Abstract
Enzyme replacement therapy (ERT) is the standard-of-care treatment of Pompe disease, a lysosomal storage disorder caused by deficiency of acid α-glucosidase (GAA). One limitation of ERT with recombinant human (rh) GAA is antibody formation against GAA. Similarly, in adeno-associated virus (AAV) vector-mediated gene transfer for Pompe disease, development of antibodies against the GAA transgene product and the AAV vector prevents therapeutic efficacy and vector readministration, respectively. Here a nondepleting anti-CD4 monoclonal antibody (mAb) was administrated intravenously prior to administration of an AAV2/9 vector encoding GAA to suppress anti-GAA responses, leading to a substantial reduction of anti-GAA immunoglobulins, including IgG1, IgG2a, IgG2b, IgG2c, and IgG3. Transduction efficiency in liver with a subsequent AAV2/8 vector was massively improved by the administration of anti-CD4 mAb with the initial AAV2/9 vector, indicating a spread of benefit derived from control of the immune response to the first AAV2/9 vector. Anti-CD4 mAb along with AAV2/9-CBhGAApA significantly increased GAA activity in heart and skeletal muscles along with a significant reduction of glycogen accumulation. Taken together, these data demonstrated that the addition of nondepleting anti-CD4 mAb with gene therapy controls humoral immune responses to both vector and transgene, resulting in clear therapeutic benefit in mice with Pompe disease.
Introduction
Pompe disease is a lysosomal storage disorder (LSD) caused by a deficiency in the activity of acid α-glucosidase (GAA), which results in progressive intralysosomal accumulation of glycogen. Pompe disease presents with a spectrum of phenotypes, ranging from a rapidly progressive infantile-onset form to slowly progressive late-onset forms. Prior to the availability of enzyme replacement therapy (ERT), the infantile-onset Pompe disease caused early death by 1 year of age deriving from muscle weakness and cardiorespiratory failure related to an underlying hypertrophic cardiomyopathy. Late-onset Pompe disease is characterized as slowly progressing skeletal muscle weakness without severe cardiac involvement. ERT with recombinant human (rh) GAA (alglucosidase alpha; Myozyme) has reduced the cardiomyopathy and prolonged survival in all Pompe disease patients.1 Furthermore, ERT significantly improved the survival rate and muscle function of presymptomatic patients.2
During ERT for Pompe disease, the administrated rhGAA provokes high antibody titers in a subset of patients, which has correlated with poor long-term outcomes.1,3,4 Pompe disease patients who lack any residual GAA protein, and therefore are incapable of inducing self-tolerance to GAA, are deemed cross-reacting immune material (CRIM) negative. CRIM-negative Pompe disease subjects are at higher risk of producing very high anti-GAA antibodies, which markedly reduce efficacy from ERT with rhGAA.5 This issue was demonstrated in the first clinical trial of ERT in Pompe disease using Chinese hamster ovary cell-derived rhGAA,5 in which the initial two CRIM-negative patients produced much higher titers of anti-GAA antibodies than did the third, CRIM-positive patient. Formation of high-titer anti-GAA antibodies correlated with markedly reduced efficacy in the CRIM-negative patients.
Current approaches to the control of immune responses in Pompe disease include broad-based immunosuppressive agents, including a variable combination of drugs such as rituximab, methotrexate, and intravenous immunoglobulin, based largely on experience form autoimmune disease and hemophilia.6–9 These agents have successfully lessened neutralizing responses to rhGAA in patients with Pompe disease, but they are associated with untoward side effects.
An established model of Pompe disease, a GAA knockout (KO) mouse, features the accumulation of lysosomal glycogen in muscle and several organs, along with excessive accumulation of autophagic substrates and impaired fusion of autophagosomes with lysosomes.10–12 GAA-KO mice are similar to CRIM-negative patients with Pompe disease with regard to immune tolerance to GAA, because the mice do not produce endogenous GAA and lack immune tolerance to introduced GAA, either in the form of ERT13 or expression from an adeno-associated virus (AAV) vector that constitutively expressed GAA.14 We previously reported a strategy for inducing immune tolerance in GAA-KO mice with an AAV vector containing a liver-specific regulatory cassette, by administering a low number of the vector particles to GAA-KO mice prior to the initiation of ERT.15 The method induced immune tolerance against administrated GAA with the increase of therapeutic efficacy in the heart and diaphragm. Efficacy from this immunomodulatory gene therapy required liver-specific hGAA expression that activated antigen-specific regulatory T-regulatory (Treg) cells.16
High-affinity antibody production requires T helper cell, and CD4-deficient mice lacking helper T cells fail to initiate antibody formation to the protein products of gene therapy.17 An in vitro study with lymphocytes showed that CD4+ and CD8+ T cells displayed an increase in expression of proinflammatory cytokines including intracellular interferon-gamma (INF-γ) and tumor necrosis factor-alpha (TNF-α), in response to rhGAA in treated patients, compared with untreated patients and healthy subjects.18 This suggests that T cells play a critical role in the immune response to rhGAA in Pompe patients. In a series of studies using a nondepleting anti-CD4 monoclonal antibody (mAb), reduced immune responses have been demonstrated in a variety of settings, including infusion of foreign proteins,19 graft rejection,20 and autoimmune diseases,21 including rheumatoid arthritis.22
Extensive preclinical studies in rodents and nonhuman primates, using nondepleting anti-CD4 mAb, have demonstrated that a short 1–2-week course of mAb treatment, in conjunction with the desired antigen, is capable of inducing long-term immune tolerance to the antigen.19–23 The use of nondepleting anti-CD4 mAb has also shown to decrease the immune response to introduced rhGAA in Pompe disease mice.24 Anti-CD4 mAb in combination with cyclosporine was effective at suppressing anticapsid antibodies in response to administration of an AAV vector, although the effect was transient.25 In the present study, we describe the inhibition of T helper cells by anti-CD4 mAb to achieve the control of immune responses to an AAV vector in GAA-KO mice. The vector administered contains a ubiquitously active regulatory cassette, and previously provoked robust immune responses in GAA-KO mice.14 We hypothesized that suppressing transgene-directed immune responses would enhance the efficacy achieved by this vector. To our knowledge, this is the first successful use of nondepleting anti-CD4 mAb alone to prevent antibody formation against a therapeutic protein in the context of gene therapy.
Materials and Methods
Preparation AAV vectors
Briefly, 293 cells were transfected with the p-trsLSP-GFP (courtesy of Dr. Douglas McCarty, Nationwide Children's Hospital, Columbus, OH) vector plasmid26 or pAAV-CBhGAApA vector plasmid,27 the AAV8 or AAV9 packaging plasmid28 (courtesy of Dr. James M. Wilson, University of Pennsylvania, Philadelphia, PA), and pAdHelper (Stratagene). Cells were harvested 48 hr following infection and freeze-thawed three times, and isolated by sucrose cushion pelleting followed by two cesium chloride gradient centrifugation steps. AAV stocks were dialyzed against three changes of Hanks buffer with 5% sorbitol added to the third dialysis, and aliquots were stored at −80°C. The number of vector DNA-containing particles was determined by DNase I digestion, DNA extraction, and Southern blot analysis. All viral vector stocks were handled according to Biohazard Safety Level 2 guidelines published by the NIH.
Animal studies
All animal procedures were performed in accordance with Duke University Institutional Animal Care and Use Committee–approved guide lines. Six-month-old GAA-KO mice were administrated 200 μl of 0.1 mg/ml anti-CD4 antibody through intravenous injection. The mice were injected with 1.0×1011 vp AAV2/9-CBhGAApA on day 0. The anti-CD4x3 group (G4; Fig. 1A) received intravenous anti-CD4 mAb (YTS177; 50 mg/kg) at day −1, +6, and +13, whereas the anti-CD4x1 (G3; Fig. 1A) was injected with anti-CD4 only on day −1. For the immune challenge with rhGAA, mice in group G3 (Fig. 1A) were injected intravenously with rhGAA (20 mg/kg) and intraperitoneally with incomplete Freund's adjuvant as described15 10 weeks after injection of the vector. The mice were euthanized at 14 or 24 weeks following vector injection for collection of tissues. GAA activity and glycogen content were analyzed as described.29
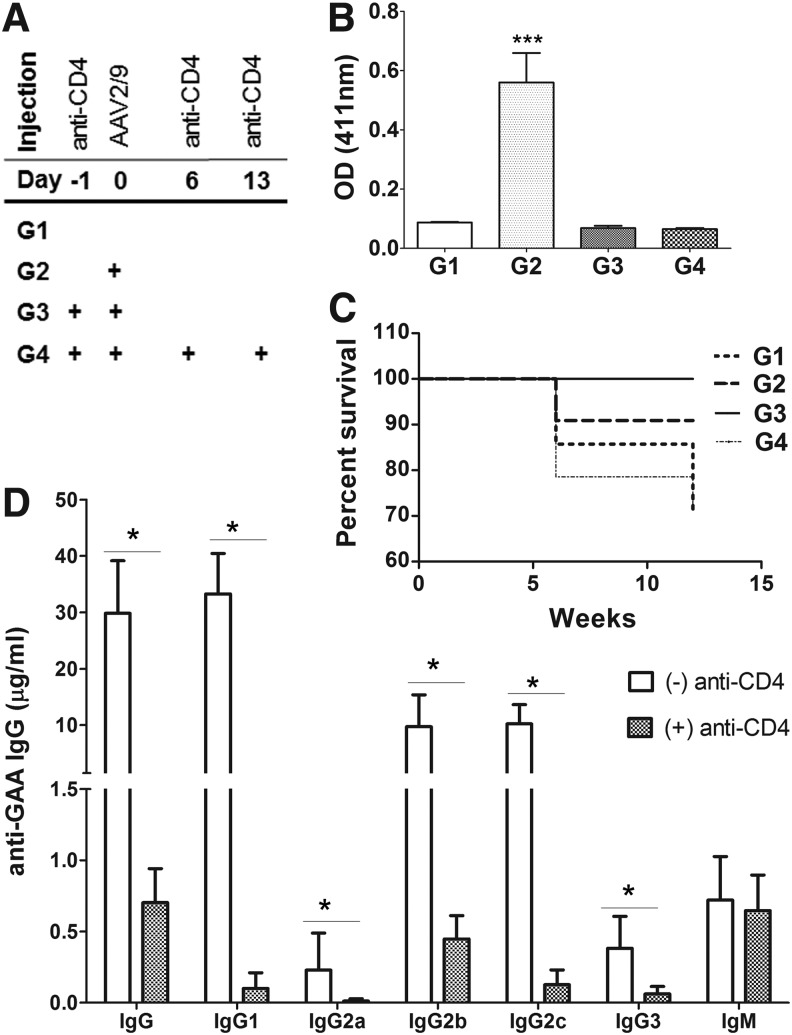
FIG. 1.
A short-term treatment with anti-CD4 mAb prevented anti-GAA formation. GAA-KO mice were treated with AAV2/9-CBhGAApA with (+) anti-CD4 mAb (either once or three times) or without (−) anti-CD4 mAb pretreatment. (A) Experimental scheme of anti-CD4 mAb treatment in GAA-KO mice. (B) Anti-GAA IgG1 at week 6 following vector administration. (C) Survival curves for groups of mice from week 0 to 12. (D) Anti-GAA subclasses at week 12. The comparison was performed between the vector alone (G2) and vector plus three doses of anti-CD4 mAb (G4) groups. Group size as follows: G1 (n=7), G2 (n=11), G3 (n=9), and G4 (n=14). *p<0.05; ***p<0.001. AAV, adeno-associated virus; GAA, α-glucosidase; KO, knockout; mAb, monoclonal antibody.
Antibody quantification
The enzyme-linked immunosorbent assay (ELISA) for anti-GAA IgG1 was performed as described.30 Briefly, rhGAA (5 μg) in carbonate buffer was coated onto each well of a 96-well plate (Costar cat. no. 3596; Corning Life Sciences) at 4°C overnight. After a wash with PBS containing 0.05% Tween 20, plasma samples with serial dilutions ranging from 1:10 to 1:200,000 were added in duplicate to rhGAA-coated plates and incubated at room temperature. The wells were washed with phosphate buffered saline (PBS) containing 0.05% Tween 20, incubated with a 1:2,500 dilution of alkaline phosphatase-conjugated sheep antimouse IgG1 at room temperature for 1 hr, and washed 3 times with PBS, and finally alkaline phosphatase substrate (p-nitrophenyl phosphate) was added. The absorbance at 411 nm was measured with a Tecan SpectraFluor microplate reader (MTX Lab Systems). All samples yielded absorbance values that were within the linear range of the assay at their respective dilutions. Absorbance values were deemed positive if both values at any given serial dilution were >0.1. In this study, screening for anti-GAA IgG1 was performed in samples diluted 1:200.
Assays of IgG subclasses
To determine the repression of antibody formation by the injection of anti-CD4, an ELISA was performed. Plates were coated with 1 μg/ml of rhGAA in carbonate buffer at 4°C overnight. Standard curves specific for murine IgG (Sigma Chemical Co.), IgG1 (R&D Systems), IgG2a (R&D Systems), IgG2b (R&D Systems), IgG2c (SouthernBiotech), IgG3 (R&D Systems), and IgM (Sigma) were also coated to the wells in seven 2-fold dilution starting from 1 μg/ml. After coating, wells were washed with PBS with 0.05% Tween 20 and blocked with PBS with 2% BSA. Plasma samples were loaded and incubated at 4°C overnight, and detection was made with specific anti-Ig antibodies conjugated with horseradish peroxidase. Detection was performed by adding to the wells 3,3′,5,5′-tetramethylbenzidine substrate (BD Biosciences), and color development was measured at 450 and 570 nm (for background subtraction) on an Enspire plate reader (Perkin Elmer) after blocking the reaction with H2SO4.
Vector readministration
To evaluate the effect of anti-CD4 in the transduction efficiency in liver, anti-CD4 mAb-treated mice were injected with the GFP expression vector, AAV2/8-LSPGFP, at 20 weeks after anti-CD4 injection. The mice were euthanized 4 weeks after the vector administration. Liver samples were immediately embedded and frozen in optimal cutting temperature medium (OCT), and stored at −80°C. The OCT-embedded samples were sectioned with 5 μm thickness and mounted on poly-L-lysine-coated slides. The slides were warmed at 60°C for 15 min to melt the OCT and washed with xylene to remove the OCT following three times washing steps with ethanol (99%, 90%, and 80%). The sectioned tissues were scanned with a confocal microscope (LSM 510 META; Carl Zeisis). After imaging, mean pixel intensity was determined by a Java-based version of NIH image software (ImageJ).
Real-time PCR was performed on liver DNA to quantify vector DNA using primers for eGFP (eGFP F 5′-GGTGT TCTGCTGGTAGTGG and eGFP R 5′-CCCTGAAGTTC ATCTGCACC) and mouse β-actin (β-actin F 5′-AGAGGG AAATCGTGCGTGAC-3′ and β-actin R 5′-CAATAGTGA TGACCTGGCCGT-3′). Plasmid DNA corresponding to 0.01 copy to 10 copies of the eGFP transgene (in 500 ng genomic DNA) was used in a standard curve. To determine the viral copy number, the ΔΔCt values of samples were compared to the standard curve.
Statistical analysis
Data analysis was performed with GraphPad Prism5 (GraphPad Software, Inc.). Multiple comparisons were performed using one-way ANOVA with Tukey's multiple comparison test. Comparison between two data sets used a homoscedastic t-test, including antibody titers in Fig. 1C, comparison of GFP expression in Fig. 2, and sex-dependent GAA activity and glycogen content in Fig. 6. Survival curves were compared with a log-rank (Mantel–Cox) test. The statistical significance of comparisons was indicated as follows: *p≤0.05; **p≤0.01, or ***p≤0.001.
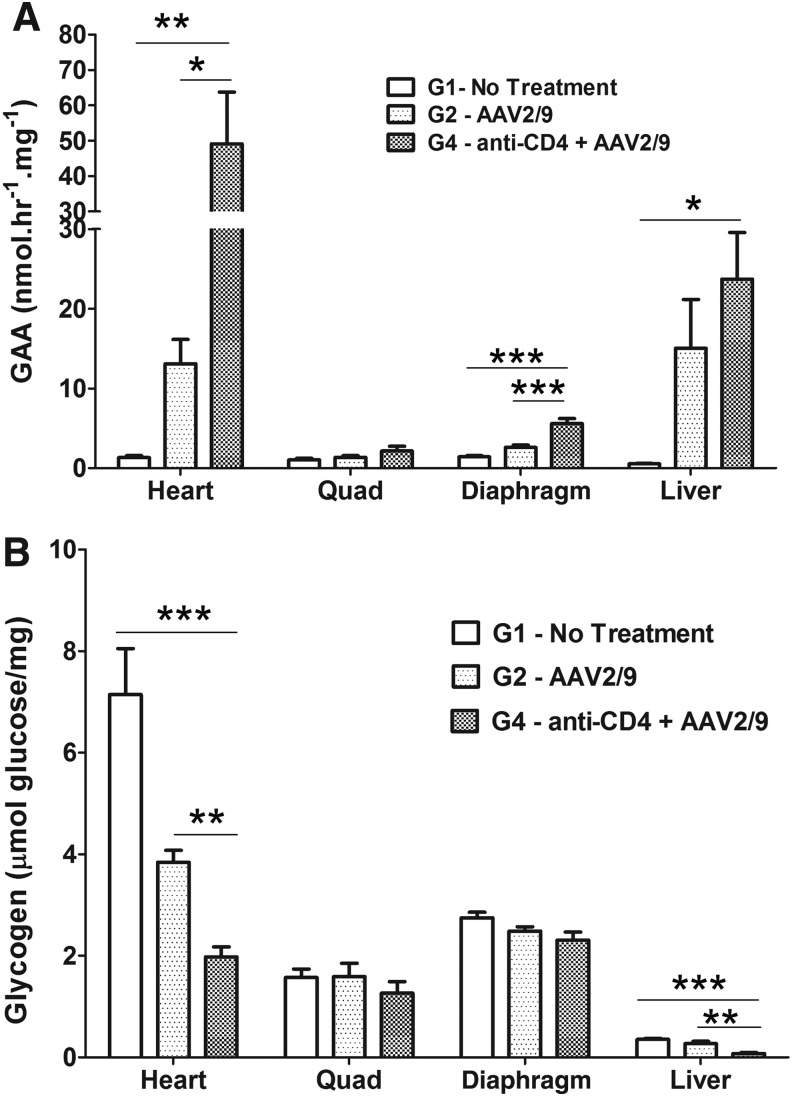
FIG. 2.
Anti-CD4 mAb enhanced biochemical correction with AAV2/9-CBhGAApA in GAA-KO mice. GAA-KO mice were treated with AAV2/9-CBhGAApA (+) anti-CD4 (3 times; n=6) or without (−) anti-CD4 (n=11) pretreatment, corresponding to groups G2 and G4, respectively. The untreated (G1) group was used for a negative control. At week 14 or 24 following injection, mice were euthanized for tissue analysis. (A) GAA activity. (B) Glycogen content. *p<0.05; **p<0.01; ***p<0.001.
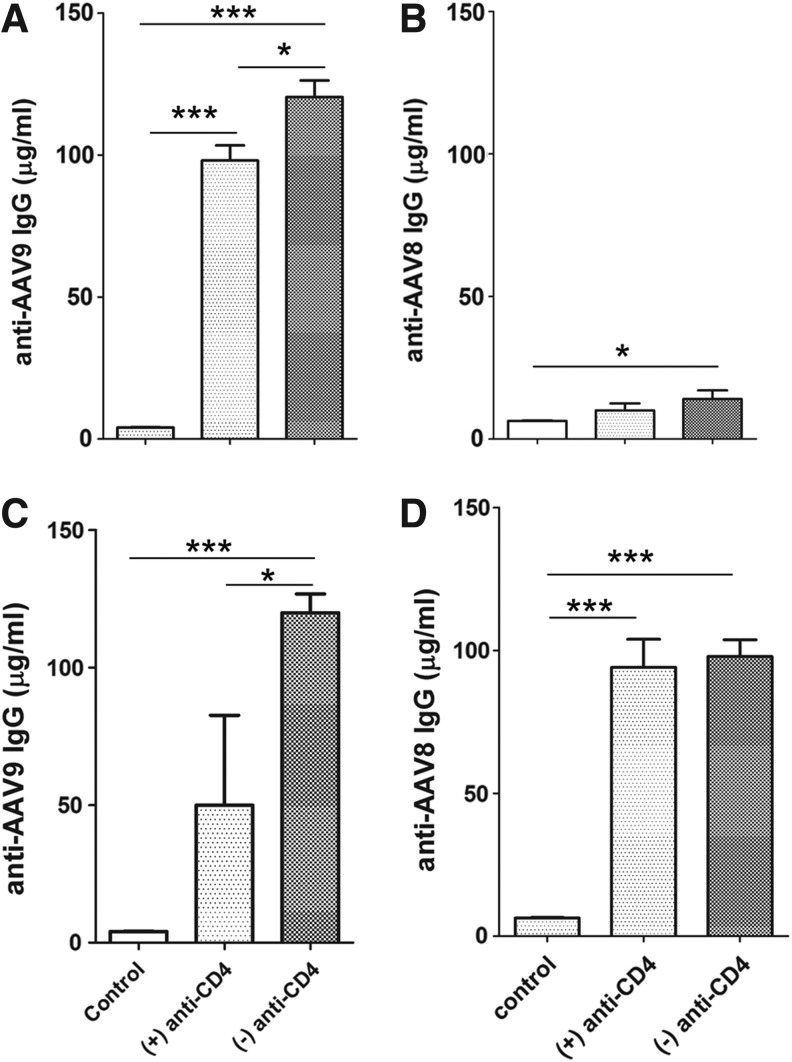
FIG. 6.
Suppression of cross-reacting anticapsid antibodies by anti-CD4. GAA-KO mice were injected with AAV2/9-CBhGAApA (+) anti-CD4 (3 times; n=6) or without (−) anti-CD4 (n=6) pretreatment. Blood was sampled for ELISA quantification of anti-AAV9 or anti-AAV8 IgG at week 12. Following the injection of AAV2/8-LSPGFPpA at week 20, blood was sampled again at week 24. (A) Anti-AAV9. (B) Anti-AAV8. (C) Anti-AAV9 at week 24. (D) Anti-AAV8 at week 24. *p<0.05; ***p<0.001.
Results
Short-term anti-CD4 mAb therapy suppressed anti-GAA in response to an immunogenic GAA expression vector
In previous studies in autoimmune disease models with rodents and nonhuman primates,31,32 immune tolerance was induced by the pretreatment with three doses of anti-CD4 mAb. We also found that the induction of anti-GAA IgG1 responses assessed at 4 weeks after injection of rhGAA in Pompe animals was prevented by administration of a nonlytic anti-CD4 antibody given prior to ERT.24 Given this effect of anti-CD4 to prevent immune responses, we elected to evaluate anti-CD4 mAb therapy to establish tolerance in the context of gene therapy (Fig. 1A). Therefore, we pretreated GAA-KO mice with anti-CD4 mAb before administering the immunogenic AAV vector, AAV2/9-CBhGAApA,14 and quantified anti-GAA IgG1at 6 weeks following vector administration (Fig. 1B). Administration of AAV2/9-CBhGAApA without anti-CD4 mAb treatment resulted in high levels of anti-GAA antibodies. In contrast, mice given the vector accompanied by anti-CD4 mAb, either as a single administration (G3) or three injections (G4), demonstrated low antibody titers no different from those of antigen-naïve mice (Fig. 1B).
Sustained immune tolerance to rhGAA has increased the efficacy of gene therapy during preclinical experiments with AAV vectors in Pompe disease.15,33 GAA-KO mice given a single pretreatment of anti-CD4 mAb were administered an immune challenge consisting of rhGAA together with adjuvant 4 weeks prior to sampling for antibody formation, and these mice maintained their state of immune tolerance (G3 in Fig. 1B). No signs of hypersensitivity were observed at the time of the rhGAA immune challenge, in contrast to the mortality that has been demonstrated in GAA-KO mice following anti-GAA antibody formation.15 Mice treated with three injections of anti-CD4 mAb experienced 29% mortality over 12 weeks despite maintaining immune tolerance to rhGAA, although the survival curve did not differ significantly from other groups of GAA-KO mice (p=0.4). Mortality was equivalent to that observed in untreated GAA-KO mice at 12 weeks (Fig. 1C). The cause of mortality was not ascertained; however, given the demonstrated lack of toxicity from anti-CD4 mAb administration, it was attributed to the frequency of handling and injection of these rather fragile mice.
Antibody formation against GAA following administration of AAV2/9-CBhGAApA alone was high titer (≥10 μg/ml serum) and comprised of IgG1, IgG2b, and IgG2c, reflecting T helper (Th)2-dependent responses at the 12-week time point (Fig. 1D). The suppression of anti-GAA IgG1 and IgG2b was maintained with small variations in titers at the 24 weeks in response to anti-CD4 mAb administration (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum), indicating the induction of stable immune tolerance to GAA.
Immunological tolerance following therapy with anti-CD4 enhances biochemical correction in the heart and skeletal muscles
To evaluate the effect of anti-CD4 mAb treatment upon biochemical correction from AAV2/9-CBhGAApA, GAA activity and glycogen quantification were performed following administration of three doses of anti-CD4 mAb with AAV2/9 (G4 as shown in Fig. 1A). GAA activity was significantly increased in heart (p<0.05) by anti-CD4 mAb administration, in comparison with vector alone (Fig. 2A). Similarly, GAA activity increased following anti-CD4 mAb treatment in the diaphragm (p<0.001) and liver (p<0.05). Glycogen content was significantly reduced in the heart and liver following anti-CD4 mAb administration, in comparison with vector alone (p<0.01, Fig. 2B). Glycogen content was slightly decreased in the diaphragm and quadriceps without achieving statistical significance.
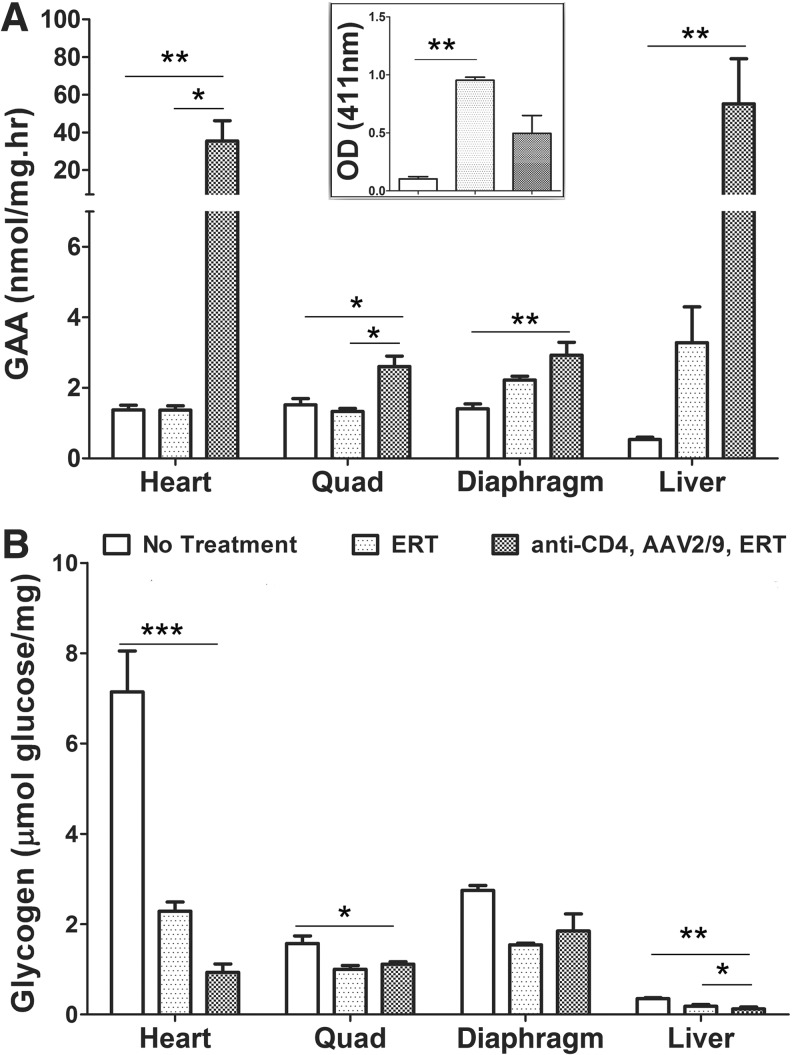
Next, we evaluated the effect of a single dose of anti-CD4 mAb prior to AAV2/9-CBhGAApA upon immune tolerance to rhGAA by performing an immune challenge with rhGAA (ERT). GAA-KO mice were given a single injection of anti-CD4 mAb 1 day prior to vector injection (G3 in Fig. 1), and subsequently mice were treated with a single injection of rhGAA with adjuvant as an immune challenge at 10 weeks following vector administration (Fig. 3). Anti-CD4 mAb suppressed antibody responses to administrated rhGAA, because anti-GAA IgG1 was not significantly elevated following the immune challenge in the anti-CD4 mAb-treated group (Fig. 3A insert). Anti-GAA IgG1 was significantly reduced in untreated control mice, in comparison with mice that received rhGAA (ERT) alone (Fig. 3A insert). The effect of vector administration in the setting of immune tolerance was apparent, because GAA activity was markedly increased in heart (p<0.01), diaphragm (p<0.01), quadriceps (p<0.05), and liver (p<0.05) of GAA-KO mice by the combination of anti-CD4 and vector (Fig. 3A). The effect of ERT alone was underestimated by quantifying GAA activity 4 weeks following rhGAA administration, because rhGAA has a short half-life.34 Glycogen accumulation was reduced in heart (p<0.001), quadriceps (p<0.05), and liver (p<0.05) following anti-CD4 and vector administration, which correlated with increased GAA activity in those tissues (Fig. 3B).
FIG. 3.
A single injection of anti-CD4 achieved biochemical correction from AAV2/9-CBhGAApA in GAA-KO mice. GAA-KO mice were administrated a single injection of anti-CD4 1 day before vector administration, and then the mice were treated rhGAA+adjuvant at week 10 following the vector injection. The mice were euthanized at week 14. (A) GAA activity. (B) Glycogen content. *p<0.05; **p<0.01; ***p<0.001.
Sex-dependent different GAA activity in liver
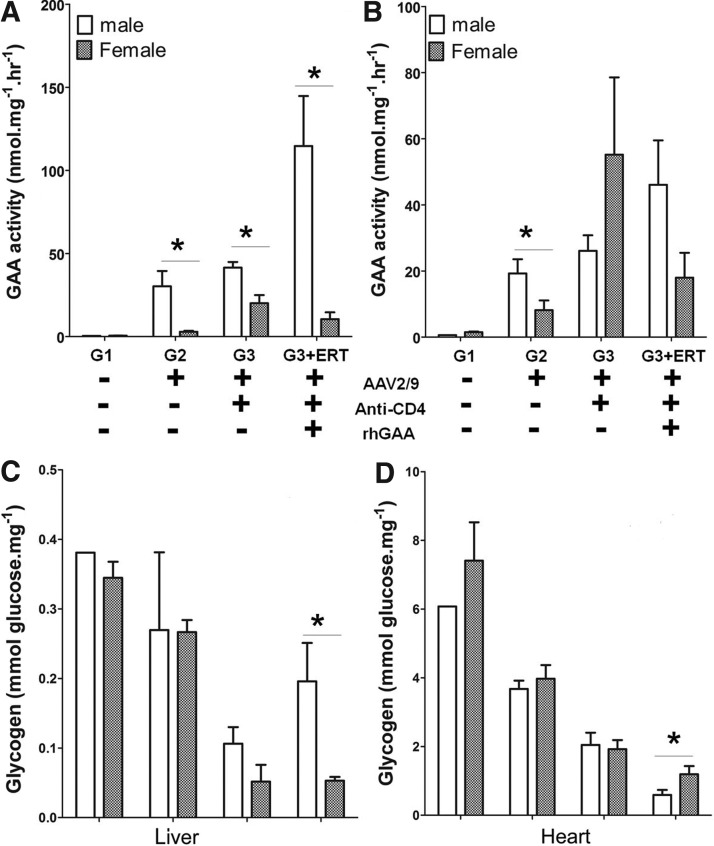
Intriguingly, GAA activity in liver was significantly higher in male mice than in females (Fig. 4A). GAA activity was slightly elevated in the heart of male mice treated with vector alone (p<0.05), in comparison with females, and trended higher in males treated with vector, anti-CD4 mAb, and rhGAA (p=0.08) (Fig. 4B). The increase of GAA activity in males, in comparison with females, did not correlate with reduced glycogen accumulation in the liver of male mice (Fig. 4C). We understand the lack of an inverse correlation between higher GAA activity and lower glycogen content in the liver, because most glycogen in the liver is cytoplasmic and not localized to the lysosomes.35 The effect of anti-CD4 mAb was greater in males, because the glycogen content of the heart of male mice was lower, in comparison with female mice, for mice treated with vector, anti-CD4, and rhGAA (p<0.05; Fig. 4D). Apparently, heart GAA was not sufficiently elevated in males treated with vector only to further lower the heart's glycogen content, in comparison with females. Formation of antibodies against GAA was slightly higher in females than in males, although this increase did not reach statistical significance (Supplementary Fig. S2A). No difference in the formation of anti-AAV9 antibodies based upon the sex of the mice was observed (Supplementary Fig. S2B).
FIG. 4.
Higher GAA expression in the liver for male mice. Biochemical correction was analyzed for male mice, in comparison with female mice. GAA activity for (A) liver and (B) heart. Glycogen content for (C) liver and (D) heart. *p<0.05.
Anti-CD4 prior to AAV2/9 administration enhanced subsequent transduction with AAV2/8
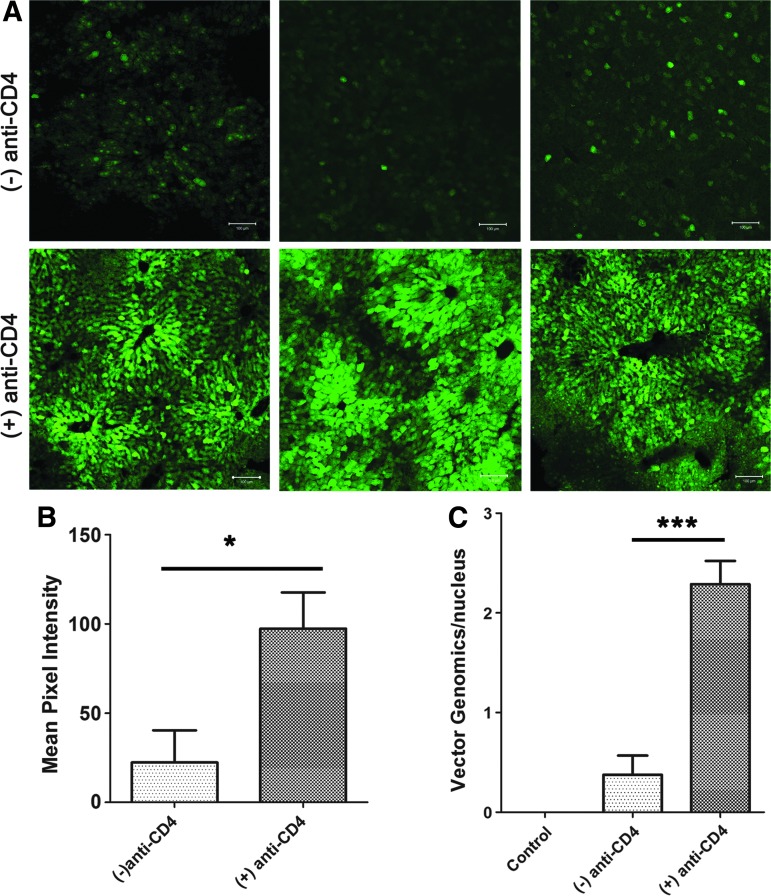
Immune responses against AAV capsids present a serious hurdle to gene therapy in Pompe disease, given the potential need to readminister an AAV vector to maintain efficacy.36–38 To evaluate the effect of anti-CD4 mAb treatment upon AAV vector readministration, GAA-KO mice initially were treated with anti-CD4 mAb and AAV2/9-CBhGAApA (G4; Fig. 1A) prior to injection of an AAV2/8 vector (AAV2/8-LSPGFP) encoding green fluorescence protein (GFP) at week 20, and were euthanized at week 24. Pretreatment with anti-CD4 mAb increased the measurable transduction of liver cells with GFP (Fig. 5A), which correlated with a significantly higher signal for fluorescence in liver by approximately 5-fold (p<0.05), in comparison with the group of mice that did not receive anti-CD4 (Fig. 5B). Pretreatment of anti-CD4 mAb significantly increased transduction as demonstrated by the 6-fold increased vector genome copy number in the liver (p<0.01; Fig. 5C). Thus, suppression of immune responses with anti-CD4 mAb enhanced transduction efficiency from AAV vector readministration.
FIG. 5.
Effect of anti-CD4 upon AAV vector readministration. GAA-KO mice were treated with AAV2/9-CBhGAApA with (+) anti-CD4 (3 times) or without (−) anti-CD4 pretreatment. At week 20, mice were injected with AAV2/8-LSPGFP (n=6 in each group), and euthanized at week 24. Liver was frozen and sectioned to detect GFP expression using confocal microscopy. Images from three different animals in each group are shown (A). The pixel intensity was analyzed with ImageJ (B). The quantity of vector genomes was also analyzed by qPCR (C). *p<0.05; ***p<0.001. Color images available online at www.liebertpub.com/hum
Anti-CD4 mAb suppresses cross-reacting anticapsid antibody formation
To evaluate the induction of immune tolerance by anti-CD4 on anticapsid responses, we analyzed anticapsid antibodies for GAA-KO mice shown in Fig. 5. These mice initially received the AAV2/9 vector encoding GAA, with or without anti-CD4 mAb. As expected, anti-AAV9 antibodies were provoked by AAV2/9 vector administration (Fig. 6A). However, the anti-AAV9 IgG response was significantly reduced by administration of anti-CD4 mAb treatment, although it was still elevated in comparison with untreated mice (p<0.05; Fig. 6A). Somewhat surprisingly, anti-AAV8 IgG levels were significantly elevated following administration of AAV2/9 without anti-CD4, in comparison with untreated GAA-KO mice (p<0.05; Fig. 6B), indicating a high degree of antibody cross reactivity between the serotypes. The latter result explained why pretreatment with anti-CD4 mAb at the time of AAV2/9 administration increased subsequent transduction with AAV2/8, as shown in Fig. 5. The presence of cross-reacting anti-AAV IgG antibodies against AAV2/8 was prevented by anti-CD4 administration (Fig. 6B), and anti-AAV formation correlated inversely with AAV2/8-LSPGFP transduction in the liver (Fig. 5A). Anti-AAV9 titers persisted for the duration of the observation period (Fig. 6C). Moreover, anti-AAV8 increased dramatically following the administration of the AAV2/8 vector at week 20, confirming that immune suppression from anti-CD4 mAb was only transient, and the mice remained immunocompetent to react against a further immune challenge (Fig. 6D).
Discussion
Multiple studies with AAV vectors encoding GAA have previously treated GAA-KO mice successfully.14,27,35,39,40 However, antibody formation remains a major obstacle for GAA gene transfer approaches in which constitutive—versus tissue-specific—expression of the therapeutic transgene is utilized to achieve disease correction.14,35,41 Despite the correlation between anti-GAA antibody formation and poor outcomes, experiments with AAV vectors containing constitutively active regulatory cassettes have not directly implicated the immune response as opposed to other potential limitations. For example, previous studies compared a vector containing a tissue-specific regulatory cassette with another vector containing a constitutively active regulatory cassette,14,35 and the latter cassette might simply be less active or might become inactivated in vivo. Our current study has identified immune responses as the cause for poor responses to a vector containing a constitutively active transgene, because blocking the activation of CD4+ lymphocytes markedly increased the efficacy of the same vector.
ERT with rhGAA has prolonged ventilator-free survival and muscle strength in patients with Pompe disease. However, approximately 35–40% of patients with classic infantile Pompe disease treated with ERT develop high, sustained antibody titers,1 which abrogated the efficacy of ERT.15,16,33,42–45 We and others previously demonstrated that liver-restricted expression of GAA could induce immune tolerance via induction of CD4+CD25+ Treg cells,16,42,45–47 and speculated that these antigen-specific Treg cells might suppress the activation of B lymphocytes by CD4+ helper T cells. This scenario in particular applies for those gene therapy applications, such as Pompe disease, in which muscle is as a target for transduction,35,48 known to be more immunogenic tissue in comparison with liver.49,37 Here we sought to determine whether a nondepleting anti-CD4 mAb would prevent the antibody response to the transgene in mice with Pompe disease injected with an immunogenic AAV vector expressing the GAA transgene under the transcriptional control of a constitutive promoter. The immunogenic AAV vector was delivered intravenously, either with or without simultaneous anti-CD4 mAb treatment. The effect of anti-CD4 mAb was demonstrated by the lack of anti-GAA antibody formation 6 weeks following vector administration (Fig. 1B), whereas mice treated with the vector alone consistently generated anti-GAA antibodies. A single injection of anti-CD4 could clearly block the formation of anti-GAA induced by the injection of AAV2/9-CBhGAApA.
The administration of AAV2/9-CBhGAApA induced the formation of diverse IgG subclasses against GAA, including anti-GAA IgG1a, IgG2b, and IgG2c, which were markedly reduced by the injection of anti-CD4 mAb. CD4+ T cells produce proinflammatory factors, including interferon-gamma (IFN-γ), and elevations of the IgG2b subclass correlated with tissue inflammation.18,50 Here we observed the suppression of IgG2b formation by pretreatment with anti-CD4 mAb; although the precise role of each IgG subclass of immune response remained to be studied, the therapeutic effect of anti-CD4 mAb appeared to last for more than 24 weeks (Supplementary Fig. S2). According to a previous report,51 the induced antibodies could interfere with the efficacy of ERT by inhibition of rhGAA uptake in the tissues. We previously reported sustained immune tolerance to rhGAA in the context of liver-specific expression of GAA using AAV2/8-LSPGAApA,47 which had a very similar beneficial effect to the current strategy of anti-CD4 mAb pretreatment and ubiquitous expression of GAA with AAV2/9-CBhGAApA. In the current study, we also evaluated the increase of GAA activity in heart and skeletal muscles using a ubiquitously expressing AAV vector with immune suppression by anti-CD4 mAb antibodies (Fig. 2A). Thus, the prevention of anti-GAA formation by any method might enhance the efficacy of GAA activity in heart and skeletal muscles, regardless of enzyme sources either transgene-mediated from gene therapy or from ERT.
Patients with two deleterious GAA mutations who are completely unable to form native enzyme are cross-reactive immunological material (CRIM)-negative; patients with presence of some residual, functioning, or nonfunctioning enzyme are CRIM-positive.1 The administered rhGAA represents a neo-antigen to infantile-onset Pompe patients, including both CRIM-negative and a significant fraction of CRIM-positive infants who form high, sustained anti-GAA antibodies during ERT.43
In a previous study, we demonstrated that an AAV2/8 vector expressed much higher GAA in male GAA-KO mice, in comparison with female mice.47 In the present study, we observed higher GAA activity in the liver of male GAA-KO mice, in comparison with female livers (Fig. 3D). However, we did not observe any difference between males and females with regard to antibody formation (Supplementary Fig. S2). Thus, the sex-dependent transgene expression with AAV vectors did not reduce the immunosuppressive effect of anti-CD4 mAb treatment.
In addition to the beneficial effect of anti-CD4 mAb therapy on transgene-mediated efficacy, we also showed enhanced transduction efficiency in the liver following administration of an AAV2/8 vector containing GFP (Fig. 5). The benefit of anti-CD4 mAb extended to readministration via reduction of cross-reacting anti-AAV9 antibodies that otherwise inhibited transduction by the AAV2/8 vector encoding GFP. Cross-reacting antibodies directed against a completely different serotype (e.g., AAV8) following administration of an AAV2/9 vector are not commonly observed in mice.52 Conversely, cross-reacting anti-AAV antibodies in response to naturally occurring AAV infection have been well-documented.53 Similarly, cross-reactive anti-AAV antibodies were stimulated by administration of AAV8 vectors in hemophilia B dogs54 and in nonhuman primates,55 and preexisting anti-AAV antibodies have been documented in large animal models.56 In our study, we observed production of cross-reactive antibodies to AAV9 following administration of AAV8 vectors. Given the risk of stimulating cross-reacting anti-AAV in a naïve subject, we have demonstrated an additional benefit from coreceptor blockade with anti-CD4 mAb in terms of facilitating readministration with an AAV vector of a new serotype.
The mechanism for inhibiting transduction with an AAV2/8 vector encoding GFP following administration of the AAV2/9 vector encoding GAA seemed to be the presence of low-level anti-AAV8. Although neutralizing antibodies were not analyzed, it is known that binding antibodies often correlate with neutralizing antibodies and binding antibodies alone can interfere with transduction of the liver.56 For example, even extremely low anti-AAV neutralizing antibody titers can result in vector neutralization,57,58 and administration of an AAV2/8 vector to nonhuman primates with preexisting binding, nonneutralizing antibodies against AAV8, resulted in sequestration of the vector in the spleen, with poor transduction of the liver.59 Therefore, the inverse correlation of liver transduction with low-level anti-AAV8 does suggest that cross-reacting anti-AAV was the likely culprit.
These results are important, as they provide one avenue to readminister the vector that does not involve the use of prolonged and intense immunosuppressive regimens; it is also an important result in the context of a systemic disease affecting children, which may require vector readministration to achieve optimal efficacy or if efficacy is lost as patients become adults. Readministration has been critical to maintaining efficacy from AAV vector-mediated gene therapy in canine glycogen storage disease38,60 and hemophilia.37 Previously, it has been suggested that the merely switching serotypes would achieve complete efficacy from readministering an AAV vector, which might be questioned in the future due to the effect of cross-reacting anti-AAV antibodies in the current study.
Heart involvement is the main cause of mortality in infantile Pompe disease, and high anti-GAA antibody titers strongly correlated with cardiomyopathy and mortality in that population.1,43 Severely affected, infantile patients have a severe deficiency of GAA in association with cardiomyopathy, whereas patients with slightly higher GAA activity have late-onset Pompe disease and no cardiomyopathy.61 The effect of the anti-CD4 mAb was important, because the biochemical correction of the heart was greatly increased in mice that did not form antibodies following anti-CD4 mAb treatment. While anti-CD4 mAb did not prevent mortality in the GAA-KO mice studied here, we would expect improved clinical outcomes if GAA deficiency could be corrected in the heart by an effective gene therapy.
At present, the control of immune responses to ERT in Pompe disease is limited to immunosuppressive agents with known toxicities, which emphasizes the need for more specific, safer immune modulation. Given this current suppression of antibody responses obtained with a short course of nondepleting anti-CD4 mAb, further preclinical development of this approach is warranted in the context of gene therapy for Pompe disease and other inherited disorders of metabolism.
Supplementary Material
Acknowledgments
This work was supported by Grant # 241701 from the Muscular Dystrophy Association of the United States and by a grant from Genethon. The AAV packaging plasmids were provided courtesy of Dr. James M. Wilson at the University of Pennsylvania (Philadelphia, PA). GAA-KO mice were provided courtesy of Dr. Nina Raben at the National Institute of Arthritis and Musculoskeletal and Skin Disorders. Nondepleting anti-CD4 mAb (YTS177) was provided by Tolerx, Inc. (Cambridge, MA).
Author Disclosure Statement
D.K. has received research/grant support from Genzyme Corporation. No competing financial interests exist for any of the other authors.
References
- 1.Kishnani PS, Goldenberg PC, Dearmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien YH, Lee NC, Thurberg BL, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics 2009;124:e1116–e1125 [DOI] [PubMed] [Google Scholar]
- 3.De Vries JM, Van Der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab 2010;101:338–345 [DOI] [PubMed] [Google Scholar]
- 4.Banugaria SG, Patel TT, Mackey J, et al. Persistence of high sustained antibodies to enzyme replacement therapy despite extensive immunomodulatory therapy in an infant with Pompe disease: need for agents to target antibody-secreting plasma cells. Mol Genet Metab 2012;105:677–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amalfitano A, Bengur AR, Morse RP, et al. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med 2001;3:132–138 [PubMed] [Google Scholar]
- 6.Markic J, Polic B, Kuzmanic-Samija R, et al. Immune modulation therapy in a CRIM-positive and IgG antibody-positive infant with Pompe disease treated with alglucosidase alfa: a case report. JIMD Rep 2012;2:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markic J, Polic B, Stricevic L, et al. Effects of immune modulation therapy in the first Croatian infant diagnosed with Pompe disease: a 3-year follow-up study. Wien Klin Wochenschr 2014;126:133–137 [DOI] [PubMed] [Google Scholar]
- 8.Messinger YH, Mendelsohn NJ, Rhead W, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med 2012;14:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banugaria SG, Prater SN, Patel TT, et al. Algorithm for the early diagnosis and treatment of patients with cross reactive immunologic material-negative classic infantile Pompe disease: a step towards improving the efficacy of ERT. PLoS One 2013;8:e67052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raben N, Roberts A, and Plotz PH. Role of autophagy in the pathogenesis of Pompe disease. Acta Myol 2007;26:45–48 [PMC free article] [PubMed] [Google Scholar]
- 11.Raben N, Schreiner C, Baum R, et al. Suppression of autophagy permits successful enzyme replacement therapy in a lysosomal storage disorder—murine Pompe disease. Autophagy 2010;6:1078–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spampanato C, Feeney E, Li L, et al. Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol Med 2013;5:691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raben N, Danon M, Gilbert AL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 2003;80:159–169 [DOI] [PubMed] [Google Scholar]
- 14.Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005;12:876–884 [DOI] [PubMed] [Google Scholar]
- 15.Sun B, Kulis MD, Young SP, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe disease. Mol Ther 2010;18:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Sun B, Osada T, et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine Pompe disease. Hum Gene Ther 2012;23:460–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao O, Dobrzynski E, Wang L, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood 2007;110:1132–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banati M, Hosszu Z, Trauninger A, et al. Enzyme replacement therapy induces T-cell responses in late-onset Pompe disease. Muscle Nerve 2011;44:720–726 [DOI] [PubMed] [Google Scholar]
- 19.Benjamin RJ, and Waldmann H. Induction of tolerance by monoclonal antibody therapy. Nature 1986;320:449–451 [DOI] [PubMed] [Google Scholar]
- 20.Qin SX, Cobbold S, Benjamin R, et al. Induction of classical transplantation tolerance in the adult. J Exp Med 1989;169:779–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayward AR, Shriber M, Cooke A, et al. Prevention of diabetes but not insulitis in NOD mice injected with antibody to CD4. J Autoimmun 1993;6:301–310 [DOI] [PubMed] [Google Scholar]
- 22.Nissler K, Pohlers D, Huckel M, et al. Anti-CD4 monoclonal antibody treatment in acute and early chronic antigen induced arthritis: influence on macrophage activation. Ann Rheum Dis 2004;63:1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karim M, Feng G, Wood KJ, et al. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: antigen-specific reactivation in vivo is critical for bystander regulation. Blood 2005;105:4871–4877 [DOI] [PubMed] [Google Scholar]
- 24.Sun B, Banugaria SG, Prater SN, et al. Non-depleting anti-CD4 monoclonal antibody induces immune tolerance to ERT in a murine model of Pompe disease. Mol Genet Metab 2014;1:446–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh JH, Cochrane M, Cobbold S, et al. Successful attenuation of humoral immunity to viral capsid and transgenic protein following AAV-mediated gene transfer with a non-depleting CD4 antibody and cyclosporine. Gene Ther 2011;19:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarty DM, Fu H, Monahan PE, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther 2003;10:2112–2118 [DOI] [PubMed] [Google Scholar]
- 27.Sun B, Zhang H, Franco LM, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005;11:57–65 [DOI] [PubMed] [Google Scholar]
- 28.Gao GP, Alvira MR, Wang L, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 2002;99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amalfitano A, Mcvie-Wylie AJ, Hu H, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A 1999;96:8861–8866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding E, Hu H, Hodges BL, et al. Efficacy of gene therapy for a prototypical lysosomal storage disease (GSD-II) is critically dependent on vector dose, transgene promoter, and the tissues targeted for vector transduction. Mol Ther 2002;5:436–446 [DOI] [PubMed] [Google Scholar]
- 31.Scully R, Qin S, Cobbold S, et al. Mechanisms in CD4 antibody-mediated transplantation tolerance: kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol 1994;24:2383–2392 [DOI] [PubMed] [Google Scholar]
- 32.Winsor-Hines D, Merrill C, O'Mahony M, et al. Induction of immunological tolerance/hyporesponsiveness in baboons with a nondepleting CD4 antibody. J Immunol 2004;173:4715–4723 [DOI] [PubMed] [Google Scholar]
- 33.Sun B, Li S, Bird A, et al. Antibody formation and mannose-6-phosphate receptor expression impact the efficacy of muscle-specific transgene expression in murine Pompe disease. J Gene Med 2010;12:881–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khanna R, Flanagan JJ, Feng J, et al. The pharmacological chaperone AT2220 increases recombinant human acid alpha-glucosidase uptake and glycogen reduction in a mouse model of Pompe disease. PLoS One 2012;7:e40776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun B, Zhang H, Franco LM, et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther 2005;11:889–898 [DOI] [PubMed] [Google Scholar]
- 36.Halbert CL, Standaert TA, Aitken ML, et al. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol 1997;71:5932–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang LL, Calcedo R, Nichols TC, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV28-mediated liver-directed gene therapy. Blood 2005;105:3079–3086 [DOI] [PubMed] [Google Scholar]
- 38.Demaster A, Luo X, Curtis S, et al. Long-term efficacy following readministration of an adeno-associated virus vector in dogs with glycogen storage disease type Ia. Hum Gene Ther 2012;23:407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraites TJ, Schleissing MR, Shanely RA, et al. Correction of the enzymatic and functional deficits in a model of Pompe disease using adeno-associated virus vectors. Mol Ther 2002;5:571–578 [DOI] [PubMed] [Google Scholar]
- 40.Mah C, Pacak CA, Cresawn KO, et al. Physiological correction of Pompe disease by systemic delivery of adeno-associated virus serotype 1 vectors. Mol Ther 2007;15:501–507 [DOI] [PubMed] [Google Scholar]
- 41.Cresawn KO, Fraites TJ, Wasserfall C, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 2005;16:68–80 [DOI] [PubMed] [Google Scholar]
- 42.Sun B, Bird A, Young SP, et al. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet 2007;81:1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banugaria SG, Prater SN, Ng YK, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med 2011;13:729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo X, Hall G, Li S, et al. Hepatorenal correction in murine glycogen storage disease type I with a double-stranded adeno-associated virus vector. Mol Ther 2011;19:1961–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P, Luo X, Bird A, et al. Deficiency in MyD88 signaling results in decreased antibody responses to an adeno-associated virus vector in murine Pompe disease. Biores Open Access 2012;1:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegler RJ, Bercury SD, Fidler J, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther 2008;19:609–621 [DOI] [PubMed] [Google Scholar]
- 47.Wang G, Young S, Bali D, et al. Assessment of toxicity and biodistribution of recombinant AAV2/8 vector-mediated immunomodulatory gene therapy in mice with Pompe disease. Mol Ther Methods Clin Dev 2014;1:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun B, Young SP, Li P, et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol Ther 2008;16:1366–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman BE, Dobrzynski E, Wang L, et al. Muscle as a target for supplementary factor IX gene transfer. Hum Gene Ther 2007;18:603–613 [DOI] [PubMed] [Google Scholar]
- 50.Nimmerjahn F, Lux A, Albert H, et al. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A 2010;107:19396–19401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu F, Ding E, Liao SX, et al. Improved efficacy of gene therapy approaches for Pompe disease using a new, immune-deficient GSD-II mouse model. Gene Ther 2004;11:1590–1598 [DOI] [PubMed] [Google Scholar]
- 52.Li H, Murphy SL, Giles-Davis W, et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol Ther 2007;15:792–800 [DOI] [PubMed] [Google Scholar]
- 53.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus types 1, 2, 5, 6, 8 and 9 in the healthy population: implications for gene therapy using AAV sectors. Hum Gene Ther 2010;21:704–771 [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Cao O, Swalm B, et al. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther 2005;12:1453–1464 [DOI] [PubMed] [Google Scholar]
- 55.Mingozzi F, Chen Y, Murphy SL, et al. Pharmacological modulation of humoral immunity in a nonhuman primate model of AAV gene transfer for hemophilia B. Mol Ther 2012;20:1410–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Louis Jeune V, Joergensen JA, Hajjar RJ, et al. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum Gene Ther Methods 2013;24:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, Lillicrap D, Patarroyo-White S, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood 2006;108:107–115 [DOI] [PubMed] [Google Scholar]
- 58.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 2006;107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Calcedo R, Bell P, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011;22:1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinstein DA, Correia CE, Conlon T, et al. Adeno-associated virus-mediated correction of a canine model of glycogen storage disease type Ia. Hum Gene Ther 2010;21:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirschhorn R, and Reuser AJJ. Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency. In: The Metabolic and Molecular Basis for Inherited Disease. Scriver CR, Beaudet AL, Sly WS, and Valle D, eds. (McGraw-Hill, New York, NY: ) 2001; pp. 3389–3419 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.