E., Riccio O., van den Born M., Vooijs M., Begthel H., Cozijnsen M., Robine S., Winton D. J., Radtke F. et al. (2005). Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959-963 10.1038/nature03659 [DOI] [PubMed] [Google Scholar]
- Xie T. and Spradling A. C. (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328-330 10.1126/science.290.5490.328 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.