Abstract
The ocular surface epithelia, including the stratified but non-keratinized corneal, limbal and conjunctival epithelium, in concert with the epidermal keratinized eyelid epithelium, function together to maintain eye health and vision. Abnormalities in cellular proliferation or differentiation in any of these surface epithelia are central in the pathogenesis of many ocular surface disorders. Goblet cells are important secretory cell components of various epithelia, including the conjunctiva; however, mechanisms that regulate goblet cell differentiation in the conjunctiva are not well understood. Herein, we report that conditional deletion of transforming growth factor β receptor II (Tgfbr2) in keratin 14-positive stratified epithelia causes ocular surface epithelial hyperplasia and conjunctival goblet cell expansion that invaginates into the subconjunctival stroma in the mouse eye. We found that, in the absence of an external phenotype, the ocular surface epithelium develops properly, but young mice displayed conjunctival goblet cell expansion, demonstrating that TGFβ signaling is required for normal restriction of goblet cells within the conjunctiva. We observed increased expression of SAM-pointed domain containing ETS transcription factor (SPDEF) in stratified conjunctival epithelial cells in Tgfbr2 cKO mice, suggesting that TGFβ restricted goblet cell differentiation directly by repressing Spdef transcription. Gain of function of Spdef in keratin 14-positive epithelia resulted in the ectopic formation of goblet cells in the eyelid and peripheral cornea in adult mice. We found that Smad3 bound two distinct sites on the Spdef promoter and that treatment of keratin 14-positive cells with TGFβ inhibited SPDEF activation, thereby identifying a novel mechanistic role for TGFβ in regulating goblet cell differentiation.
Keywords: Conjunctiva, Differentiation, Goblet cells, SPDEF, TGFβ signaling, Mouse
INTRODUCTION
The ocular surface epithelia, including the stratified but non-keratinized corneal, limbal, and conjunctival epithelium, in concert with the epidermal, keratinized eyelid epithelium, function together to enable eye health and vision (Swamynathan, 2013). The transparent cornea is essential for vision, and increases in cellular stratification and keratinization of the cornea result in vision loss or blindness and are associated with many severe ocular surface diseases (Li et al., 2007). The limbus exists at the transition between corneal and conjunctival epithelium, and is a stem cell niche containing limbal stem cells that migrate centripetally to maintain the cornea (Pellegrini et al., 1999; Swamynathan, 2013). Conjunctival epithelium, which encompasses the palpebral conjunctiva, the fornix and the bulbar conjunctiva, lines the inner surface of the eyelid, is composed of stratified epithelium interspersed with goblet cells, and adjoins the cornea at the limbus (Wei et al., 1993, 1997; Pellegrini et al., 1999). Goblet cells are important secretory cell components of various epithelia, including the lung (Park et al., 2007; Chen et al., 2009; Tompkins et al., 2009), the intestine (Radtke and Clevers, 2005) and the conjunctiva (Wei et al., 1993, 1995, 1997; Mantelli and Argueso, 2008). Within the conjunctiva, goblet cells secrete aqueous mucins that protect the surface of the eye by contributing to the tear film to maintain lubrication, clear molecules and maintain mucosal barrier integrity (Wei et al., 1993; Mantelli and Argueso, 2008). Abnormalities in goblet cell function are associated with dry eye syndrome and other drying diseases (Mantelli and Argueso, 2008; Marko et al., 2013; Zhang et al., 2013), and lead to perturbations of the ocular surface epithelium that negatively affect eye health and vision. Mechanisms that regulate goblet cell differentiation in the conjunctiva are not well understood, thereby limiting therapeutic options for ocular surface disorders, such as dry eye syndrome, largely to analgesic measures, such as artificial tears (Cornec et al., 2014).
Although the precise regulatory networks governing goblet cell differentiation in the conjunctiva are unknown, conjunctival phenotypes in murine models have identified Krüppel-like family 4 (KLF4) (Swamynathan et al., 2007; Gupta et al., 2011) and 5 (KLF5) (Kenchegowda et al., 2011), SAM pointed domain-containing ETS transcription factor (SPDEF) (Marko et al., 2013) and the Notch signaling pathway (Zhang et al., 2013) as essential factors required for goblet cell differentiation in the mouse conjunctiva, as deletion of these genes, or inhibition of Notch signaling, results in conjunctiva that lack goblet cells. SPDEF plays a crucial role in goblet cell differentiation in multiple organs, including the lung (Park et al., 2007), the intestine (Gregorieff et al., 2009; Noah et al., 2010) and the conjunctiva (Marko et al., 2013). Although upstream regulators of SPDEF have been reported in the lung (Ren et al., 2013) and the intestine (Aronson et al., 2014), less is known about its upstream regulators in the conjunctiva.
Transforming growth factor β (TGFβ) has an established role in controlling epithelial cell proliferation and differentiation (Derynck et al., 1998; Feng and Derynck, 2005). In the presence of TGFβ ligand, the transmembrane serine/threonine kinases TGFβ receptor I (TGFβRI) and receptor II (TGFβRII) heterodimerize to phosphorylate Smad2 (Derynck et al., 1998; Massague and Wotton, 2000). Phosphorylated Smad2 and Smad3 form a heteromeric complex in the cytoplasm with Smad4, the common mediator of TGFβ and bone morphogenetic protein (BMP) signaling, which translocates to the nucleus and directly binds DNA to effect transcription of target genes (Derynck et al., 1998; Massague and Wotton, 2000; McNairn et al., 2013a). BMP signaling, via Smad4, has been implicated in eyelid development and differentiation of the mouse conjunctiva at early stages, but loss of components of canonical TGFβ signaling failed to produce a perinatal phenotype using Le-Cre (Huang et al., 2009). Although TGFβ signaling is important for corneal epithelial wound healing (Terai et al., 2011), and loss of Tgfbr2 in CD4+ T cells induces an immune response in the eye (DePaiva et al., 2011), a cell-autonomous function for TGFβ signaling in conjunctival epithelial cell fate or goblet cell differentiation has not been identified.
Here, we report that conditional deletion of Tgfbr2 in keratin 14 (K14)-positive stratified epithelia causes ocular surface epithelial hyperplasia and conjunctival goblet cell expansion that invaginates into the subconjunctival stroma in the mouse eye. We found that the ocular surface epithelium develops properly in the absence of TGFβ signaling, but young asymptomatic mice displayed conjunctival goblet cell expansion, demonstrating that TGFβ signaling is required for restriction of goblet cells differentiation within the conjunctiva. The adult hyperplastic Tgfbr2-deficient palpebral conjunctiva retained its conjunctival identity but displayed increased SPDEF expression, not only in goblet cells but also in stratified conjunctival epithelial cells. Overexpression of SPDEF in K14-positive epithelia resulted in the formation of ectopic goblet cells in the eyelid and peripheral cornea in adult mice. We hypothesized that TGFβ restricted goblet cell differentiation directly by repressing Spdef transcription. We found that Smad3 bound two distinct sites on the Spdef promoter and that treatment of K14-positive cells with TGFβ inhibited SPDEF activation, thereby identifying a novel mechanistic role for TGFβ in the regulation of goblet cell differentiation.
RESULTS
Tgfbr2 conditional deletion in K14-expressing cells results in progressive periorbital tissue expansion with narrowing of the palpebral fissure
Murine ocular surface epithelium is derived from K14-expressing cells (Pajoohesh-Ganji et al., 2012; Zhang et al., 2013). Mice that lack Tgfbr2 in stratified epithelia expressing K14 (cKO mice; K14-Cre×Tgfbr2flox/flox), including skin, oral and anogenital epithelia, are susceptible to squamous cell carcinoma formation (Lu et al., 2006; Guasch et al., 2007). Here, we report that these mice also develop progressive periorbital tissue expansion with narrowing of the palpebral fissure upon aging. To lineage trace Tgfbr2-deficient cells in the eye, we backcrossed K14-Cre×Tgfbr2flox/flox mice with an eYFP reporter strain (R26R-eYFPflox-STOP-flox) (Srinivas et al., 2001) (Fig. 1A), such that K14-positive epithelial cells, including those of the ocular surface epithelium, lacked Tgfbr2 and expressed YFP (McCauley and Guasch, 2013). The external appearance of juvenile Tgfbr2 cKO eyes, between birth and 8 months of age, appeared indistinguishable from the eyes of age-matched wild-type mice; however, by ∼9 months of age, the periocular tissue of Tgfbr2 cKO mice became grossly swollen and enlarged, with excessive mucous discharge and marked narrowing of the palpebral fissure (Table 1 and Fig. 1B). YFP fluorescence was detected in both wild-type (K14-Cre×Tgfbr2+/+×R26R-eYFPflox-STOP-flox) and Tgfbr2 cKO skin and eyelid epithelium, demonstrating efficient targeting by K14-Cre (Fig. 1B). We confirmed expression of YFP in the ocular surface epithelium of adult wild-type mice, and verified the normal cell-surface expression pattern of TGFβRII in the basal layer of eyelid, conjunctival and corneal epithelia (supplementary material Fig. S1A-C). Tgfbr2 cKO ocular surface epithelium also expressed YFP, indicating its derivation from K14-expressing cells, but lacked expression of TGFβRII in eyelid, conjunctival and corneal epithelia (supplementary material Fig. S1D-F). Additionally, the loss of Tgfbr2 was directly demonstrated at the mRNA level in YFP-positive cells isolated from Tgfbr2 cKO eyes (Fig. 1C,D), providing evidence that the loss of Tgfbr2 in the ocular surface epithelium caused ocular pathology in these mice.
Fig. 1.
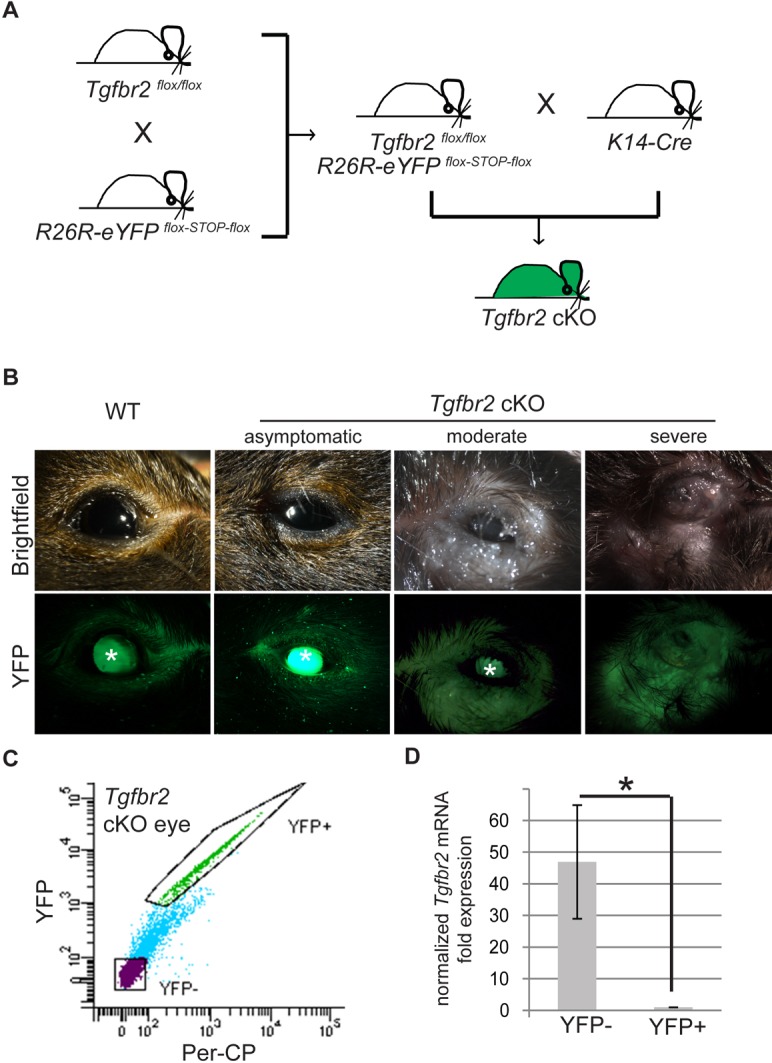
Tgfbr2 conditional deletion in K14-expressing cells results in progressive periorbital tissue expansion with narrowing of the palpebral fissure. (A) Triple transgenic mice were obtained by crossing Tgfbr2flox/flox mice with R26R-eYFPflox-STOP-flox mice and K14-Cre mice. (B) External appearance of K14-Cre×Tgfbr2+/+×R26R-eYFPflox-STOP-flox wild-type and K14-Cre×Tgfbr2flox/flox×R26R-eYFPflox-STOP-flox (Tgfbr2 cKO) eyes showing representative examples of mice with an asymptomatic, a moderate and a severe phenotype. Asterisks indicate that the lens is autofluorescent. (C,D) YFP-positive and YFP-negative cells were isolated by FACS from dissected eyes of cKO mice and subjected to mRNA extraction and qPCR. Fluorescence in the PerCP channel was used to exclude autofluorescence. Data represent the mean±s.d.; Student's t-test, *P=0.007.
Table 1.
Summary of abnormalities observed in Tgfbr2-deficient eyes
Tgfbr2-deficient mice develop extensive abnormalities in eyelid, corneal and conjunctival epithelia
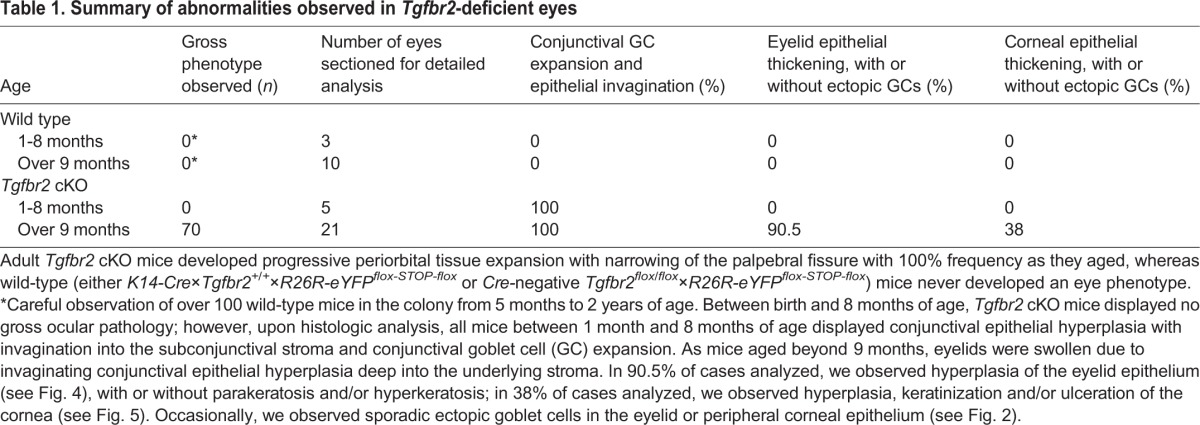
In order to characterize the ocular phenotype, we first assessed the ocular histology of Tgfbr2 cKO mice and age-matched wild-type controls by Hematoxylin and Eosin (supplementary material Fig. S2) and periodic acid-Schiff's (PAS) staining (Fig. 2). The eyelid swelling observed in Tgfbr2 cKO mice was due to marked conjunctival epithelial hyperplasia with epithelial cell nests and epithelial cell-lined cystic spaces invaginating into the underlying stroma (Fig. 2B). Some mice developed a more severe phenotype with additional abnormalities, including thickened, keratinized and/or ulcerated corneal epithelium, thickened eyelid epithelium with parakeratosis and/or hyperkeratosis, and variable occurrence of ectopic goblet cells in the peripheral cornea and squamous eyelid epithelium (Table 1, Fig. 1B, Fig. 2A,B; supplementary material Fig. S2). Given that Tgfbr2 cKO mice are known to be susceptible to squamous cell carcinoma (Lu et al., 2006; Guasch et al., 2007), Tgfbr2 cKO eyes were histologically evaluated for features of malignancy. The invaginating hyperplastic Tgfbr2 cKO conjunctival epithelium lacked dysplastic cytological features and mitotic activity characteristic of squamous cell carcinoma, and the surrounding stroma lacked the desmoplastic response typical of invasive carcinomas. Furthermore, analysis of LM332 (formerly called kalinin or laminin 5), a basement membrane marker that, when discontinuous, is indicative of invasion (Barsky et al., 1983), revealed a continuous and intact basement membrane throughout the invaginated hyperplastic Tgfbr2 cKO conjunctiva (supplementary material Fig. S3). The histopathology of the Tgfbr2 cKO ocular phenotype shared features with inverted mucoepidermoid papillomas and inverted follicular keratosis, which are benign lesions. By 9 months of age, all Tgfbr2 cKO mice analyzed displayed gross ocular pathology (Table 1). There was a marked increase in goblet cell density at the expense of stratified, non-goblet epithelial cells in the conjunctival fornix of symptomatic Tgfbr2 cKO mice (Fig. 2A′,B′). Furthermore, goblet cells in the expanded and invaginated Tgfbr2 cKO conjunctiva expressed Muc5AC (Fig. 2C,D), a mucin normally produced by human and murine conjunctival goblet cells (Mantelli and Argueso, 2008). Taken together, these results suggest that TGFβ signaling may play a previously unidentified role in ocular surface epithelial homeostasis and differentiation.
Fig. 2.
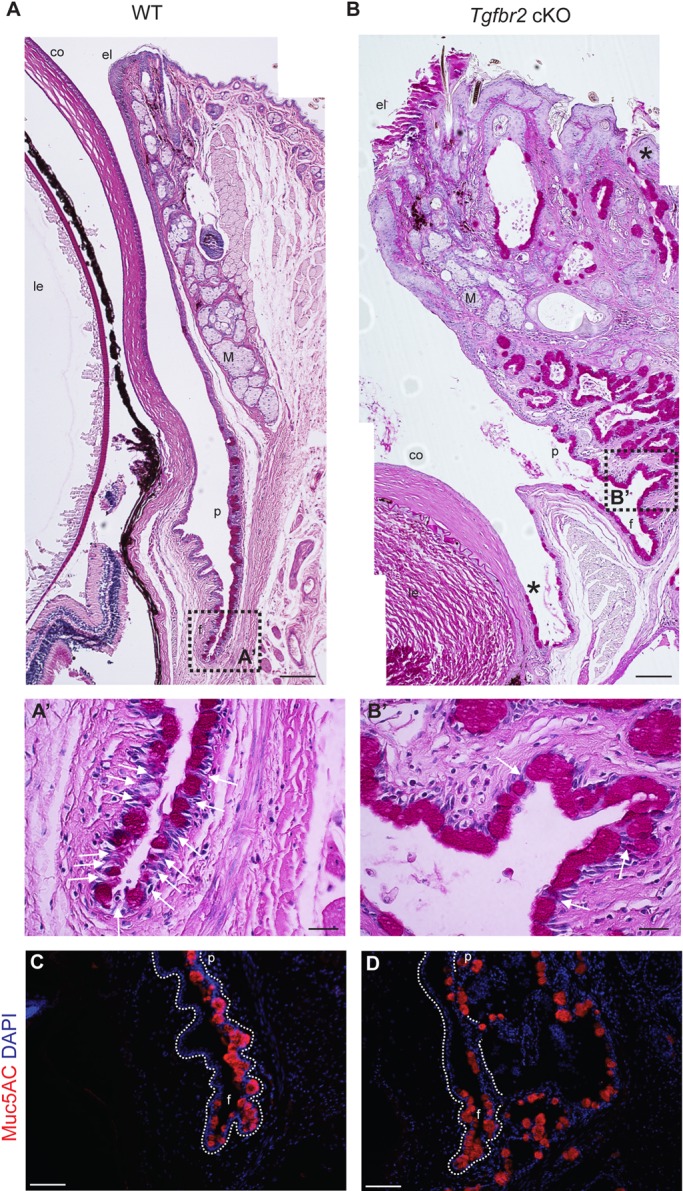
Tgfbr2-deficient mice develop eyelid, corneal and conjunctival epithelial hyperplasia. (A-B′) Combined PAS and Hematoxylin and Eosin staining demonstrated extensive squamous and mucous epithelial hyperplasia with invaginations into the underlying stroma, involving the palpebral conjunctiva, fornix and eyelids of symptomatic Tgfbr2 cKO mice compared with sections of comparable regions from age-matched wild-type mice. Ectopic goblet cells (magenta) were found within Tgfbr2 cKO hyperplastic eyelid epithelium and peripheral corneal epithelium (B, asterisks). Higher magnification of the boxed areas are shown in A′ and B′. White arrows indicate non-goblet cell stratified conjunctival epithelial cells interspersed between goblet cells. (C,D) Goblet cells within the expanded and invaginated Tgfbr2 cKO conjunctiva expressed Muc5AC. Dotted lines indicate the basal layer. DAPI counterstains nuclei in blue. co, cornea; le, lens; f, fornix; p, palpebral conjunctiva; M, Meibomian gland; el, eyelid; f, fornix. Scale bars: 100 µm in A,B,C,D; 20 µm in A′,B′.
Tgfbr2-deficient ocular surface epithelia differentiate properly, but Tgfbr2-deficient conjunctiva begins to invaginate by 6 weeks of age
To determine whether Tgfbr2 cKO eyes developed normally, we examined expression of ocular surface differentiation markers in asymptomatic 6-week-old mice (Fig. 3). We found no difference between wild-type and Tgfbr2 cKO mice in keratin 10 expression in the skin and eyelid epithelium (Fig. 3A,B) or in keratin 12 expression in the corneal epithelium (Fig. 3C,D). Paired box homeotic gene 6 (Pax6), a master regulator of corneal epithelial differentiation (Li et al., 2007), which is essential for maintenance of corneal cell fate (Ouyang et al., 2014), is also normally expressed in Tgfbr2 cKO mice (Fig. 3E,F). Although no difference in keratin 13 expression was observed between wild-type and Tgfbr2 cKO conjunctiva, we found that the Tgfbr2 cKO conjunctiva had keratin 13-positive papillary epithelial hyperplasia in the absence of external symptoms (Fig. 3G,H; supplementary material Fig. S4A,B). Furthermore, we observed an increase in goblet cell density in asymptomatic Tgfbr2 cKO conjunctiva between 6 weeks and 5 months of age, with a paucity of intervening non-goblet cell stratified epithelium, compared with K14-Cre-negative littermate conjunctiva (supplementary material Fig. S4C,D). These data demonstrate that, although the ocular surface epithelium develops normally in the absence of TGFβ signaling, TGFβ is required for normal restriction of goblet cell differentiation in the conjunctiva (supplementary material Fig. S4E).
Fig. 3.
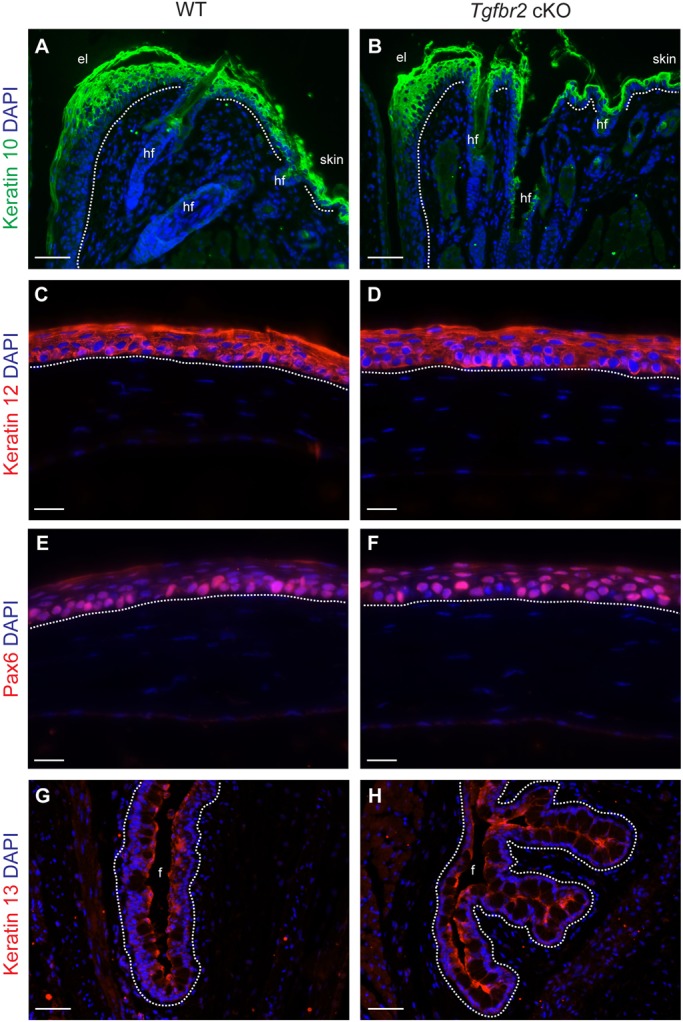
Tgfbr2-deficient ocular surface epithelia differentiate properly. (A-F) Immunofluorescent staining with antibodies against keratin 10 (A,B), keratin 12 (C,D), Pax6 (E,F) and keratin 13 (G,H) on eyes dissected from 6-week-old mice indicated that the eyelid (A,B), cornea (C-F) and conjunctiva (G,H) developed normally in Tgfbr2 cKO mice. In the absence of external symptoms, the Tgfbr2 cKO conjunctiva was noted to invaginate into the subepithelial stroma by 6 weeks of age (H). Dotted lines indicate the basal layer. DAPI counterstains nuclei in blue. el, eyelid; hf, hair follicle; f, fornix. Scale bars: 50 µm in A,B,G,H; 20 µm in C-F.
Adult Tgfbr2-deficient eyelid epithelium is hyperplastic
Although Tgfbr2-deficient eyes differentiated properly at early stages, we observed marked changes in eyelid, corneal and conjunctival epithelium in adult Tgfbr2 cKO mice (Table 1, Fig. 2; supplementary material Fig. S2). Because TGFβ is known to regulate epithelial cellular proliferation and differentiation (McNairn et al., 2013a,b), we questioned whether the K14-positive ocular surface epithelia in adult Tgfbr2 cKO mice was hyperproliferative. The eyelid epithelium of adult Tgfbr2 cKO mice was expanded, containing three to five additional p63-positive basal layers and three to five additional keratin 10-positive suprabasal layers compared with wild-type eyelid epithelium (Fig. 4A-D). Quantification of 5-bromo-2-deoxyuridine (BrdU) staining revealed that Tgfbr2 cKO eyelid epithelium was hyperproliferative, compared with wild type (Fig. 4E-G).
Fig. 4.
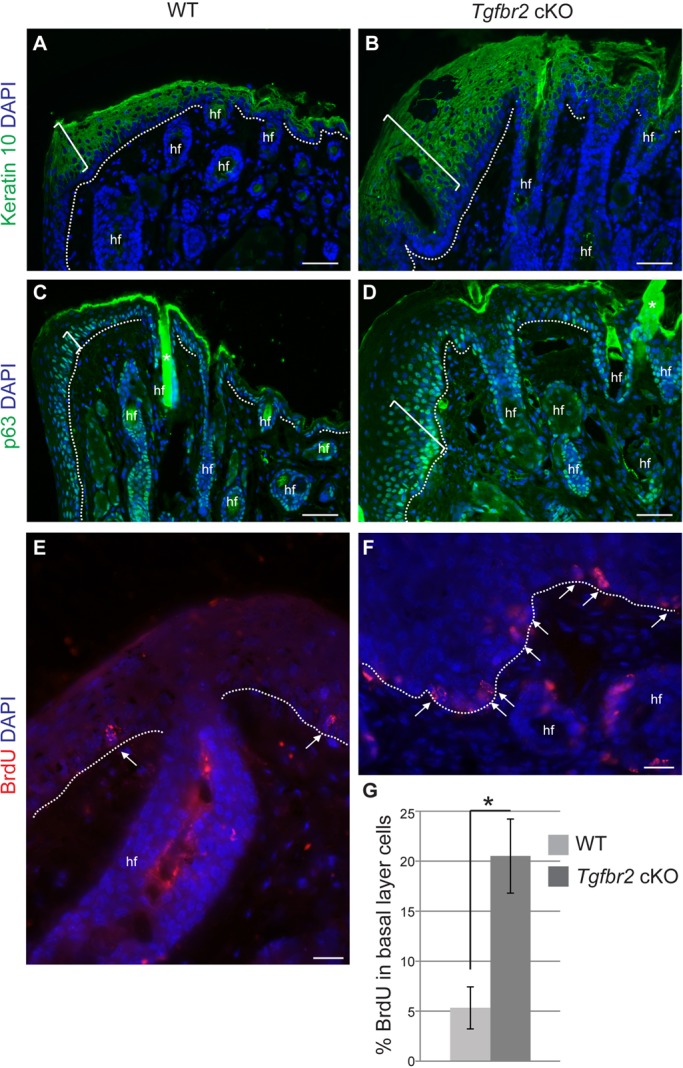
The Tgfbr2-deficient eyelid becomes hyperplastic with age. Immunofluorescence staining with antibodies against the suprabasal marker keratin 10 (A,B), the basal marker p63 (C,D) and BrdU (E,F) indicated that the Tgfbr2 cKO eyelid epithelium (B) was hyperplastic with increased epithelial layers (brackets) compared with wild type. (E-G) Quantification (G) of BrdU immunofluorescence staining (E,F, arrows) indicated that the Tgfbr2 cKO eyelid epithelium was hyperproliferative. Data represent the mean number of BrdU-positive cells in relation to total basal epithelial cells±s.d.; Student's t-test; *P=0.003. hf, hair follicle. Asterisks in C,D indicate autofluorescence originating from the hair shaft. Scale bars: 50 µm in A-D; 20 µm in E,F.
The hyperplastic Tgfbr2-deficient adult cornea becomes keratinized
Of all adult Tgfbr2 cKO mice analyzed, ∼38% displayed severely affected corneal epithelium (Table 1). Adult Tgfbr2 cKO mice without severe corneal pathology displayed a reduction in the number of stratified corneal epithelial layers and a weakening of expression of the corneal epithelial marker keratin 12 (Fig. 5A,B). However, in Tgfbr2 cKO mice with severely affected corneal pathology, keratin 12 expression was completely lost in the corneal epithelium, which was associated with an increase in epithelial stratification (Fig. 5C). Loss of keratin 12 expression occurred progressively, beginning with loss of expression in the central cornea as the number of corneal epithelial layers increased (supplementary material Fig. S5). We were interested to see whether the expression of other corneal markers was lost or affected in Tgfbr2 cKO mice. We observed no change in Pax6 expression between wild-type and Tgfbr2 cKO corneal epithelium (Fig. 5D-F), suggesting that Pax6 functions either upstream or independently of the TGFβ signaling pathway in this context. The murine cornea normally expresses p63 in the basal layer; p63 staining revealed an expansion of the corneal basal layer in severely affected Tgfbr2 cKO mice (Fig. 5G-I). The severely affected Tgfbr2 cKO cornea became keratinized, as the expanded suprabasal layers expressed keratin 10 (Fig. 5L). We did not find any keratin 10 expression in adult wild-type or adult asymptomatic Tgfbr2 cKO corneal epithelium (Fig. 5J,K). Quantification of BrdU staining revealed that severely affected Tgfbr2 cKO corneal epithelia was hyperproliferative compared with wild-type or asymptomatic adult Tgfbr2 cKO (Fig. 5M-P).
Fig. 5.
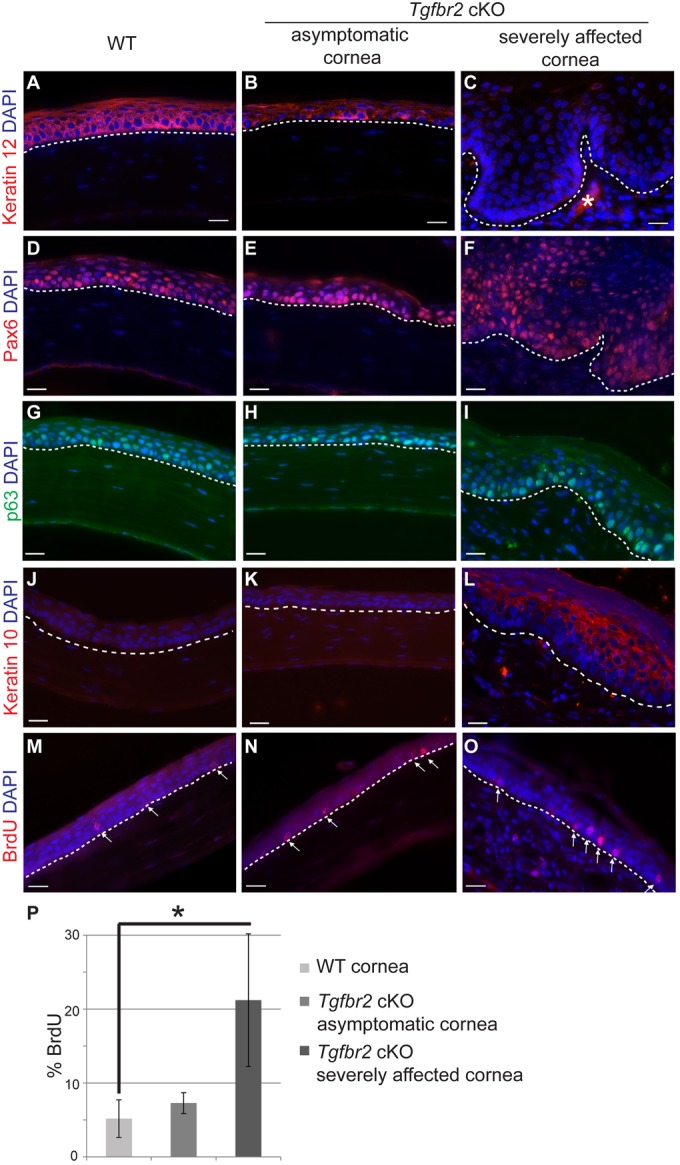
The hyperplastic Tgfbr2-deficient adult cornea becomes keratinized. (A-C) Immunofluorescence staining with antibodies against keratin 12 (A-C), Pax6 (D-F), p63 (G-I), keratin 10 (J-L) and BrdU (M-O) indicated that the corneal epithelium of adult Tgfbr2 cKO mice becomes keratinized with increasing phenotypic severity. (M-P) Quantification (P) of BrdU immunofluorescence staining (M-O, arrows) indicated that the severely affected Tgfbr2 cKO corneal epithelium was hyperproliferative compared with wild-type and asymptomatic Tgfbr2 cKO adult corneal epithelium. Data represent the mean number of BrdU-positive cells in relation to total basal epithelial cells ±s.d.; Student's t-test; *P=0.04. Dotted lines indicate the basal layer. Asterisk in C denotes autofluorescence in the stroma. DAPI counterstains nuclei in blue. Scale bars: 20 µm.
The hyperplastic Tgfbr2-deficient palpebral conjunctiva is hyperproliferative
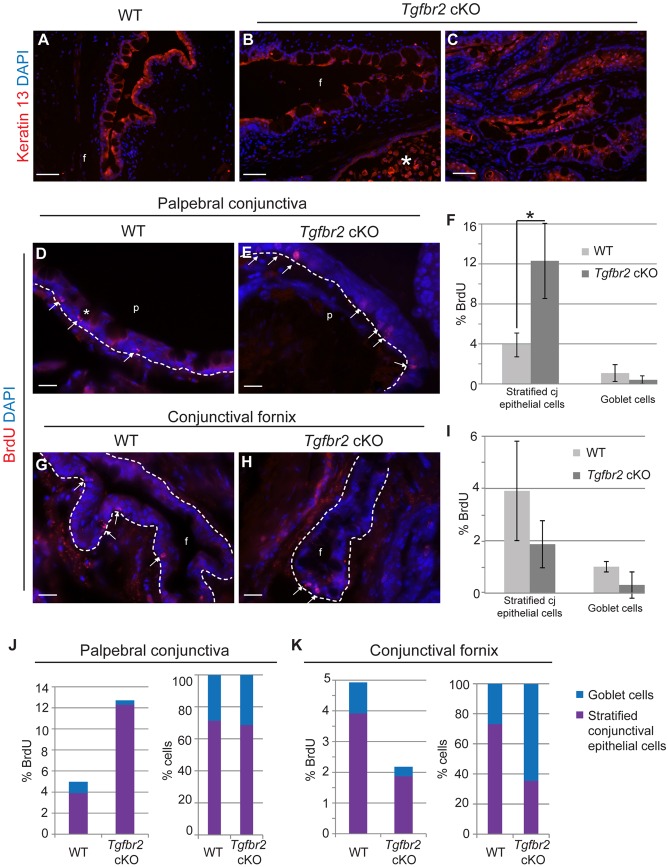
The hyperplastic Tgfbr2 cKO conjunctiva retained keratin 13 expression, a marker of normal conjunctival epithelium (Fig. 6A-C). Quantification of BrdU staining indicated that the Tgfbr2 cKO palpebral conjunctival epithelium was hyperproliferative compared with wild-type palpebral conjunctiva (Fig. 6D-F), but there was no significant difference in proliferation in the conjunctival fornix (Fig. 6G-I) between wild-type and Tgfbr2 cKO mice. Quantification of the ratio of BrdU-positive cells to total basal epithelial cells demonstrated that the stratified conjunctival epithelial cells, but not goblet cells, were hyperproliferative in Tgfbr2 cKO palpebral conjunctiva, whereas the ratio of stratified epithelial cells to goblet cells was unchanged (Fig. 6J). Conversely, quantification of the ratio of BrdU-positive cells to total basal epithelial cells in the conjunctival fornix revealed no significant difference in proliferation between wild-type and Tgfbr2 cKO mice, whereas the ratio of stratified epithelial cells to goblet cells reflected an expansion of the goblet cell compartment (Fig. 6K). The lack of BrdU-positive goblet cells indicated that the increase in goblet cell density observed in the Tgfbr2 cKO conjunctiva was a result of aberrant differentiation rather than goblet cell proliferation.
Fig. 6.
Tgfbr2-deficient stratified conjunctival epithelial cells, but not goblet cells, are hyperproliferative. (A-I) Immunofluorescence staining with antibodies against keratin 13 (A-C) revealed positive keratin 13 expression in the Tgfbr2 cKO conjunctival epithelium (B), including the hyperplastic conjunctival epithelium (C). (D-I) Quantification (F,I) of BrdU immunofluorescence staining (D,E,G,H, arrows) indicated that the Tgfbr2 cKO palpebral conjunctiva, but not the fornix, was hyperproliferative compared with wild type. (J,K) Quantification of the ratio of BrdU-positive cells to total basal epithelial cells demonstrated that the stratified conjunctival epithelial cells, but not goblet cells, were hyperproliferative in Tgfbr2 cKO palpebral conjunctiva, whereas the ratio of stratified epithelial cells to goblet cells remained unchanged (J). Quantification of the ratio of BrdU-positive cells to total basal epithelial cells in the conjunctival fornix demonstrated no significant difference in proliferation between wild-type and Tgfbr2 cKO mice, whereas the ratio of stratified epithelial cells to goblet cells reflected an expansion of the goblet cell compartment (K). Asterisk in B denotes autofluorescence originating from inflammatory cells. Asterisk in D indicates a rare BrdU-positive goblet cell. Data represent the mean number of BrdU-positive cells in relation to total basal epithelial cells±s.d.; Student's t-test; *P=0.02. cj, conjunctival; p, palpebral conjunctiva; f, conjunctival fornix. Scale bars: 50 µm in A-C; 20 µm in D,E,G,H.
The hyperplastic Tgfbr2-deficient stratified palpebral conjunctiva exhibits increased SPDEF expression associated with the goblet cell expansion
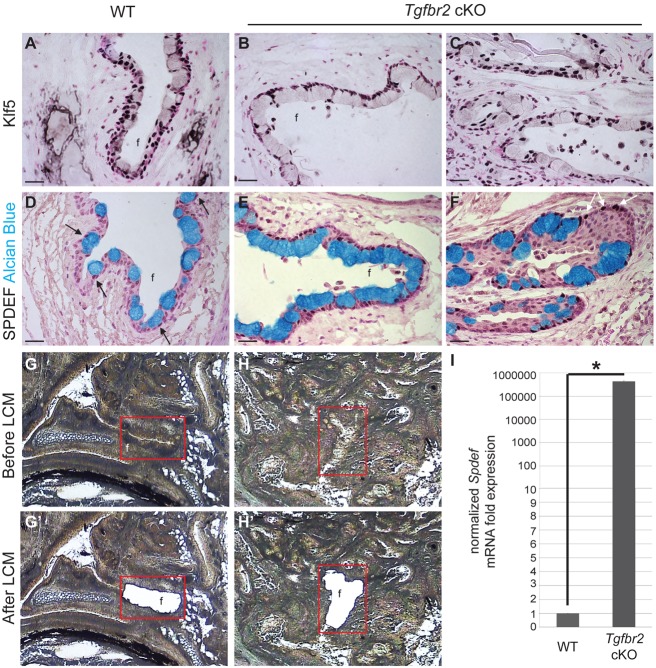
We next sought to identify whether the hyperplastic conjunctival epithelium invaginating into the subconjunctival stroma of Tgfbr2 cKO mice retained normal conjunctival identity. We confirmed that Tgfbr2 cKO conjunctiva, despite the expansion and marked abundance of goblet cells, expressed normal markers of conjunctival epithelium, including keratin 13 (Fig. 6A-C), KLF5 (Fig. 7A-C) and SPDEF (Fig. 7D-F). Interestingly, although KLF5 staining appeared consistent between wild-type and Tgfbr2 cKO conjunctiva, SPDEF staining appeared markedly increased in the nuclei of goblet cells, as well as abundantly expressed in the nuclei of interspersed stratified cells in the hyperplastic Tgfbr2-deficient conjunctival epithelium (Fig. 7D-F). We confirmed that Spdef mRNA expression was significantly increased in Tgfbr2-deficient conjunctival epithelium isolated by laser-capture microdissection (Fig. 7G-I). These data suggest that the increased conjunctival stratified epithelial SPDEF expression may promote goblet cell expansion in Tgfbr2-deficient mice.
Fig. 7.
The hyperplastic Tgfbr2-deficient conjunctiva exhibits increased SPDEF expression associated with the goblet cell expansion. (A-C) Immunohistochemistry on the conjunctival epithelium in wild-type (A) and Tgfbr2 cKO mice (B), and the invaginated conjunctival epithelium within the subepithelial stroma of Tgfbr2 cKO eyes (C) showed similar expression of KLF5. (D-F) Alcian Blue-positive goblet cells within the conjunctiva of wild-type mice expressed SPDEF (D, black arrows). Alcian Blue-positive goblet cells (black arrows), as well as some stratified Alcian Blue-negative epithelial cells (white arrows) within the conjunctiva (E) and the epithelial invaginations (F) of Tgfbr2 cKO mice strongly expressed SPDEF. Nuclear Fast Red stains all nuclei in red. Scale bars: 20 µm. (G-H′) Conjunctival fornix (G,H) from wild-type (G,G′) and Tgfbr2 cKO (H,H′) mice were subjected to laser-capture microdissection and RNA extraction. (I) Tgfbr2 cKO conjunctival epithelium expressed Spdef over 400,000 fold more than in wild type. Data represent the mean±s.d.; Student's t-test, *P=0.00006. f, fornix.
Overexpression of SPDEF in K14-positive epithelium results in ectopic goblet cell formation and upregulation of goblet cell-associated genes
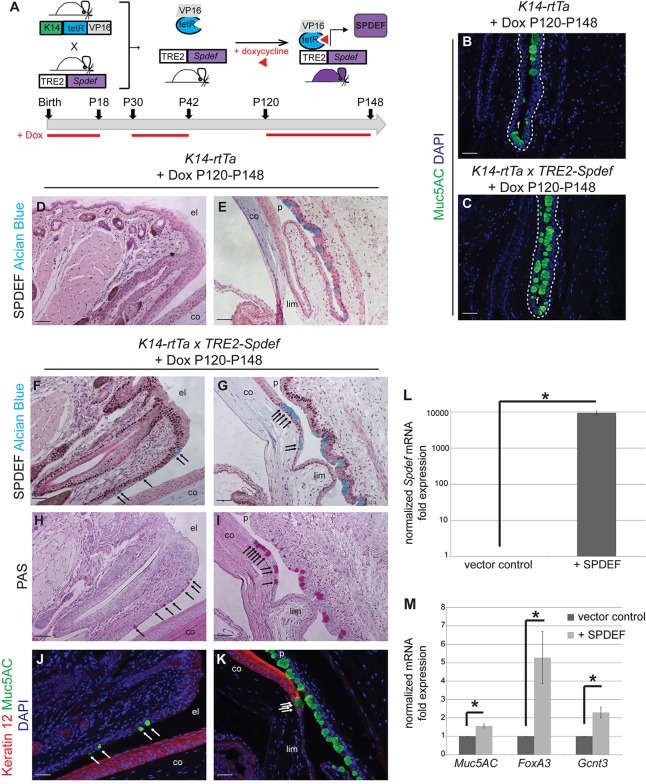
To test whether epithelial expression of SPDEF was sufficient to drive goblet cell differentiation in the ocular surface epithelia, we bred mice to express SPDEF in all K14-positive cells, including those of the ocular surface epithelia, in a doxycycline-inducible manner (K14-rtTA×TRE2-Spdef) (Fig. 8A). When doxycycline was administered continuously from birth, K14-rtTA×TRE2-Spdef mice displayed growth retardation compared with K14-rtTA littermates, and died by 3 weeks of age. Analysis of these mice at postnatal day 18 (P18) revealed abundant nuclear SPDEF expression in skin, eyelid and conjunctival epithelium, and patchy expression in the corneal epithelium in K14-rtTA×TRE2-Spdef mice compared with K14-rtTA alone; however, no additional or ectopic goblet cells were found outside the conjunctiva, and no changes in goblet cell density in the conjunctiva were observed (supplementary material Fig. S6). Doxycycline-induced SPDEF expression in adult mice, from P30 to P42 or from P120 to P148, resulted in an overt phenotype in K14-rtTA×TRE2-Spdef mice, which is characterized by progressive periorbital tissue expansion with narrowing of the palpebral fissure (supplementary material Fig. S7A), reminiscent of the external phenotype observed in Tgfbr2 cKO mice (Fig. 1). We found increased goblet cell density in the palpebral conjunctiva and conjunctival fornix of doxycycline-induced adult K14-rtTA×TRE2-Spdef mice compared with K14-rtTA littermates (Fig. 8B,C), suggesting that the level of SPDEF expression in the conjunctiva may correlate with goblet cell density. We confirmed abundant nuclear expression of SPDEF in K14-positive epithelia of doxycycline-induced adult K14-rtTA×TRE2-Spdef mice, including the skin, eyelid and conjunctiva (Fig. 8D-G; supplementary material Fig. S7B,C), but observed only occasional nuclear SPDEF expression in the cornea (supplementary material Fig. S7E). SPDEF expression was limited to goblet cell nuclei in the conjunctival epithelium in doxycycline-treated K14-rtTA littermates (Fig. 8D,E), replicating the normal pattern observed in wild-type animals, as expected (Fig. 7D). Although no ectopic goblet cells were observed in the skin or cornea of doxycycline-induced adult K14-rtTA×TRE2-Spdef mice (supplementary material Fig. S7B-E), clusters of ectopic goblet cells were found interspersed in the eyelid epithelium and in the peripheral cornea (Fig. 8F-K; supplementary material Fig. S8), indicating that overexpression of SPDEF is sufficient to drive goblet cell differentiation in adult K14-positive ocular epithelia. Lentiviral-mediated overexpression of SPDEF (Chen et al., 2009) in K14-positive cells resulted in modest yet significant upregulation of goblet cell-associated genes, such as Muc5aC, Foxa3 and Gcnt3 (Fig. 8L,M), confirming the in vivo observation that SPDEF is sufficient for goblet cell differentiation in K14-positive stratified epithelia.
Fig. 8.
Overexpression of SPDEF in K14-positive epithelium results in ectopic goblet cell formation in vivo and upregulation of goblet cell-associated genes in vitro. (A) K14-rtTA mice were crossed to TRE2-Spdef mice and fed doxycycline to induce SPDEF expression in K14-positive epithelium at three different timepoints (P0, P30 and P120), and analyzed at P18, P42 and P148, respectively. (B,C) Immunofluorescence staining with an antibody against Muc5AC revealed that adult K14-rtTA×TRE2-Spdef mice (C), which were induced with doxycycline from P120 to P148, exhibited increased goblet cell density in the conjunctival fornix, compared with K14-rtTA control littermates (B). Dotted lines indicate the basal layer. (D-K) Immunohistochemistry with an antibody against SPDEF revealed that adult K14-rtTA control mice expressed SPDEF exclusively in the nuclei of conjunctival goblet cells (D,E). However, adult K14-rtTA×TRE2-Spdef mice, induced at P120, expressed SPDEF abundantly throughout eyelid epithelium and conjunctival epithelium (F,G), and formed ectopic Alcian Blue-positive goblet cells in the eyelid epithelium (arrows, F) and peripheral cornea (arrows, G), which also expressed PAS (H,I, arrows) and Muc5AC (J,K, arrows). (J,K) Co-immunofluorescence staining with an antibody against keratin 12 revealed goblet cells in the keratin 12-positive peripheral corneal epithelium. (L,M) Lentiviral infection of K14-positive cells with the full-length Spdef gene resulted in a 10,000-fold enrichment of Spdef mRNA compared with cells infected with empty vector control (*P=0.00004) (L), and significantly upregulated genes associated with goblet cell differentiation, including Muc5aC (*P=0.00005), Foxa3 (*P=0.006) and Gcnt3 (*P=0.002) (M). Data represent the mean±s.d.; Student's t-test. Scale bars: 50 µm. f, fornix; el, eyelid; co, cornea; p, palpebral conjunctiva; lim, limbus.
TGFβ negatively regulates SPDEF in a Smad3-dependent manner
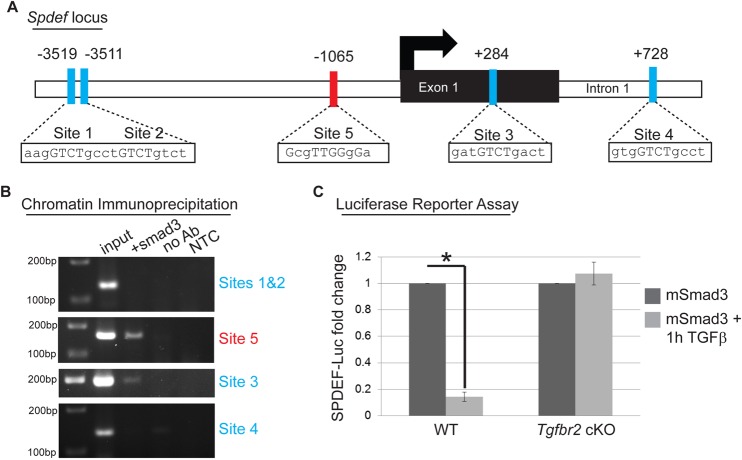
Because loss of function of Tgfbr2 and gain of function of Spdef both resulted in expansion of the goblet cell compartment in the mouse eye, we hypothesized that TGFβ directly repressed Spdef to control goblet cell differentiation. Inspection of the promoter region of the mouse Spdef gene revealed five putative Smad-binding elements (SBE) that are evolutionarily conserved and not found in repeat regions of the genome (Fig. 9A). Four of these sites contained the consensus SBE sequence GTCT (sites 1-4) (Derynck et al., 1998; Massague and Wotton, 2000), whereas one site (site 5) contained the consensus repressive SBE sequence GNNTTGGNGN (Kerr et al., 1990; Frederick et al., 2004). We performed chromatin immunoprecipitation to determine whether Smad3, a canonical effector of TGFβ signaling, bound the potential SBE in the Spdef promoter. Smad3 bound to the Spdef promoter at sites 3 and 5, but not sites 1, 2 or 4 (Fig. 9B), indicating that Spdef may be a previously unidentified target of TGFβ signaling. To test directly whether TGFβ regulated the transcriptional activity of Spdef, we performed luciferase reporter assays. Overexpression of Smad3 in K14-positive cells and treatment with TGFβ resulted in an 85% reduction in luciferase activity driven by 7 kb of the mouse Spdef promoter (Fig. 9C). In parallel, we performed these luciferase promoter assays using K14-positive cells from Tgfbr2 cKO mice and found no change in the transcriptional activity of Spdef after treatment with TGFβ (Fig. 9C), indicating that loss of TGFβRII abolishes regulation of Spdef in response to TGFβ signaling. Taken together, these data indicate that TGFβRII is essential for modulating Spdef expression and goblet cell differentiation in K14-positive cells.
Fig. 9.
TGFβ negatively regulates SPDEF in a Smad3-dependent manner. (A) Promoter analysis using MatInspector (Genomatrix) revealed five putative Smad-binding elements (SBEs) in the Spdef promoter, including four SBEs containing the consensus sequence GTCT (sites 1-4), and one repressive SBE containing the consensus sequence GNNTTGGNGN (site 5). All five sites are evolutionarily conserved and are not within repeat regions. Sites 1 and 2 are located 3511 bp upstream of the transcription start site (TSS, black arrow) of the Spdef gene. Site 3 is located within exon 1 of the Spdef gene and Site 4 is located within the first intron of the Spdef gene. The inhibitory Site 5 is located 1065 bp upstream of the TSS of Spdef. (B) Chromatin immunoprecipitation with an anti-Smad3 antibody was used to isolate DNA fragments that were amplified by primers designed to flank sites 3 and 5 in the Spdef promoter, but not sites 1 and 2 or site 4 after overexpressing Smad3 in NIH3T3 cells and treating with TGFβ1 (2 ng/ml) for 24 h. Non-template (NTC) and no-antibody (no Ab) controls were used to verify the specificity of binding. (C) SPDEF-Luciferase activity was reduced by 85% after overexpressing Smad3 in wild-type keratinocytes and treating with TGFβ1 (2 ng/ml) for 1 h compared with transfected cells without treatment. SPDEF-Luciferase activity was unchanged in Tgfbr2 cKO keratinocytes overexpressing Smad3 and treated with TGFβ1 (2 ng/ml) for 1 h compared with untreated Tgfbr2 cKO keratinocytes. Data represent the mean±s.d.; Student's t-test, *P=0.000002.
DISCUSSION
Loss of TGFβ signaling in the ocular surface epithelium is not sufficient to drive carcinogenesis
Present findings elucidate a new role for TGFβ signaling in the ocular surface epithelium. We found that loss of TGFβ signaling in the conjunctiva results in goblet cell expansion and conjunctival epithelial hyperplasia. TGFβ signaling has been widely implicated in cancer, and can function as both a tumor suppressor and tumor promoter. Loss of either Smad3 (Maggio-Price et al., 2006) or Smad4 (Takaku et al., 1999), the common mediator Smad, has been reported to cause gastric and duodenal mucinous adenocarcinoma, characterized by an abundance of goblet cells. Similarly, mucinous squamous cell carcinoma-like tumors have been reported in the peri-orbital region when αv-integrin, a cell-surface protein that interacts with TGFβ in the extracellular matrix, is conditionally deleted using a neuronal specific GFAP-Cre recombinase (McCarty et al., 2008). Transition zones are unique regions between two distinct types of epithelia (McNairn and Guasch, 2011), which develop spontaneous malignant tumors when TGFβ is compromised. These spontaneous tumors occur at the anorectal (Guasch et al., 2007; Honjo et al., 2007; Yoshinaga et al., 2008), the gastrointestinal (Bleuming et al., 2007) and the gastric transition zones (Nam et al., 2012). Loss of TGFβ signaling is not sufficient on its own to cause spontaneous tumors in non-transition zone epithelia, including the tongue (Bian et al., 2009), the skin (Guasch et al., 2007), the pancreas (Ijichi et al., 2006), the intestine (Takaku et al., 1999) or the colon (Maggio-Price et al., 2006), in which additional insults are required to drive carcinogenesis. Intriguingly, the limbal transition zone is highly susceptible to tumor formation in humans (Lavker et al., 2004), but a link to loss of TGFβ signaling has not been established. The goblet cell expansion and conjunctival epithelial hyperplasia observed in our Tgfbr2 cKO mice does not have the morphological features indicative of a malignant lesion, and maintains an intact basement membrane. Moreover, no invasive tumor was detected in the limbus, supporting the concept that consequences of epithelial TGFβ deficiency differ contextually. Our results indicate that loss of TGFβ signaling in the ocular surface epithelium is sufficient to promote epithelial hyperplasia and goblet cell differentiation but is not sufficient for progression to carcinoma.
A new role for TGFβ in regulating cellular differentiation
This is the first report of TGFβ directly regulating goblet cell differentiation in any system, although TGFβ has an established role in controlling differentiation in other epithelia. In the sebaceous (McNairn et al., 2013b) and salivary (Janebodin et al., 2013) glands, inhibition of TGFβ signaling in vitro results in an increase of differentiated, mature structures. Our findings support this trend, by which the loss of TGFβ signaling in the conjunctival epithelium results in an expansion of differentiated goblet cells. Although it is known that goblet cells and stratified conjunctival epithelial cells share a common progenitor (Wei et al., 1997; Pellegrini et al., 1999), definitive goblet cell precursor cells have not been identified. We found strong nuclear expression of SPDEF in both goblet cells as well as stratified conjunctival epithelial cells in Tgfbr2 cKO mice, but only detected SPDEF in the nuclei of goblet cells in wild-type conjunctiva. Transgenic overexpression of SPDEF in adult K14-positive epithelium resulted in abundant nuclear SPDEF expression in the skin, eyelid and conjunctiva; however, we did not observe ectopic goblet cell formation in the skin, suggesting that the skin is a specialized type of epithelium that uses mechanisms to control cellular differentiation that differ from mechanisms operating in the ocular surface epithelium. SPDEF was only sporadically expressed in the nuclei of the corneal epithelium, indicating that, although the cornea is derived from K14-expressing cells, K14 may not be the most efficient driver for transgenic studies involving the cornea. However, transgenic overexpression of SPDEF was sufficient to drive goblet cell metaplasia in the eyelid epithelium and the peripheral cornea, similar to the mechanism of goblet cell metaplasia described in the lung (Park et al., 2007; Chen et al., 2009) and the intestine (Noah et al., 2010), indicating that SPDEF is sufficient to drive goblet cell differentiation in some K14-positive ocular cell types. Only a subset of SPDEF-expressing K14-positive epithelial cells underwent metaplasia into goblet cells in vivo, suggesting that these epithelia employ additional mechanisms to restrict goblet cell differentiation. Our data demonstrate that TGFβ is one such factor required for restriction of goblet cell differentiation in adult mice, and that it acts upstream of Spdef.
TGFβ is a novel regulator of SPDEF
We found that Smad3 bound two distinct sites on the Spdef promoter and that treatment of K14-positive cells with TGFβ inhibited SPDEF activation, thereby identifying a novel mechanistic role for TGFβ in the regulation of goblet cell differentiation. Although the role of Spdef in goblet cell differentiation is consistent between the lung, the intestine and the conjunctiva, transcriptional control of Spdef remains unclear, especially in the conjunctiva. Conditional deletion of Klf4 (Swamynathan et al., 2007; Gupta et al., 2011) or Klf5 (Kenchegowda et al., 2011), which are structurally related and expressed in the ocular surface epithelium, results in a loss of conjunctival goblet cells and reduced Spdef mRNA expression (Gupta et al., 2011; Marko et al., 2013). Gene expression analysis of Klf4-deficient mice yielded Spdef, but not elements of the TGFβ signaling pathway, as direct targets of KLF4 in the conjunctiva (Gupta et al., 2011). We detected no change in KLF5 expression in Tgfbr2 cKO conjunctiva, suggesting that TGFβ may function independently of KLF4/5 in a novel mechanism of transcriptional regulation of goblet cell differentiation. Because SPDEF is required for goblet cell differentiation in many organs, our findings support the concept that TGFβ may have a more global role in spatial or temporal restriction of goblet cell differentiation.
TGFβ as a therapeutic target for human goblet cell pathologies
SPDEF is known to play a role in goblet cell function in humans. SPDEF is expressed in the nuclei of healthy human conjunctival goblet cells, and samples obtained from individuals with Sjögren's syndrome, an autoimmune disorder that causes dry eye, display decreased Spdef mRNA expression (Marko et al., 2013). Individuals with Sjögren's syndrome also display hyposalivation and exogenous TGFβ1 production in the salivary gland of mice results in a dry mouth phenotype (Hall et al., 2010), supporting the possibility of TGFβ as a therapeutic target for this type of disorder. Treatment for dry eye, including Sjögren's syndrome, currently relies largely on managing symptoms of disease with artificial tears. Our findings present exciting opportunities for development of therapeutic strategies that target TGFβ signaling to treat disorders of goblet cell differentiation, including dry eye syndromes, Sjögren's syndrome and mucinous adenocarcinoma, in humans.
MATERIALS AND METHODS
Mice and genotyping
The conditional knockout Tgfbr2flox/flox×K14-Cre mouse model (Leveen et al., 2002; Guasch et al., 2007) is derived in a pure C57BL/6 background and backcrossed (McCauley and Guasch, 2013) into a mouse reporter containing an enhanced yellow fluorescent protein gene (EYFP) inserted into the Gt(ROSA)26Sor locus (Srinivas et al., 2001) and called R26R-eYFPflox-STOP-flox (Jackson Laboratory). Control mice were either Tgfbr2flox/flox×R26R-eYFPflox-STOP-flox or Tgfbr2+/+×R26R-eYFPflox-STOP-flox×K14-Cre, all in a C57BL/6 background.
Mice expressing SPDEF were in a mixed background and were generated by crossing K14-rtTA mice (Nguyen et al., 2006) (Jackson Laboratory) with TRE2-Spdef mice (Park et al., 2007). Control mice were K14-rtTA alone. To induce transgene expression, adult compound transgenic mice were fed with Dox-chow (doxycycline 1 g/kg chow, Bioserv) ad libitum. Neonates were administered doxycycline at birth (P0) by feeding the nursing mother with Dox-chow ad libitum.
All experiments were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee and were carried out using standard procedures. Genotyping was conducted by PCR of tail skin DNA using mouse Cre, Tgfbr2, EYFP, K14-rtTA and TRE2-Spdef primers, as described previously (Soriano, 1999; Nguyen et al., 2006; Guasch et al., 2007; Park et al., 2007).
Histological analysis
After sacrificing mice by carbon dioxide inhalation, eyes, including the skin, intact eyelids and eyeball, were dissected and fixed in 4% paraformaldehyde for 48 h. The samples were then dehydrated and embedded in paraffin or cryopreserved in 30% sucrose and embedded in OCT compound (Tissue-Tek), and stored at –80°C as previously described (McCauley and Guasch, 2013). Deparaffinized sections (5 µm) were stained with Hematoxylin and Eosin (Ventana Medical Systems), periodic acid-Schiff (PAS; Sigma-Aldrich) or Alcian Blue (Poly Scientific) according to the manufacturer's instructions.
Immunostainings and antibodies
Deparaffinized tissue sections (5 µm) were subjected to antigen retrieval and immunostaining as previously described (Tompkins et al., 2009). Frozen tissue sections (10 µm) were subjected to immunofluorescence labeling as previously described (Runck et al., 2010). Antibodies used and image acquisition are described in the methods in the supplementary material.
Detection of cellular proliferation
Mice were injected intraperitoneally with 10 mg/ml of 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich) 2 h before sacrifice, and eyes were harvested, sectioned and stained as described in the methods in the supplementary material, with additional treatment of slides with 1 N HCl for 40 min at 37°C prior to incubation with antibody against BrdU (Abcam, ab6326, 1/100). The percentages of BrdU-positive cells were determined by counting the total number of nuclei in the basal layer and the number of BrdU-positive basal epithelial cells using a 40× objective in z-stack combined images. Data represent quantification of eyes from three wild-type and six Tgfbr2 cKO mice.
Fluorescence-activated cell sorting (FACS)
Eyes, including the surrounding skin, intact eyelids and eyeball, were removed from Tgfbr2flox/flox×R26R-eYFPflox-STOP-flox×K14-Cre mice and dissociated into a single cell suspension as previously described (McCauley and Guasch, 2013). Dead cells were excluded using 7-amino-actinomycin D (7-AAD; eBioscience) incorporation and the remaining live YFP-positive and YFP-negative cells were collected directly into cell lysis buffer containing β-mercaptoethanol using a FACS Aria II (BD) and FACSDiva software (BD). Sorted cells were vortexed and stored at –80°C until RNA extraction.
Laser-capture microdissection
Eyes were dissected from adult wild-type and Tgfbr2 cKO mice and processed for laser capture microdissection as previously described (Potter and Brunskill, 2012). Once samples were obtained, RNA was isolated using a ZR RNA MicroPrep kit (Zymo Research) and amplified using the Ovation RNA-Seq System V2 (Nugen) according to the manufacturer's directions before being subjected to qPCR as described below.
Real-time PCR
Total RNA was isolated using a Qiagen RNeasy Mini Kit and used to produce cDNA (Maxima first strand cDNA synthesis kit, Fermentas). Real-time PCR was performed using the CFX96 real-time PCR System, CFX Manager Software and SsoFast EvaGreen Supermix reagents (Bio-Rad) or StepOnePlus real-time PCR system and TaqMan reagents (Applied Biosystems). All reactions were run three times in triplicate and analyzed using the ΔΔCT method with relative expression normalized to Gapdh or 18S. Primers are described in the methods in the supplementary material.
Isolation of primary keratinocytes and cell culture
Wild-type and Tgfbr2 cKO keratinocytes were isolated from newborn C57BL/6 mice at P1. Detailed protocol is described in the methods in the supplementary material. Loss of Tgfbr2 has been verified by qPCR and loss of TGFβ responsiveness confirmed as previously described (Guasch et al., 2007).
Chromatin immunoprecipitation
NIH3T3 cells were seeded in 10 cm plates at 80% confluence and transiently transfected with a pCMV-driven mouse Smad3 (Sino Biological) using X-treme Gene transfection reagent (Roche Applied Science) for 24 h, then treated with recombinant human TGFβ1 (R&D Systems, 2 ng/ml) for an additional 24 h. Cells were crosslinked with 1% formaldehyde and subjected to ChIP using an antibody against Smad3 (Abcam, ab28379) using a ChIP assay kit (Millipore) according to the manufacturer's instructions. After purification, DNA obtained from the ChIP assay was used as PCR templates to verify the interaction between DNA and protein, using primers designed to amplify distinct sites in the mouse Spdef promoter. Primers are described in the methods in the supplementary material. PCR products were then subjected to gel electrophoresis on a 3% agarose gel using a molecular weight marker to verify the size of migrating bands.
Cloning and luciferase reporter assay
Using SalI and EcoRI restriction enzymes, a 7634 bp fragment of the mouse Spdef promoter, containing ∼3.7 kb upstream of the transcriptional start site, the first exon (399 bp) and the first intron (∼3.5 kb), was isolated from the WI1 - 1250E21 Fosmid containing the mouse Spdef gene (SpectraGenetics). The fragment was gel purified and ends were blunted with Klenow fragment followed by alkaline phosphatase to prevent re-ligation. In parallel, the pGL3 Basic Luciferase Vector (Promega) was cut with the blunt-end cutting SmaI restriction enzyme, followed by alkaline phosphatase to prevent re-ligation. The Quick Ligase kit (NEB) was used to combine the opened pGL3 Basic Vector with the 7634 bp Spdef fragment, and the resulting ligation was transformed into DH5α competent cells and selected on LB-ampicillin plates overnight. Colonies were subjected to enzymatic digestion followed by sequencing to confirm the integration.
Primary wild-type and Tgfbr2 cKO keratinocytes were seeded in six-well plates at 80% confluence and were transiently transfected with the pGL3 basic luciferase vector containing 7634 bp of the mouse Spdef promoter and pCMV-mSmad3 (Sino Biological) using X-treme gene transfection reagent (Roche Applied Science). The pcDNA3.1 empty vector and pCMV-β-galactosidase vector (Addgene) were used to normalize total DNA and transfection efficiency, respectively. A Foxa3 expression vector (Chen et al., 2009, 2014) was used as a positive control. Forty-eight hours after transfection, cells were treated with recombinant human TGFβ1 (R&D Systems, 2 ng/ml) for 1 h at 37°C before measuring luciferase activity using a Luciferase Assay System kit (Promega). Experiments were performed three times in triplicate and statistical significance was determined using a paired two-tailed Student's t-test.
The SPDEF-expressing lentivirus has been previously described (Chen et al., 2009). Seventy-two hours after infection, cells were trypsinized and GFP+ cells were isolated by FACS and processed for RNA extraction and qPCR.
Supplementary Material
Acknowledgements
We thank Dr Abbot Spaulding and Dr Keith Stringer for initial pathological interpretations; Angie Keiser and Joe Kitzmiller for assisting with the KLF5 and SPDEF immunohistochemistry; Andrew Potter for assisting with laser-capture microdissection; and Dr Anil Jegga for expertise in analyzing the Spdef promoter.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
H.A.M., C.-Y.L. and G.G. conceived and designed the experiments; H.A.M., C.-Y.L., A.C.A., Y.Z. and G.G. performed the experiments; H.A.M., C.-Y.L., K.A.W.-B., J.A.W. and G.G. analyzed the data; and H.A.M. and G.G. wrote the paper.
Funding
All flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children's Hospital Medical Center, supported in part by the National Institutes of Health (NIH) [AR-47363, DK78392 and DK90971]. This work was supported by grants from the V Foundation and the Sidney Kimmel Foundation (G.G.), and the NIH [EY21501 to C.-Y.L. and Heart, Lung and Blood Institute HL095580 to J.A.W.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.117804/-/DC1
References
- Aronson B. E., Stapleton K. A., Vissers L. A. T. M., Stokhuijzen E., Bruijnzeel H. and Krasinski S. D. (2014). Spdef deletion rescues the crypt cell proliferation defect in conditional Gata6 null mouse small intestine. BMC Mol. Biol. 15, 3 10.1186/1471-2199-15-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky S., Siegal G., Jannotta F. and Liotta L. (1983). Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab. Invest. 49, 140-147. [PubMed] [Google Scholar]
- Bian Y., Terse A., Du J., Hall B., Molinolo A., Zhang P., Chen W., Flanders K. C., Gutkind J. S., Wakefield L. M. et al. (2009). Progressive tumor formation in mice with conditional deletion of TGF-β signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 69, 5918-5926 10.1158/0008-5472.CAN-08-4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuming S. A., He X. C., Kodach L. L., Hardwick J. C., Koopman F. A., ten Kate F. J., van Deventer S. J. H., Hommes D. W., Peppelenbosch M. P., Offerhaus G. J. et al. (2007). Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 67, 8149-8155 10.1158/0008-5472.CAN-06-4659 [DOI] [PubMed] [Google Scholar]
- Chen G., Korfhagen T. R., Xu Y., Kitzmiller J., Wert S. E., Maeda Y., Gregorieff A., Clevers H. and Whitsett J. A. (2009). SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Korfhagen T. R., Karp C. L., Impey S., Xu Y., Randell S. H., Kitzmiller J., Maeda Y., Haitchi H. M., Sridharan A. et al. (2014). Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am. J. Respir. Crit. Care Med. 189, 301-313 10.1164/rccm.201306-1181OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornec D., Jamin C. and Pers J.-O. (2014). Sjogren's syndrome: where do we stand, and where shall we go? J. Autoimmun. 51, 109-114 10.1016/j.jaut.2014.02.006 [DOI] [PubMed] [Google Scholar]
- DePaiva C. S., Volpe E. A., Gandhi N. B., Zhang X., Zheng X., Pitcher J. D. III, Farley W. J., Stern M. E., Niederkorn J. Y., Li D.-Q. et al. (2011). Disruption of TGF-β signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PLoS ONE 6, 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Zhang Y. and Feng X.-H. (1998). Transcriptional activators of TGF-β responses: Smads. Cell 95, 737-740 10.1016/S0092-8674(00)81696-7 [DOI] [PubMed] [Google Scholar]
- Feng X.-H. and Derynck R. (2005). Specificity and versatility in TGF-β signaling through smads. Annu. Rev. Cell Dev. Biol. 21, 659-693 10.1146/annurev.cellbio.21.022404.142018 [DOI] [PubMed] [Google Scholar]
- Frederick J. P., Liberati N. T., Waddell D. S., Shi Y. and Wang X.-F. (2004). Transforming growth factor β-mediated transcriptional repression of c-myc is dependent on direct binding of smad3 to a novel repressive smad binding element. Mol. Cell. Biol. 24, 2546-2559 10.1128/MCB.24.6.2546-2559.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A., Stange D. E., Kujala P., Begthel H., van den Born M., Korving J., Peters P. J. and Clevers H. (2009). The Ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology 137, 1333-1345.e3 10.1053/j.gastro.2009.06.044 [DOI] [PubMed] [Google Scholar]
- Guasch G., Schober M., Pasolli H. A., Conn E. B., Polak L. and Fuchs E. (2007). Loss of TGFβ signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313-327 10.1016/j.ccr.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D., Harvey S. A. K., Kaminski N. and Swamynathan S. K. (2011). Mouse conjunctival forniceal gene expression during postnatal development and its regulation by Kruppel-like factor 4. Invest. Opthalmol. Vis. Sci. 52, 4951-4962 10.1167/iovs.10-7068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. E., Zheng C., Swaim W. D., Cho A., Nagineni C. N., Eckhaus M. A., Flanders K. C., Ambudkar I. S., Baum B. J. and Kulkarni A. B. (2010). Conditional overexpression of TGF-β1 disrupts mouse salivary gland development and function. Lab. Invest. 90, 543-555 10.1038/labinvest.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo Y., Bian Y., Kawakami K., Molinolo A., Longenecker G., Boppana R., Larsson J., Karlsson S., Gutkind J. S., Puri R. K. et al. (2007). TGF-β receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle 6, 1360-1366 10.4161/cc.6.11.4268 [DOI] [PubMed] [Google Scholar]
- Huang J., Dattilo L. K., Rajagopal R., Liu Y., Kaartinen V., Mishina Y., Deng C.-X., Umans L., Zwijsen A., Roberts A. B. et al. (2009). FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development 136, 1741-1750 10.1242/dev.034082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi H., Chytil A., Gorska A. E., Aakre M. E., Fujitani Y., Fujitani S., Wright C. V. E. and Moses H. L. (2006). Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-β signaling in cooperation with active Kras expression. Genes Dev. 20, 3147-3160 10.1101/gad.1475506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janebodin K., Buranaphatthana W., Ieronimakis N., Hays A. L. and Reyes M. (2013). An in vitro culture system for long-term expansion of epithelial and mesenchymal salivary gland cells: role of TGF-β1 in salivary gland epithelial and mesenchymal differentiation. BioMed Res. Int. 2013, 1-20 10.1155/2013/815895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchegowda D., Swamynathan S., Gupta D., Wan H., Whitsett J. and Swamynathan S. K. (2011). Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands, abnormal cornea and loss of conjunctival goblet cells. Dev. Biol. 356, 5-18 10.1016/j.ydbio.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. D., Miller D. B. and Matrisian L. M. (1990). TGF-β1 inhibition of transin/stromelysin gene expression is mediated through a Fos binding sequence. Cell 61, 267-278 10.1016/0092-8674(90)90807-Q [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Tseng S. C. G. and Sun T.-T. (2004). Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp. Eye Res. 78, 433-446 10.1016/j.exer.2003.09.008 [DOI] [PubMed] [Google Scholar]
- Leveen P., Larsson J., Ehinger M., Cilio C. M., Sundler M., Sjostrand L. J., Holmdahl R. and Karlsson S. (2002). Induced disruption of the transforming growth factor b type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood 100, 560-568 10.1182/blood.V100.2.560 [DOI] [PubMed] [Google Scholar]
- Li W., Chen Y.-T., Hayashida Y., Blanco G., Kheirkah A., He H., Chen S.-Y., Liu C.-Y. and Tseng S. C. G. (2007). Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J. Pathol. 214, 114-122 10.1002/path.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.-L., Herrington H., Reh D., Weber S., Bornstein S., Wang D., Li A. G., Tang C.-F., Siddiqui Y., Nord J. et al. (2006). Loss of transforming growth factor-β type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 20, 1331-1342 10.1101/gad.1413306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio-Price L., Treuting P., Zeng W., Tsang M., Bielefeldt-Ohmann H. and Iritani B. M. (2006). Helicobacter infection is required for inflammation and colon cancer in Smad3-deficient mice. Cancer Res. 66, 828-838 10.1158/0008-5472.CAN-05-2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F. and Argüeso P. (2008). Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 8, 477-483 10.1097/ACI.0b013e32830e6b04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko C. K., Menon B. B., Chen G., Whitsett J. A., Clevers H. and Gipson I. K. (2013). Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am. J. Pathol. 183, 35-48 10.1016/j.ajpath.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. and Wotton D. (2000). Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 19, 1745-1754 10.1093/emboj/19.8.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H., Barry M., Crowley D., Bronson R. T., Lacy-Hulbery A. and Hynes R. O. (2008). Genetic ablation of αv integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. Am. J. Pathol. 172, 1740-1747 10.2353/ajpath.2008.070700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley H. A. and Guasch G. (2013). Serial orthotopic transplantation of epithelial tumors in single-cell suspension. Methods Mol. Biol. 1035, 231-245 10.1007/978-1-62703-508-8_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNairn A. J. and Guasch G. (2011). Epithelial transition zones: merging microenvironments, niches, and cellular transformation. Eur. J. Dermatol. 21, 21-28. [DOI] [PubMed] [Google Scholar]
- McNairn A. J., Brusadelli M. and Guasch G. (2013a). Signaling moderation: TGF-β in exocrine gland development, maintenance, and regulation. Eur. J. Dermatol. 23, 31-38. [DOI] [PubMed] [Google Scholar]
- McNairn A. J., Doucet Y., Demaude J., Brusadelli M., Gordon C. B., Uribe-Rivera A., Lambert P. F., Bouez C., Breton L. and Guasch G. (2013b). TGFβ signaling regulates lipogenesis in human sebaceous glands. BMC Dermatol. 13, 2 10.1186/1471-5945-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam K. T., O'Neal R., Lee Y. S., Lee Y. C., Coffey R. J. and Goldenring J. R. (2012). Gastric tumor development in Smad3-deficient mice initiates from forestomach/glandular transition zone along the lesser curvature. Lab. Invest. 92, 883-895 10.1038/labinvest.2012.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H., Rendl M. and Fuchs E. (2006). Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127, 171-183 10.1016/j.cell.2006.07.036 [DOI] [PubMed] [Google Scholar]
- Noah T. K., Kazanjian A., Whitsett J. and Shroyer N. F. (2010). SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp. Cell Res. 316, 452-465 10.1016/j.yexcr.2009.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H., Xue Y., Lin Y., Zhang X., Xi L., Patel S., Cai H., Luo J., Zhang M., Zhang M. et al. (2014). WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 511, 358-361 10.1038/nature13465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A., Pal-Ghosh S., Tadvalkar G. and Stepp M. A. (2012). Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells 30, 2032-2043 10.1002/stem.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.-S., Korfhagen T. R., Bruno M. D., Kitzmiller J. A., Wan H., Wert S. E., Hershey G. K. K., Chen G. and Whitsett J. A. (2007). SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978-988 10.1172/JCI29176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G., Golisano O., Paterna P., Lambiase A., Bonini S., Rama P. and De Luca M. (1999). Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 145, 769-782 10.1083/jcb.145.4.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S. and Brunskill E. W. (2012). Laser capture. Methods Mol. Biol. 886, 211-221 10.1007/978-1-61779-851-1_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F. and Clevers H. (2005). Self-renewal and cancer of the gut: two sides of a coin. Science 307, 1904-1909 10.1126/science.1104815 [DOI] [PubMed] [Google Scholar]
- Ren X., Shah T. A., Ustiyan V., Zhang Y., Shinn J., Chen G., Whitsett J. A., Kalin T. V. and Kalinichenko V. V. (2013). FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol. Cell. Biol. 33, 371-386 10.1128/MCB.00934-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runck L. A., Kramer M., Ciraolo G., Lewis A. G. and Guasch G. (2010). Identification of epithelial label-retaining cells at the transition between the anal canal and rectum in mice. Cell Cycle 9, 3039-3045 10.4161/cc.9.15.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.-S., William C. M., Tanabe Y., Jessell T. M. and Constantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 10.1186/1471-213X-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan S. K. (2013). Ocular surface development and gene expression. J. Ophthalmol. 2013, 1-22 10.1155/2013/103947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamynathan S. K., Katz J. P., Kaestner K. H., Ashery-Padan R., Crawford M. A. and Piatigorsky J. (2007). Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells. Mol. Cell. Biol. 27, 182-194 10.1128/MCB.00846-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku K., Miyoshi H., Matsunaga A., Oshima M., Sasaki N. and Taketo M. M. (1999). Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res. 59, 6113-6117. [PubMed] [Google Scholar]
- Terai K., Call M. K., Liu H., Saika S., Liu C.-Y., Hayashi Y., Chikama T.-I., Zhang J., Terai N., Kao C. W.-C. et al. (2011). Crosstalk between TGF-β and MAPK signaling during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 52, 8208-8215 10.1167/iovs.11-8017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins D. H., Besnard V., Lange A. W., Wert S. E., Keiser A. R., Smith A. N., Lang R. and Whitsett J. A. (2009). Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS ONE 4, e8248 10.1371/journal.pone.0008248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z.-G., Wu R.-L., Lavker R. M. and Sun T.-T. (1993). In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Invest. Ophthalmol. Vis. Sci. 34, 1814-1828. [PubMed] [Google Scholar]
- Wei Z.-G., Cotsarelis G., Sun T.-T. and Lavker R. M. (1995). Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest. Ophthalmol. Vis. Sci. 36, 236-246. [PubMed] [Google Scholar]
- Wei Z.-G., Lin T., Sun T.-T. and Lavker R. M. (1997). Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest. Ophthalmol. Vis. Sci. 38, 753-761. [PubMed] [Google Scholar]
- Yoshinaga K., Obata H., Jurukovski V., Mazzieri R., Chen Y., Zilberberg L., Huso D., Melamed J., Prijatelj P., Todorovic V. et al. (2008). Perturbation of transforming growth factor (TGF)-β1 association with latent TGF-β bindisng protein yields inflammation and tumors. Proc. Natl. Acad. Sci. USA 105, 18758-18763 10.1073/pnas.0805411105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lam O., Nguyen M.-T. T., Ng G., Pear W. S., Ai W., Wang I.-J., Kao W. W.-Y. and Liu C.-Y. (2013). Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development 140, 594-605 10.1242/dev.082842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.