Abstract
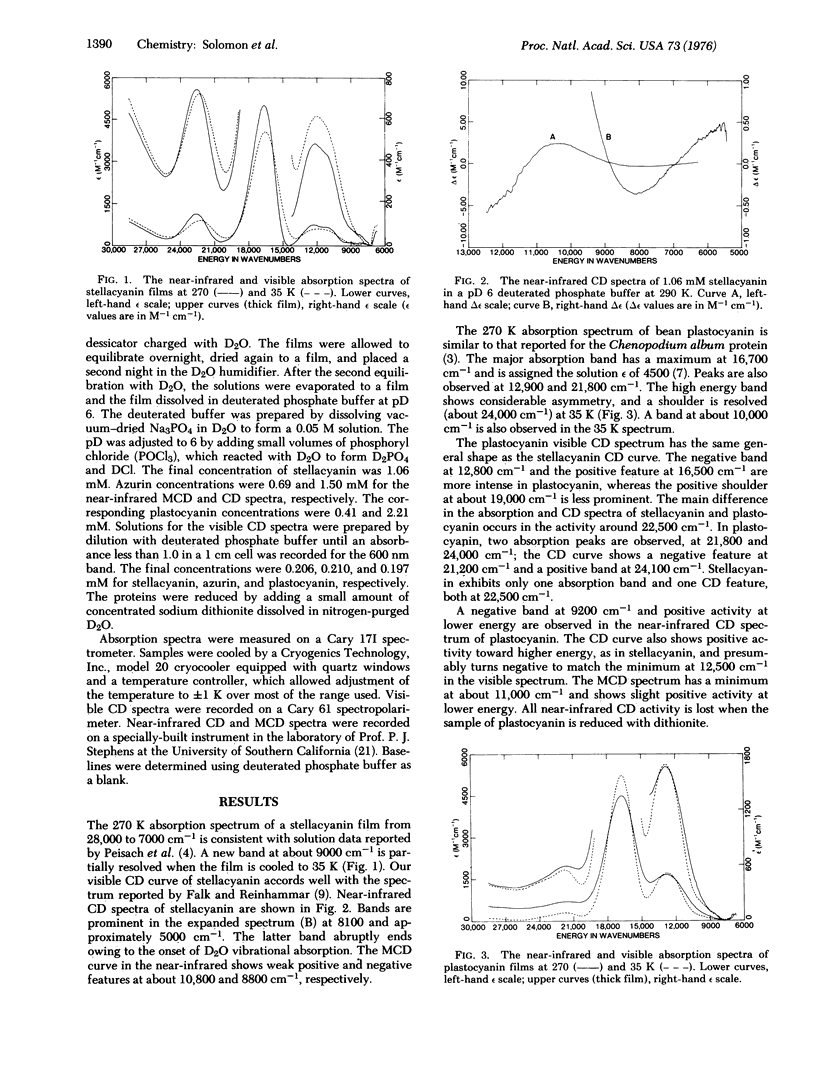
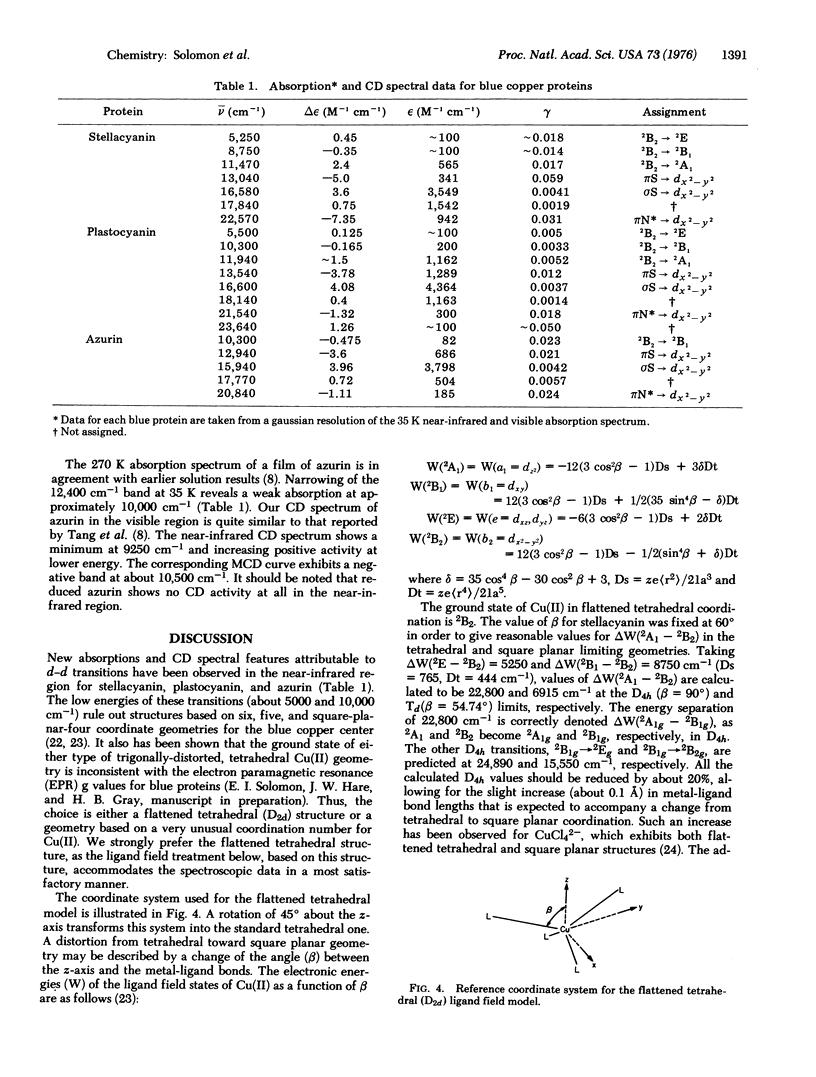
Low temperature absorption, circular dichroism, and magnetic circular dichroism spectral studies of the blue copper proteins Rhus vernicifera stellacyanin, bean plastocyanin, and Pseudomonas aeruginosa azurin have been made. Low energy bands attributable to the d-d transitions 2B2 leads to 2E and 2B2 leads to 2B1 in a flattened tetrahedral (D 2d) copper-(II) center are observed in these proteins at about 5000 and 10,000 cm-1, respectively. The band positions accord well with ligand field calculations based on a tetrahedral structure that is distorted approximately 6 degrees toward a square plane. The ligands in this flattened tetrahedral coordination unit in bean plastocyanin are identified from various spectroscopic experiments as His-38, Cys-85, His-88, and a deprotonated peptide nitrogen (N) a few residues above His-38.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg W. E., Peisach J. The optical and magnetic properties of copper in Chenopodium album plastocyanin. Biochim Biophys Acta. 1966 Oct 10;126(2):269–273. doi: 10.1016/0926-6585(66)90063-x. [DOI] [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F. Cupric ion in blue proteins. J Chem Phys. 1968 May 15;48(10):4398–4404. doi: 10.1063/1.1668007. [DOI] [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F., Maria H. J. Optical and magnetic properties of Pseudomonas azurins. Biochim Biophys Acta. 1968 Feb 19;154(2):342–351. doi: 10.1016/0005-2795(68)90048-2. [DOI] [PubMed] [Google Scholar]
- Bryce G. F., Gurd F. R. Visible spectra and optical rotatory properties of cupric ion complexes of L-histidine-containing peptides. J Biol Chem. 1966 Jan 10;241(1):122–129. [PubMed] [Google Scholar]
- Falk K. E., Reinhammar B. Visible and near-infrared circular dichroism of some blue copper proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):84–90. doi: 10.1016/0005-2795(72)90182-1. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ulrich E. L., Berg S. P., Krogmann D. W. Nuclear magnetic resonance studies of the copper binding sites of blue copper proteins: oxidized, reduced, and apoplastocyanin. Biochemistry. 1975 Oct 7;14(20):4428–4433. doi: 10.1021/bi00691a014. [DOI] [PubMed] [Google Scholar]
- McMillin D. R., Rosenberg R. C., Gray H. B. Preparation and spectroscopic studies of cobalt(II) derivatives of blue copper proteins. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4760–4762. doi: 10.1073/pnas.71.12.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne P. R., Wells J. R., Ambler R. P. The amino acid sequence of plastocyanin from French bean (Phaseolus vulgaris). Biochem J. 1974 Dec;143(3):691–701. doi: 10.1042/bj1430691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne P. R., Wells J. R. Structural and molecular weight studies on the small copper protein, plastocyanin. J Biol Chem. 1970 Apr 10;245(7):1566–1574. [PubMed] [Google Scholar]
- Miskowski V., Tang S. P., Spiro T. G., Shapiro E., Moss T. H. The copper coordination group in "blue" copper proteins: evidence from resonance Raman spectra. Biochemistry. 1975 Mar 25;14(6):1244–1250. doi: 10.1021/bi00677a024. [DOI] [PubMed] [Google Scholar]
- Peisach J., Levine W. G., Blumberg W. E. Structural properties of stellacyanin, a copper mucoprotein from Rhus vernicifera, the Japanese lac tree. J Biol Chem. 1967 Jun 25;242(12):2847–2858. [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Siiman O., Young N. M., Carey P. R. Resonance raman spectra of "blue" copper proteins and the nature of their copper sites. J Am Chem Soc. 1976 Feb 4;98(3):744–748. doi: 10.1021/ja00419a017. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Clendening P. J., Gray H. B., Grunthaner F. J. Letter: Direct observation of sulfur coordination in bean plastocyanin by X-ray photoelectron spectroscopy. J Am Chem Soc. 1975 Jun 25;97(13):3878–3879. doi: 10.1021/ja00846a087. [DOI] [PubMed] [Google Scholar]
- Stigbrand T., Sjöholm I. Circular dichroism studies on the copper protein umecyanin. Biochim Biophys Acta. 1972 Apr 15;263(2):244–257. doi: 10.1016/0005-2795(72)90077-3. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Hirayama Y., Tanaka H., Ishizu K. Copper(II) complex of sulfur-containing peptides. Characterization and similarity of electron spin resonance spectrum to the chromophore in blue copper proteins. J Am Chem Soc. 1975 Sep 17;97(19):5577–5581. doi: 10.1021/ja00852a043. [DOI] [PubMed] [Google Scholar]
- Tang S. P., Coleman J. E., Myer Y. P. Conformational studies of copper proteins. Pseudomonas blue protein and Polyporus laccase. J Biol Chem. 1968 Aug 25;243(16):4286–4297. [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. R. Purification and properties of a proteolytic enzyme from French beans. Biochem J. 1965 Oct;97(1):228–235. doi: 10.1042/bj0970228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. W., Jr, Kasperian M. H., Martin R. B. Binding of copper (II) to potentially tridentate amino acid ligands. J Am Chem Soc. 1970 Sep 9;92(18):5365–5372. doi: 10.1021/ja00721a013. [DOI] [PubMed] [Google Scholar]
- Wilson E. W., Jr, Martin R. B. Penicillamine deprotonations and interactions with copper ions. Arch Biochem Biophys. 1971 Feb;142(2):445–454. doi: 10.1016/0003-9861(71)90508-x. [DOI] [PubMed] [Google Scholar]



