A new anti-LAP antibody that enhances inflammation in vivo
Keywords: experimental autoimmune encephalomyelitis, inflammation, latency-associated peptide, regulatory T cells, TGF-beta
Abstract
Regulatory T cells (Tregs) play a critical role in the maintenance of immunological tolerance. The best-characterized Tregs are those expressing the transcription factor Foxp3 and in vivo modulation of Foxp3 Tregs has been employed to study their role in immune homeostasis. Latency-associated peptide (LAP) is a membrane-bound TGF-β complex that has also been shown to play a role in Treg function and oral tolerance. We developed a novel anti-mouse LAP mAb that allowed us to investigate the effect of targeting LAP in vivo on immune function and on anti-CD3-induced oral tolerance. We found that in vivo anti-LAP mAb administration led to a decrease in the number of CD4+LAP+ Tregs in spleen and lymph nodes without affecting CD4+Foxp3+ Tregs. Spleen cells from anti-LAP-injected mice proliferated more in vitro and produced increased amounts of IL-2, IL-17 and IFN-γ. Moreover, injection of anti-LAP antibody abrogated the protective effect of oral anti-CD3 on experimental autoimmune encephalomyelitis (EAE). Finally, in vivo anti-LAP administration prior to myelin oligodendrocyte glycoprotein immunization resulted in severe EAE in the absence of pertussis toxin, which is used for EAE induction. Our findings demonstrate the importance of CD4+LAP+ T cells in the control of immune homeostasis and autoimmunity and provides a new tool for the in vivo investigation of murine LAP+ Tregs on immune function.
Introduction
Regulatory T cells (Tregs) are key mediators of immunologic homeostasis and are essential to prevent autoimmunity and limit chronic inflammatory diseases. The transcription factor Foxp3 has been extensively studied and is considered to be a master regulator of Treg development and function (1–3). Additionally, the cytokine TGF-β has been shown to be involved in the induction of Foxp3 expression on CD4+ T cells (4, 5). Foxp3+ Tregs are an important source of TGF-β and TGF-β plays a crucial role in controlling autoimmune diseases (6).
We and others have demonstrated the importance of the membrane-bound latency-associated peptide (LAP)/TGF-β complex in Treg function (7–11). TGF-β is synthesized as pro-TGF-β and is then intracellularly processed by furin proprotein convertase to form a latent complex, consisting of TGF-β associated with LAP (12). Activated Tregs express LAP on the surface through the anchoring molecule glycoprotein A repetitions predominant (GARP) (13, 14) and it has been proposed that Tregs mediate their suppressive function by presenting active TGF-β to effector cells in a cell contact-dependent manner (7). LAP-expressing Tregs are also able to confer infectious tolerance by inducing Foxp3 expression on naive CD4+ T cells in in vitro coculture experiments (11). It has been demonstrated that LAP+ T cells suppress disease in animal models of colitis (9, 15), lupus (16), atherosclerosis (17) and in experimental autoimmune encephalomyelitis (EAE) (18, 19). Furthermore, we have demonstrated a link between LAP+ T cells and oral tolerance as oral anti-CD3 induces CD4+LAP+ T cells that suppress EAE in a TGF-β-dependent mechanism (20). Tregs play an important role in oral tolerance (21–24) and orally administered antigen induces the secretion of TGF-β (25, 26) and TGF-β-secreting Treg cells termed Th3 cells (27). Thus, LAP expression on the cell surface may be one of the features of Th3-type Tregs induced by oral tolerance.
In vivo depletion of Tregs has been used as an important tool to investigate the role of Tregs in immune system homeostasis and control of autoimmunity. Treg depletion is usually accomplished either by the administration of anti-CD25 mAb (28) or by the use of DEREG (DEpletion of REGulatory T cells) mice carrying a DTR-eGFP transgene under the control of an additional Foxp3 promoter, allowing specific depletion of Tregs by administration of diphtheria toxin (29). Both methods used to deplete Foxp3+ regulatory T cells demonstrate the important role Treg cells play in the balance between immunity and tolerance (30).
Given the importance of LAP+ T cells on immune regulation, we generated a murine-specific anti-LAP mAb (31) and in the present study we investigated for the first time the effect of in vivo administration of anti-LAP mAb on immune regulation and oral tolerance.
Methods
Mice
C57BL/6 and RAG-1 deficient (RAG-1−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Foxp3-GFP knock-in mice were obtained from Dr Vijay K. Kuchroo (Harvard Medical School, Cambridge, MA, USA). All mice were housed in a specific pathogen-free environment according to the animal protocol guidelines of the Committee on Animals of Harvard Medical School, which also approved the experiments.
Antibodies and reagents
Antibody specific to CD3 (145-2C11) (BD Biosciences, San Jose, CA, USA) was used to stimulate T cells in vitro. Fluorescent anti-mouse mAbs used in flow cytometry were CD4 (GK 1.5), CD8a (53–6.7) and LAP (TW7-16B4), purchased from Biolegend, San Diego, CA, USA, and Foxp3 (FJK-16s), IL-17 (eBio17B7), GARP (YGIC86), F4/80 (BM8) and IFN-γ (XMG1.2) purchased from eBiosciences, Santa Clara, CA, USA. Fixable Viablity Dye eFluor 780 (FVD eFluor 780) from eBiosciences was used for cell viability studies. For Fcγ receptor blocking, we used CD16/CD32-specific antibody (BioXCell). Anti-LAP (clone TW7-16B4) mAb used for in vivo treatment was purified from hybridoma generated in our laboratory by Taka Oida (31) and the isotype control (IC; unspecific mouse IgG1 clone MOPC-21) was purchased from BioXcell. The P3U1 cell line expressing mouse TGF-β1 (P3U1-mTGF-β1) was generated in our laboratory by Taka Oida as previously described (31).
Oral administration of anti-CD3 antibody and in vivo anti-LAP treatment
In studies of oral tolerance induction, mice were orally treated with 5 μg of hamster IgG anti-mouse CD3-specific antibody (clone 145-2C11) or hamster IgG control antibody (both from BioXCell) dissolved in 200 μl of PBS by gastric intubation with an 18-gauge stainless steel feeding needle (Thomas Scientific) once a day for five consecutive days. In EAE experiments, we immunized mice 1 day after the last feeding. In other experiments, lymphoid organs were taken 1 day after the last oral treatment without subsequent immunization. For in vivo anti-LAP treatment, mice were injected intra-peritoneally (i.p.) with 50 μg of anti-LAP-specific antibody (clone TW7-16B4) or mouse IgG1 IC antibody (BioXCell) dissolved in 200 μl of PBS once a day for five consecutive days. In some experiments, EAE was induced in anti-LAP or IC-treated mice 1 day after the last antibody injection. In other experiments, lymphoid organs were taken 1 day after the last antibody injection without subsequent immunization.
Induction and evaluation of EAE
Mice were injected subcutaneously (s.c.) in both flanks with 100 μg myelin oligodendrocyte glycoprotein (MOG)35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) dissolved in PBS emulsified in an equal volume of CFA (Difco) supplemented with 5mg ml−1 Mycobacterium tuberculosis H37Ra. Pertussis toxin (PT; List Biological Laboratories) was injected i.p. (150ng) on the day of immunization and 48h later. Clinical assessment of EAE was performed daily after disease induction according to the following criteria: 0, no disease; 1, tail paralysis; 2, hindlimb weakness or partial paralysis; 3, complete hindlimb paralysis; 4, forelimb and hindlimb paralysis; and 5, moribund state. Mean clinical scores on separate days were calculated by adding scores of individual mice and dividing total number of mice in each group, including mice that did not develop signs of EAE.
Suppression assay
For the in vitro suppression assay, 1×105 sorted CD4+ Foxp3+ T cells from anti-LAP or IC-treated mice cells were cultured at a 1:1 ratio with syngeneic naive responder CD4+ T cells (CD4+CD62L+CD44−Foxp3−) previously stained with CellTrace Violet according to the manufacturer’s recommendation (CellTrace Violet proliferation kit; Invitrogen). Cells were stimulated with 1 μg ml−1 of soluble anti-CD3 in the presence of mitomycin-treated antigen-presenting cells (APCs) in 200 μl of Iscove’s Modified Dulbecco’s Medium supplemented with 10% fetal bovine serum (FBS) in 96-well round-bottom plates. Proliferation was assessed 72h later by flow cytometry based on the dilution of the CellTrace Violet dye on responder cells. In another in vitro suppression assay with similar conditions as described above, anti-LAP or IC antibodies were added (50 µg ml−1) directly to the coculture of nTregs (CD4+Foxp3+ T cells) and naive CD4+ T cells.
T-cell proliferation
Cells were cultured in quadruplicates at 2.0×105 per well in the presence of various concentrations of anti-CD3 antibody or MOG35-55 peptide in 96-well round-bottom microtiter plates (Corning, Tewksbury, MA, USA) for 72h at 37°C with 5% CO2 in a humid incubator. Cultures were pulsed with 0.25 μCi tritiated thymidine (3H-Thymidine; PerkinElmer, Waltham, MA, USA) for the last 12h. 3H-Thymidine incorporation was measured (CPM) using a liquid scintillation beta counter (Wallac; PerkinElmer).
Cytokine analysis
Spleens and lymph nodes were harvested from mice, and single-cell suspensions were prepared. Cells were cultured at 2.0×105 per well in 96-well U-bottom plates with 20 μg ml−1 MOG35-55 peptide or 1 μg ml−1 of anti-CD3 in IMDM medium supplemented with 10% FBS. For TGF-β detection, cells were cultured in X-VIVO 15 serum-free medium (Lonza). Supernatants were harvested at 72h of culture and the concentrations of indicated cytokines were measured by quantitative capture ELISA according to the guidelines of the manufacturer (BD Biosciences).
Flow cytometry
Cells were washed (1700 r.p.m., 5min at 4°C) with FACS buffer (Mg2+ and Ca2+ free HBSS with 2% FBS, 0.4% 0.5M EDTA and 2.5% 1M HEPES). Fcγ receptors were blocked by incubation with anti-CD16/CD32 antibody for 10min at 4°C. Cells were then stained with fluorescent anti-mouse cell surface molecule antibodies for 25min at 4°C in dark. After staining, cells were washed again with FACS buffer before flow cytometry (BD™ LSR II; Becton Dickinson, Franklin Lakes, NJ, USA). For Foxp3 staining, cells were fixed and permeabilized after initial staining of surface antigens. After fixing/permeabilization, cells were incubated with anti-mouse Foxp3 antibody for 25min at 4°C in the dark and then washed again in FACS buffer. For intracellular cytokine staining, cells were stimulated with PMA (50ng ml−1) and ionomycin (1000ng ml−1) in culture medium containing 1 μl ml−1 GolgiSTOP (BD Biosciences) for 4h at 37°C with 5% CO2 in a humid incubator. After incubation, cells were fixed and permeabilized before being stained. All FACS results show events gated on CD4+ T cells. All FACS data were analyzed using FlowJo software (TreeStar).
Adoptive transfer
Spleen cells from Foxp3-GFP knock-in mice were first enriched using CD4 microbeads isolation kit (Miltenyi Biotec). CD4+LAP−Foxp3− T cells were then sorted and injected (1×106 cells per mouse) intravenously into RAG1−/− mice. Twenty-four hours later, recipient mice were treated daily, for 10 consecutive days, with 5 μg of oral anti-CD3 or IC.
Antibody-dependent cell-mediated phagocytosis assay
Thioglycollate-elicited peritoneal exudate cells (thio-PEC) were used as effector cells. To obtain thio-PEC, mice were injected i.p. with 1ml of 3% thioglycollate (Sigma Aldrich, St Louis, MO, USA). Five days later, peritoneal cells were collected and the contaminating erythrocytes were lysed. The target cells, P3U1-mTGFβ-1 cells, were first labeled with CellTrace violet (Invitrogen), incubated with 10 μg ml−1 of TW7-16B4 anti-LAP mAb or mouse IgG1 for 30min on ice and washed. Thio-PEC at a target/effector ratio of 1:2 were added and incubated for 2h at 37°C. The cells were then stained with anti-F4/80 Ab-allophycocyanin and analyzed by flow cytometry.
Complement-mediated cytotoxicity
Anti-LAP was added to P3U1-mTGF-β1 cells (1×106 ml−1) in complete medium supplemented by mouse serum, inactivated or not by incubation at 56°C for 30min. Cells were incubated for 3h at 37°C and cell lysis was determined by viability dye staining (FVD eFluor 780) according to the manufacturer’s instructions and analyzed by flow cytometry.
Statistical analysis
GraphPad Prism 6.0 was used for statistical analysis (unpaired, two-tailed Student’s t-test or one-way ANOVA, followed by Tukey’s multiple comparisons). Differences were considered statistically significant with a P value of <0.05.
Results
Effect of in vivo anti-LAP mAb administration on CD4+LAP+ T cells and immune function
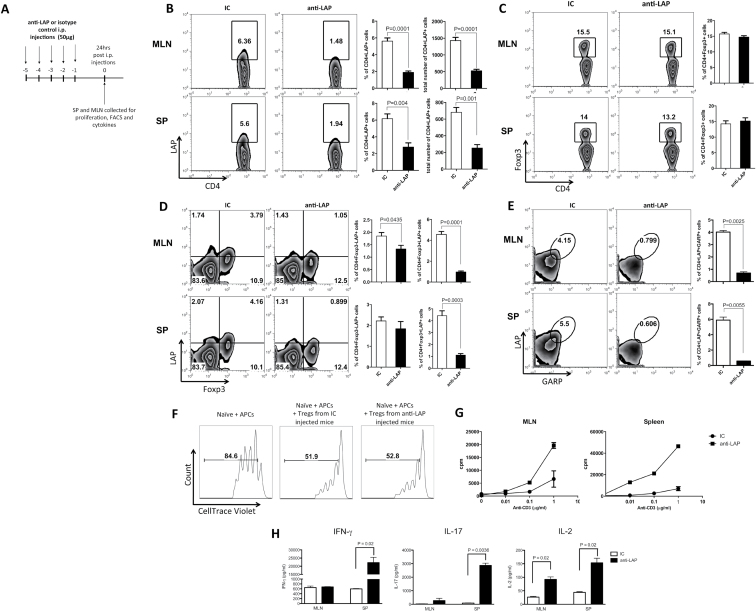
Given the important regulatory activity of CD4+LAP+ T cells in experimental models, we generated a novel anti-mouse LAP mAb that binds to LAP on the surface of mouse cells as we found that monoclonal anti-human LAP antibody does not cross-react with mouse LAP (31). Our murine-specific anti-LAP mAb provided the opportunity to investigate the role of LAP+ Tregs in vivo and to determine whether targeting LAP+ Tregs in vivo affected Foxp3 Tregs. To study LAP+ cells in vivo, we treated mice daily for five consecutive days with anti-LAP (TW7-16B4 clone) i.p. (Fig. 1A). We found that anti-LAP administration significantly reduced CD4+LAP+ T cells in spleen and mesenteric lymph nodes (MLNs) (Fig. 1B) without affecting the CD4+Foxp3+ T-cell population percentage (Fig. 1C), although it decreased LAP expression mainly on CD4+Foxp3+ T cells (Fig. 1D). GARP (LRRC32) has been described as the anchoring molecule for surface LAP (13, 14). In vivo anti-LAP treatment also reduced the number of LAP+GARP+ double-positive CD4 T cells (Fig. 1E), and a reduction was also observed on the CD4+GARP+ T-cell total population (Supplementary Figure 1A, available at International Immunology Online). Anti-LAP administration did not affect LAP expression on CD8+ T cells (Supplementary Figure 1B, available at International Immunology Online), a previously described T-cell population with regulatory activity (32).
Fig. 1.
Anti-LAP in vivo administration induces a pro-inflammatory phenotype in naive mice. C57BL/6J mice were injected i.p. with 50 μg of anti-LAP (TW7-16B4 clone) or IC for 5 days and 24h after the last injection, MLNs and spleen cells were harvested (A). FACS staining showing the percentages of CD4+LAP+ (B), CD4+Foxp3+ (C), CD4+Foxp3+LAP+ and CD4+GARP+LAP+ T cells (E). To access in vitro-suppressive function of Tregs, CD4+Foxp3+ T cells sorted from Foxp3-GFP knock-in mice treated with 50 μg of anti-LAP (TW7-16B4 clone) or IC daily for 5 days were cocultured for 3 days with CellTrace Violet-stained naive (CD4+CD62L+CD44−Foxp3−) T cells from Foxp3-GFP knock-in mice in the presence of mitomycin-treated APCs at a 1:1 ratio and stimulated with 1 μg ml−1 of purified anti-CD3 (F). Spleen and MLN cells from anti-LAP or IC-treated mice were activated in vitro for 72h with different concentrations of anti-CD3 for proliferation assay (G). IL-2, IL-17 and IFN-γ were measured in the supernatant of spleen and MLN cell cultures stimulated with 1 μg ml−1 of anti-CD3 for 72h (H).
Although a reduction of LAP expression on Foxp3+ CD4 T cells was observed following in vivo anti-LAP treatment (Fig. 1D), the in vitro-suppressive function of these cells was not affected as Foxp3+ cells from anti-LAP or IC-treated mice were equally suppressive (Fig. 1F). The addition of anti-LAP directly to the in vitro suppression assay did not affect the regulatory function of Foxp3+ CD4 T cells either (Supplementary Figure 1C, available at International Immunology Online).
In addition, spleen and MLN cells from anti-LAP-treated mice proliferated more vigorously and produced more IFN-γ, IL-17 and IL-2 when activated with anti-CD3 in vitro for 72h (Fig. 1G and H, respectively). These results indicate that reduction of CD4+LAP+ T cells by in vivo anti-LAP promoted a shift toward a pro-inflammatory immunological profile under steady-state conditions.
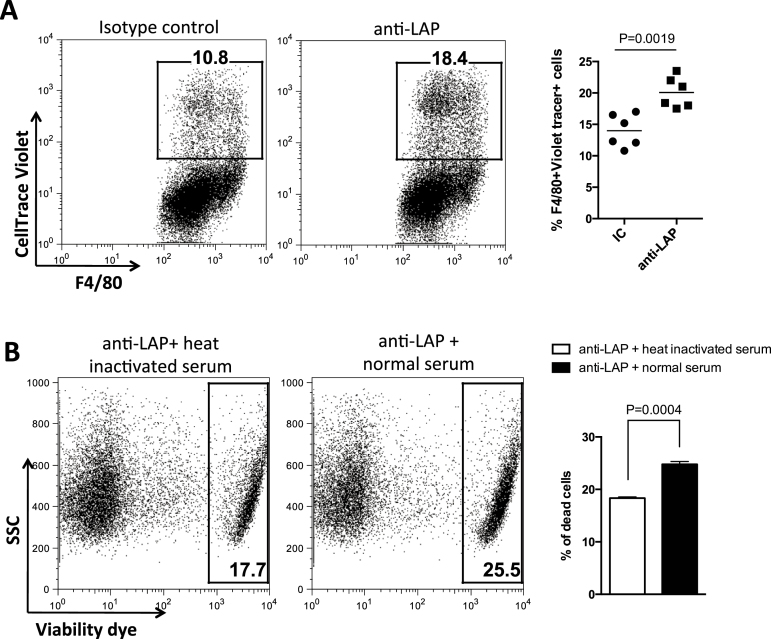
In studies of Foxp3+ Treg depletion, investigators asked whether phagocytosis could be demonstrated in an in vitro system (28). The TW7-16B4 anti-LAP mAb is a mouse IgG1 antibody and through its Fc domain, could potentially activate cellular effector mechanisms for antibody-dependent cell-mediated cytotoxicity or antibody-dependent cell-mediated phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC). To investigate these mechanisms, we established an in vitro ADCP assay using thio-PEC from wild-type mice as effector cells; a flow cytometry profile of thio-PEC showed that >90% express F4/80, a pan macrophage marker (Supplementary Figure 2A, available at International Immunology Online). Although LAP+ Tregs could be used as target cells, LAP+ Treg isolation requires antibody binding to LAP on the cell surface, which would interfere with the ADCP assay. Thus, we used P3U1-mTGF-β1 cells previously generated in our laboratory (31). This cell line expresses high levels of murine LAP on the surface and does not express F4/80 (Supplementary Figure 2B, available at International Immunology Online). As shown in Fig. 2(A), anti-LAP antibody significantly enhanced the percentage of CellTrace Violet-labeled P3U1-mTGF-β1 cells that were phagocytosed by F4/80+ macrophages. We also investigated complement-mediated lysis (CDC) and found that in the presence of anti-LAP, CDC (abrogated after heat inactivation of serum) caused an increase in P3U1-mTGF-β1 target cell death (Fig. 2B), indicating that lysis by CDC is another mechanism through which anti-LAP mAb can affect LAP+ cells.
Fig. 2.
LAP+ cell depletion is partially dependent on antibody-dependent macrophage phagocytosis and complement lysis. To confirm the role of macrophages on CD4+LAP+ T-cell depletion, the ADCP assay was performed using thio-PEC from wild-type mice as effectors and CellTrace Violet-labeled P3U1-mTGF-β1 cells as targets. P3U1-mTGF-β1 were incubated with 10 μg ml−1 of anti-LAP or IC and then cocultured for 2h at 37°C with thio-PEC at a target/effector ratio of 1:2. Cells were then stained with anti-F4/80 antibody and analyzed by flow cytometry (A). Complement-mediated lysis of P3U1-mTGF-β1 cells was determined by viability dye staining and flow cytometry analysis after incubation with anti-LAP and normal or heat-inactivated mouse serum for 3h (B).
Effect of in vivo anti-LAP mAb administration on CD4+LAP+ T cells, GARP expression and IL-17/IFN-γ responses after active immunization
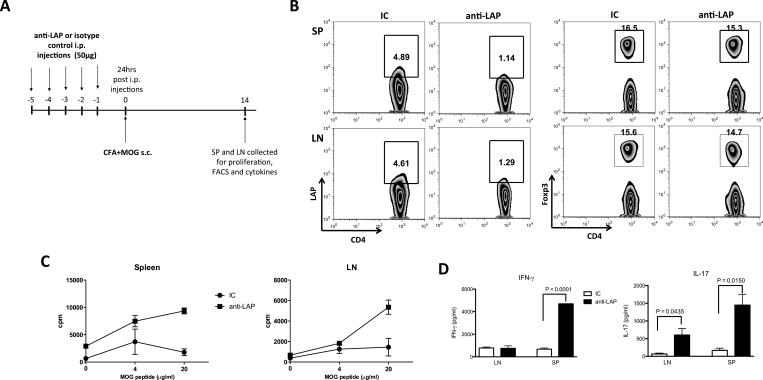
To investigate the effect of anti-LAP treatment during an inflammatory immunological response, mice treated with anti-LAP antibody were then immunized with MOG35-55 in CFA. Two weeks after immunization, mice were sacrificed and spleen and inguinal lymph node (iLN) cells harvested for in vitro recall response (Fig. 3A). We found that the percentage of CD4+LAP+ T cells was decreased following anti-LAP administration even 14 days after immunization and the last anti-LAP injection, whereas the number of CD4+Foxp3+ T cells was not affected (Fig. 3B), although LAP expression on Foxp3+ CD4 T cells was again reduced (Supplementary Figure 3A, available at International Immunology Online). Similarly to what was observed in unimmunized mice, GARP+LAP+ double-positive CD4 T cells were also reduced (Supplementary Figure 3B, available at International Immunology Online).
Fig. 3.
Anti-LAP administration enhances the pro-inflammatory immune response following MOG35-55 immunization. C57BL/6J mice were injected i.p. with 50 μg of anti-LAP (TW7-16B4 clone) or IC for 5 days and 24h after the last injection, mice were immunized subcutaneously with MOG35-55 +CFA. Fourteen days later, iLNs and spleen cells were harvested (A). FACS staining showing the percentage of CD4+LAP+ and CD4+Foxp3+ T cells in spleen and lymph nodes of anti-LAP or IC-treated mice (B). Spleen and lymph node cells from anti-LAP or IC-treated mice were activated in vitro for 72h with different concentrations of MOG35-55 peptide for the proliferation assay (C). IFN-γ and IL-17 were measured in the supernatant of spleen and iLNs cell cultures stimulated with 100 μg ml−1 of MOG35-55 peptide for 72h (D).
In vitro re-stimulation of spleen and iLN cells from anti-LAP-treated mice with MOG35-55 peptide showed a marked increase in proliferation (Fig. 3C). Anti-LAP-treated mice also produced higher amounts of IL-17 and IFN-γ after in vitro re-stimulation with MOG35-55 peptide (Fig. 3D). Thus, anti-LAP administration promotes a stronger MOG-specific inflammatory response after MOG35-55 immunization.
In vivo anti-LAP administration abrogates the protective effect of oral anti-CD3 in EAE
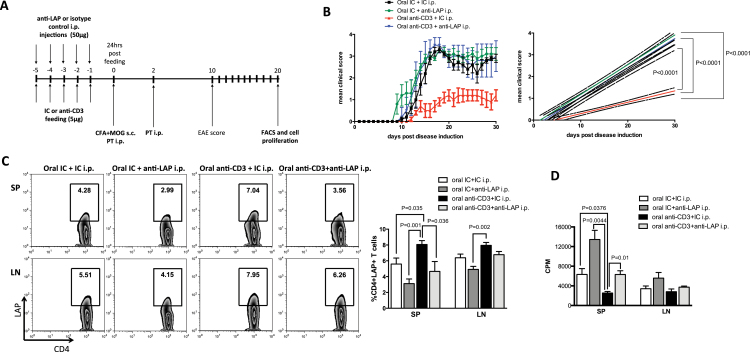
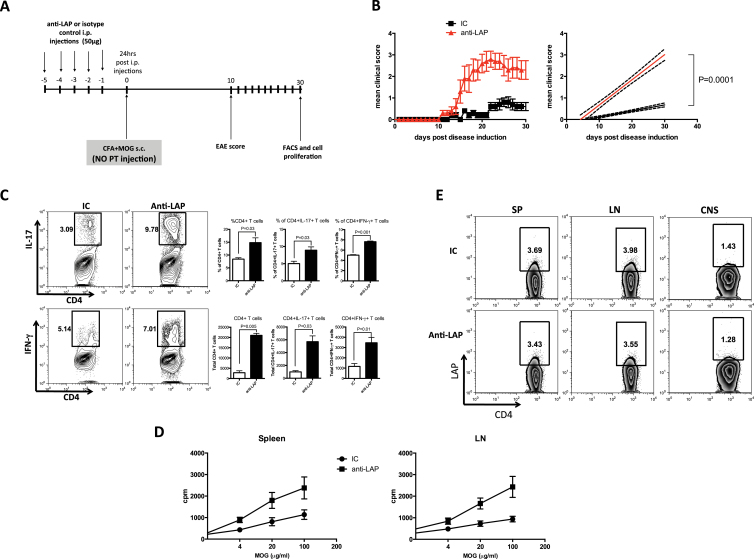
We have shown that the induction of CD4+LAP+ T cells by oral anti-CD3 suppresses EAE (20). To test whether in vivo anti-LAP administration interferes with oral tolerance induced by anti-CD3 feeding, we treated C57BL/6 mice concomitantly with oral anti-CD3 and anti-LAP i.p. followed by immunization with MOG35-55 in CFA for EAE induction. Mice also received PT injection i.p. on the day of the immunization and 48h later (Fig. 4A). We found that anti-LAP administration reversed oral tolerance induced by anti-CD3 (Fig. 4B). Mice treated orally with anti-CD3 showed an increase in CD4+LAP+ T cells in the spleen 20 days after EAE induction, which was reversed by anti-LAP administration (Fig. 4C). Additionally, spleen cells from oral anti-CD3-treated mice displayed reduced proliferation after MOG35-55 re-stimulation and this reduction was abrogated by anti-LAP administration (Fig. 4D). These results are consistent with the effect on the EAE clinical score (Fig. 4B). Anti-LAP administration in IC-fed mice did not lead to a more severe EAE (Fig. 4B).
Fig. 4.
Anti-LAP administration abrogates oral tolerance induced by oral anti-CD3. C57BL/6J mice were orally treated with 5 μg of anti-CD3 or its IC for five consecutive days and concomitantly injected i.p. with 50 μg of anti-LAP (TW7-16B4 clone) or IC for 5 days. Twenty-four hours after the last injection and oral treatment, mice were immunized subcutaneously with MOG35-55+CFA and also received PT i.p., injection that was repeated 48h later for EAE induction (A). EAE progression was followed and the disease score was monitored from day 10–30 (B). Some mice were sacrificed 14 days after immunization and iLNs and spleen cells were harvested for FACS staining and proliferation. The percentage of CD4+LAP+ T cells is shown on gated CD4+ T cells (C). Spleen and iLN cells from anti-LAP or IC-treated mice were activated in vitro for 72h with 100 μg ml−1 of MOG35-55 peptide for the proliferation assay (D).
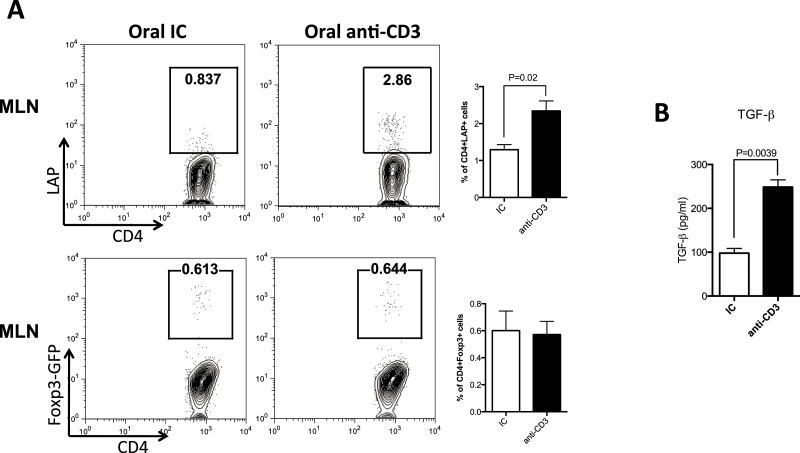
Anti-LAP mAb may affect oral tolerance by depleting LAP+ T cells and/or by preventing the induction of new LAP-expressing cells. To determine whether LAP+ T-cell expansion after oral anti-CD3 came from cells previously expressing LAP or from de novo induction of LAP expression on previously LAP−cells, we transferred CD4+LAP−Foxp3− T cells from Foxp3-GFP knock-in mice into Rag-1−/− mice and fed them anti-CD3. We found that oral anti-CD3 induced LAP expression with no increase in Foxp3 expression on the transferred cells (Fig. 5A). MLN cells from anti-CD3-treated mice secreted more TGF-β upon in vitro activation with anti-CD3 (Fig. 5B), which is involved in the establishment of a tolerogenic milieu in the gut.
Fig. 5.
Oral anti-CD3 induces LAP expression on T cells that were LAP−Foxp3−. CD4+LAP−Foxp3− T cells were sorted from Foxp3-GFP knock-in mice and transferred into RAG-1−/− recipient mice. Recipients were treated orally for 10 days with 5 μg of anti-CD3 or IC and 24h after the last treatment, MLN cells were harvested and stained for CD4 and LAP and analyzed by FACS (A). MLN cells of anti-CD3- or of IC-treated recipient mice were activated in vitro with 1 μg ml−1 of anti-CD3 and TGF-β was measured in the supernatant after 72h of cell culture (B).
Effect of in vivo anti-LAP administration on EAE induced without PT
Our results indicate that in vivo anti-LAP administration induces a pro-inflammatory immunologic response with increased proliferation and IL-17 and IFN-γ secretion, cytokines known to be important in the pathogenesis of EAE (33, 34). Although we did not observe enhancement of MOG-induced EAE in C57BL/6 mice given anti-LAP antibody (Fig. 4B), we asked whether in vivo administration of anti-LAP antibody would affect EAE if EAE was induced in a sub-optimal fashion. Thus, we investigated whether anti-LAP administration enhanced EAE in mice not given PT injection (Fig. 6A), a compound classically used to facilitate EAE induction (35, 36). Mice immunized with MOG35-55 in CFA without PT only developed very mild EAE (Fig. 6B). We found, however, that anti-LAP administration prior to MOG35-55 immunization in the absence of pertussis led to the development of severe EAE (Fig. 6B), accompanied by increased Th1 and Th17 infiltration in the central nervous system (CNS) (Fig. 6C). Spleen and iLN cells from anti-LAP-treated mice also displayed increased proliferation after in vitro activation with MOG35-55 (Fig. 6D). No differences in the frequency of CD4+LAP+ T cells were observed at 30 days after immunization (Fig. 6E), suggesting that CD4+LAP+ T cells play an important role in the induction phase of EAE as opposed to after disease is established.
Fig. 6.
Anti-LAP in vivo administration exacerbates EAE. C57BL/6J mice were injected i.p. with 50 μg of anti-LAP (TW7-16B4 clone) or IC for 5 days and 24h after last injection, mice were immunized s.c. with MOG35-55+CFA without PT i.p. injection for EAE induction (A). EAE progression was followed and disease score was monitored from day 10–30 (B). Mice were sacrificed at day 30 after immunization and iLNs, spleen and CNS-infiltrating cells were harvested for FACS staining and proliferation. The percentage and total number of CD4+IL-17+ or CD4+IFN-γ+ in CNS are shown (C). Spleen and lymph node cells from anti-LAP or IC-treated mice were activated in vitro for 72h with different concentrations of MOG35-55 peptide for the proliferation assay (D). FACS staining with the percentage of CD4+LAP+ T cells in spleen, iLNs and CNS is shown (E). FACS results show events gated on CD4+ T cells.
Discussion
The expression of TGF-β on the cell surface of Tregs has been described as playing an important role in Treg-mediated suppression (7, 11) and LAP+ Tregs play a key role in oral tolerance induction (37). Using a novel mouse-specific anti-LAP mAb we were able to investigate the importance of LAP+ cells in vivo. We found that in vivo administration of anti-LAP mAb to naive mice induced a pro-inflammatory phenotype associated with increased T-cell proliferation and increased IL-17 and IFN-γ secretion. These effects may in part be due to the depletion of LAP+ T cells since anti-LAP antibody binding in vitro caused increased ADCP and CDC. Down-modulation (endocytosis) of surface LAP may also occur. Although LAP expression was decreased on Foxp3+ CD4 T cells following anti-LAP administration, the number of Foxp3+ CD4 T cells did not change and Foxp3 Tregs retained their suppressive properties. This may in part be because only a small percentage of Foxp3+ CD4 T cells express LAP in vivo and overall, the activity of total Foxp3+ CD4 T-cell population was not directly affected by anti-LAP administration.
The effect of in vivo anti-LAP was also evident in an autoimmune inflammatory scenario. In EAE, PT acts to increase permeabilization of the blood–brain barrier facilitating the migration of pathogenic T cells to the CNS (36, 38, 39) and also promotes development of encephalitogenic Th17 cells (35, 40). Without PT, only mild EAE results. We found that MOG/CFA immunization in the absence of PT resulted in severe EAE when anti-LAP was administered. This was associated with increased T-cell proliferation and infiltration of Th1 and Th17 cells into the CNS. Thus, it appears that the high incidence and severity of EAE in the absence of PT injection is due to enhanced T-cell activation and induction of Th1 and Th17 cells secondary to the loss of LAP+ Treg activity. Interestingly, we did not observe worsening of EAE by anti-LAP administration in animals given PT, suggesting that LAP+ Tregs may not be effective when there is maximal induction of EAE.
We have previously reported the importance of LAP+ T cells in oral tolerance and we found here that in vivo anti-LAP administration completely abolished the tolerogenic effect of oral anti-CD3 by affecting CD4+LAP+ T cells. Moreover, we found that surface LAP induction following oral anti-CD3 occurs in a population of T cells without prior regulatory function. Thus, oral anti-CD3 may act not only by expanding an existing population of regulatory T cells but also by de novo induction of CD4+LAP+ Tregs. In this context, LAP expression appears to be a key marker of Th3-type Tregs induced by oral anti-CD3 (20). Our group has also shown that CD4+LAP+ Tregs play an important role in mucosal tolerance induced by nasal anti-CD3 administration. In this instance, regulatory activity was dependent on IL-10 secretion (16, 41). Thus, LAP+ expression on Tregs is not only related to TGF-β-dependent Tregs but may also be related to Tr1-type Treg cells. The induction of TGF-β versus IL-10-dependent Tregs appears to be dependent on the mucosal site of induction of the Tregs population (42). In the nasal anti-CD3 model, we have shown that the induction of CD4+LAP+IL-10+ T cells is dependent on IL-27 produced by upper airway resident dendritic cells and is controlled by the transcription factors aryl hydrocarbon receptor and c-Maf (41).
Tregs not only serve to modulate autoimmunity, but enhanced Treg activity can promote malignancy (43, 44). It has been shown that membrane-bound TGF-β is expressed on the surface of malignant B cells in B-cell non-Hodgkin lymphoma and this correlates with the inhibition of proliferation and cytokine production of intratumoral T cells (45). Furthermore, studies of colorectal cancer in humans have shown that CD4+LAP+ T cells that accumulate in tumor sites are more suppressive than Foxp3+ Tregs and are also associated with disease progression (46, 47). Thus, it is possible that anti-LAP antibody administration could have a role in the therapy of diseases where excessive immune regulation contributes to the disease process.
In summary, our findings demonstrate the importance of CD4+LAP+ T cells on the control of immune homeostasis and autoimmunity and provides a new tool for the in vivo investigation of murine LAP+ Tregs on immune function.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institutes of Health (R01 AI43458 to H.L.W); Susan Furbacher Conroy Fellowship (2002A002263 to A.P. da C).
Supplementary Material
Acknowledgement
We thank Deneen Kozoriz for cell sorting.
Conflict of interest statement: The authors declared no conflicts of interest.
References
- 1. Campbell D. J., Koch M. A. 2011. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohkura N., Kitagawa Y., Sakaguchi S. 2013. Development and maintenance of regulatory T cells. Immunity. 38:414. [DOI] [PubMed] [Google Scholar]
- 3. Sakaguchi S., Vignali D. A., Rudensky A. Y., Niec R. E., Waldmann H. 2013. The plasticity and stability of regulatory T cells. Nat. Rev. Immunol. 13:461. [DOI] [PubMed] [Google Scholar]
- 4. Chen W., Jin W., Hardegen N., et al. 2003. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198:1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peng Y., Laouar Y., Li M. O., Green E. A., Flavell R. A. 2004. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl Acad. Sci. USA. 101:4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li M. O., Sanjabi S., Flavell R. A. 2006. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 25:455. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura K., Kitani A., Strober W. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 194:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura K., Kitani A., Fuss I., et al. 2004. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 172:834. [DOI] [PubMed] [Google Scholar]
- 9. Oida T., Zhang X., Goto M., et al. 2003. CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J. Immunol. 170:2516. [DOI] [PubMed] [Google Scholar]
- 10. Di Giacinto C., Marinaro M., Sanchez M., Strober W., Boirivant M. 2005. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J. Immunol. 174:3237. [DOI] [PubMed] [Google Scholar]
- 11. Andersson J., Tran D. Q., Pesu M., et al. 2008. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J. Exp. Med. 205:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi M., Zhu J., Wang R., et al. 2011. Latent TGF-β structure and activation. Nature. 474:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stockis J., Colau D., Coulie P. G., Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur. J. Immunol. 39:3315. [DOI] [PubMed] [Google Scholar]
- 14. Tran D. Q., Andersson J., Wang R., Ramsey H., Unutmaz D., Shevach E. M. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc. Natl Acad. Sci. USA. 106:13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forster K., Goethel A., Chan C. W., Zanello G., Streutker C., Croitoru K. 2012. An oral CD3-specific antibody suppresses T-cell-induced colitis and alters cytokine responses to T-cell activation in mice. Gastroenterology. 143:1298. [DOI] [PubMed] [Google Scholar]
- 16. Wu H. Y., Quintana F. J., Weiner H. L. 2008. Nasal anti-CD3 antibody ameliorates lupus by inducing an IL-10-secreting CD4+ CD25- LAP+ regulatory T cell and is associated with down-regulation of IL-17+ CD4+ ICOS+ CXCR5+ follicular helper T cells. J. Immunol. 181:6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sasaki N., Yamashita T., Takeda M., et al. 2009. Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation. 120:1996. [DOI] [PubMed] [Google Scholar]
- 18. Chen M. L., Yan B. S., Bando Y., Kuchroo V. K., Weiner H. L. 2008. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J. Immunol. 180:7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X., Reddy J., Ochi H., Frenkel D., Kuchroo V. K., Weiner H. L. 2006. Recovery from experimental allergic encephalomyelitis is TGF-beta dependent and associated with increases in CD4+LAP+ and CD4+CD25+ T cells. Int. Immunol. 18:495. [DOI] [PubMed] [Google Scholar]
- 20. Ochi H., Abraham M., Ishikawa H., et al. 2006. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat. Med. 12:627. [DOI] [PubMed] [Google Scholar]
- 21. Weiner H. L., da Cunha A. P., Quintana F., Wu H. 2011. Oral tolerance. Immunol. Rev. 241:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hadis U., Wahl B., Schulz O., et al. 2011. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 34:237. [DOI] [PubMed] [Google Scholar]
- 23. Sun J. B., Raghavan S., Sjöling A., Lundin S., Holmgren J. 2006. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3-CD25- CD4+ regulatory T cells. J. Immunol. 177:7634. [DOI] [PubMed] [Google Scholar]
- 24. Zhang X., Izikson L., Liu L., Weiner H. L. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245. [DOI] [PubMed] [Google Scholar]
- 25. Faria A. M., Maron R., Ficker S. M., Slavin A. J., Spahn T., Weiner H. L. 2003. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-beta/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J. Autoimmun. 20:135. [DOI] [PubMed] [Google Scholar]
- 26. Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. 1992. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc. Natl Acad. Sci. USA. 89:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y., Kuchroo V. K., Inobe J., Hafler D. A., Weiner H. L. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 265:1237. [DOI] [PubMed] [Google Scholar]
- 28. Setiady Y. Y., Coccia J. A., Park P. U. 2010. In vivo depletion of CD4+FOXP3+ Treg cells by the PC61 anti-CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur. J. Immunol. 40:780. [DOI] [PubMed] [Google Scholar]
- 29. Lahl K., Loddenkemper C., Drouin C., et al. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakaguchi S., Ono M., Setoguchi R., et al. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8. [DOI] [PubMed] [Google Scholar]
- 31. Oida T., Weiner H. L. 2010. TGF-β induces surface LAP expression on murine CD4 T cells independent of Foxp3 induction. PLoS One. 5:e15523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen M. L., Yan B. S., Kozoriz D., Weiner H. L. 2009. Novel CD8+ Treg suppress EAE by TGF-beta- and IFN-gamma-dependent mechanisms. Eur. J. Immunol. 39:3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jäger A., Dardalhon V., Sobel R. A., Bettelli E., Kuchroo V. K. 2009. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 183:7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peters A., Pitcher L. A., Sullivan J. M., et al. 2011. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 35:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hofstetter H. H., Grau C., Buttmann M., et al. 2007. The PLPp-specific T-cell population promoted by pertussis toxin is characterized by high frequencies of IL-17-producing cells. Cytokine. 40:35. [DOI] [PubMed] [Google Scholar]
- 36. Linthicum D. S., Munoz J. J., Blaskett A. 1982. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell. Immunol. 73:299. [DOI] [PubMed] [Google Scholar]
- 37. da Cunha A. P., Weiner H. L. 2012. Induction of immunological tolerance by oral anti-CD3. Clin. Dev. Immunol. 2012:425021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brückener K. E., el Bayâ A., Galla H. J., Schmidt M. A. 2003. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J. Cell Sci. 116(Pt 9):1837. [DOI] [PubMed] [Google Scholar]
- 39. Yong T., Meininger G. A., Linthicum D. S. 1993. Enhancement of histamine-induced vascular leakage by pertussis toxin in SJL/J mice but not BALB/c mice. J. Neuroimmunol. 45:47. [DOI] [PubMed] [Google Scholar]
- 40. Chen X., Howard O. M., Oppenheim J. J. 2007. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. J. Immunol. 178:6123. [DOI] [PubMed] [Google Scholar]
- 41. Wu H. Y., Quintana F. J., da Cunha A. P., et al. 2011. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One. 6:e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiner H. L. 2001. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat. Immunol. 2:671. [DOI] [PubMed] [Google Scholar]
- 43. Belkaid Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875. [DOI] [PubMed] [Google Scholar]
- 44. Nishikawa H., Sakaguchi S. 2010. Regulatory T cells in tumor immunity. Int. J. Cancer. 127:759. [DOI] [PubMed] [Google Scholar]
- 45. Yang Z. Z., Grote D. M., Ziesmer S. C., et al. 2013. Soluble and membrane-bound TGF-β-mediated regulation of intratumoral T cell differentiation and function in B-cell non-Hodgkin lymphoma. PLoS One. 8:e59456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scurr M., Ladell K., Besneux M., et al. 2014. Highly prevalent colorectal cancer-infiltrating LAP+ Foxp3− T cells exhibit more potent immunosuppressive activity than Foxp3+ regulatory T cells. Mucosal Immunol. 7:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahalingam J., Lin Y. C., Chiang J. M., et al. 2012. LAP+CD4+ T cells are suppressors accumulated in the tumor sites and associated with the progression of colorectal cancer. Clin. Cancer Res. 18:5224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.