Abstract
Background
While accumulating clinical trials have focused on the impact of cell-therapy in patients with acute MI and ischemic cardiomyopathy, there are fewer efforts to examine cell-based therapy in patients with non-ischemic cardiomyopathy (NICM). We hypothesized that cell-therapy could have a similar impact in NICM.
Methods/Results
The POSIDEON-DCM trial is a phase I/II trial designed to address autologous vs. allogeneic bone marrow derived MSCs in patients with NICM. In this study, cells will be administered transendocardially with the NOGA injection-catheter system to patients (n=36) randomly allocated to two treatments groups: Group 1 (n=18 auto-hMSCs) and Group 2 (n=18 allo-hMSCs). The primary and secondary objectives are, respectively, to demonstrate the safety and efficacy of allo-hMSCS vs. auto-hMSCs in patients with NICM.
Conclusions
This study will establish safety of transendocardial injection of stem cells (TESI), compare phenotypic outcomes, and offer promising advances in the field of cell-based therapy in patients with NICM.
Keywords: Bone Marrow Cells, Dilated Cardiomyopathy, Stem Cells, Heart Failure
Introduction
Non-ischemic dilated cardiomyopathy (NICM) is a complex disorder, associated with many primary and secondary etiologic factors, affecting 5 to 8 per 100,000 persons per year1. As with other causes of heart failure2, the morbidity and mortality of NICM remains high despite recent advances in pharmacological and device therapy. The age spectrum affected by NICM not only includes children and adults, but also neonates1. NICM more commonly affects middle-aged males than females. Although ischemic cardiomyopathy is more prevalent than NICM, these two diagnoses account for equal number of heart transplantations performed1.
Recently, cell based therapies have evolved in treating various ischemic3–5 and dilated cardiomyopathies6, yet there is no clear cut consensus about which type of stems cells should be used and how should they be delivered to the affected myocardium7. The only definitive therapy for NICM remains heart transplantation, which is only available to a specific patient population. Cellular cardiomyoplasty for chronic heart failure has been studied less extensively than for acute MI, but represents a potentially important alternative for this disease.
The purpose of the POSEIDON-DCM study is to address several key questions regarding cell-based therapy in patients with NICM. This study will address the safety of intramyocardial injections of bone marrow hMSCs in patients with NICM, and importantly, will compare safety and efficacy of allogeneic vs. autologous therapy in this population. Additionally, the study design incorporates important mechanistic sub-studies. This trial will advance emerging insights from early stage trials of cell therapy for ischemic heart disease to a population with substantial unmet needs, those with non-ischemic disorders of heart muscle.
Methods
Study objectives
The primary objective of the study is to demonstrate the safety of allogeneic hMSCs delivered by transendocardial injections (TESI) in patients with nonischemic dilated cardiomyopathy (DCM), and the secondary objective is to compare the safety as well as efficacy of allogeneic hMSCs to autologous hMSCs in the same patient population.
Study design
This is a pilot study, intended as a safety assessment prior to a full comparator study, and cells will be administered via The Biosense Webster Myostar NOGA injection catheter system. Cell administration will be tested in 36 patients equally divided in two groups
All patients will provide written informed consent on the University of Miami Institutional Review Board approved protocol. Then, upon successfully fulfilling inclusion exclusion criteria (Tables 1 & 2), patients will be randomized in 1:1 ratio to one of the 2 following treatment strategies:
Table 1.
Major Inclusion Criteria
|
|
Table 2.
Major Exclusion Criteria
|
Group 1 (18 patients) - Auto-hMSCs: 20 million cell/ml delivered transendocardially in a dose of 0.5 ml per injection x 10 injections for a total of 1 x 108 (100 million) auto-hMSCs.
Group 2 (18 patients) - Allo-hMSCs: 20 million cell/ml delivered transendocardially in a dose of 0.5 ml per injection x 10 injection for a total of 1 x 108 (100 million) allo-hMSCs.
If the patient is randomized to group 1 (auto-hMSCs) and the auto-hMSCs do not expand to the required dose of 1 x 108 cells, then each patient will receive the maximum number of cells available, not to be less 0.8 x 108 (80 millions) cells. For patients randomized to Group 1 (auto-hMSCs), the cells will be derived via bone marrow aspiration (BMA) approximately 4–6 weeks prior to cardiac catheterization. For patients randomized to Group 2 (allo-hMSCs), the cells will be supplied by an allogeneic human mesenchymal stem cell source manufactured at the University of Miami Cell Production Facility8. The injections will be administered transendocardially during cardiac catheterization using the Biosense Webster MyoStar NOGA Catheter system (Figure 1A), which will be provided by Johnson & Johnson. Following the injection procedure, patients will be hospitalized for a minimum of 2 days and then have a follow up visit at 2 weeks post cardiac catheterization, and at months 2, 3, 6, and 12 post-cardiac catheterization to complete all the safety and efficacy assessments. (See Table 3 for complete end points).
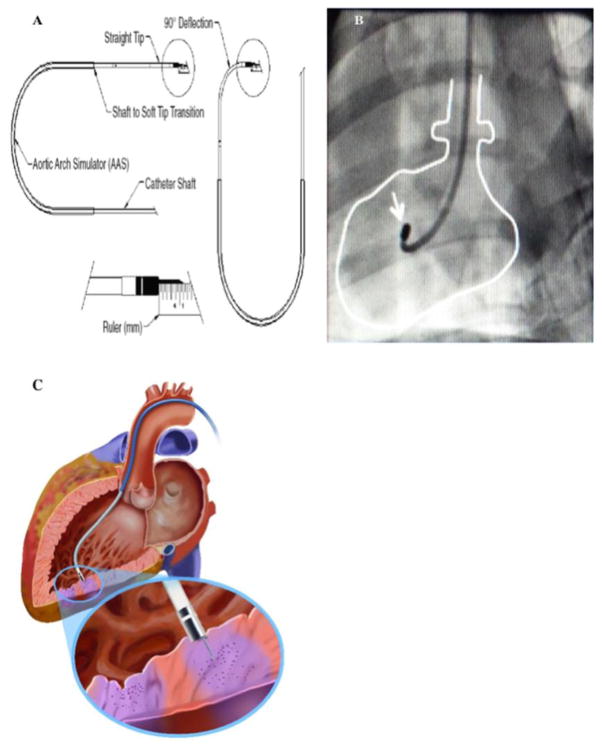
Fig. 1. NOGA-MYOSTAR injection catheter.
Injection catheter parts shown in (A), and heart tracing in (B) shows catheter within left ventricle under fluoroscopy; white arrow points to tip of catheter. (C) displays an exteriorized needle tip, engaged with endocardium, injecting cells (purple).
Table 3.
Time and Events
| Study Procedure | Screening (−10 to−5 wk) | Baseline visit 1 (− 6 to −2 wk) | Baseline visit 2 (−6 to −2 wk) | Day 1 | Day 2 | Day 14±3 | Month 2 | Month 3 | Month 6 | Month 12 |
|---|---|---|---|---|---|---|---|---|---|---|
| Informed consent | X | |||||||||
| History and physical | X | X | X | X | X | X | X | X | X | |
| Vital signs | X | X | X | X | X | X | X | X | X | |
| 12- lead EKG | X(##) | X | X | X | X | X | X | X | ||
| Concomitant medications | X | X | X | X | X | X | X | X | ||
| Randomization | X | |||||||||
| Bone marrow aspiration | X(Gp. 1) | |||||||||
| Catheterization | X | |||||||||
| Endomyocardial biopsy | X | |||||||||
| Investigational agent | X | |||||||||
| Stand post procedure care | X | X | ||||||||
| CT assessment of heart, chest abdomen and pelvis | X | |||||||||
| MRI assessment of the heart | X | X | ||||||||
| Echocardiogram | X | X($) | X | X | X | X | ||||
| Treadmill determination of pssseak VO2 | X | X | X | |||||||
| Six Minute walk test | X | X | X | |||||||
| NYHA functional class | X | X | X | X | X | |||||
| MLHF questionnaire | X | X | X | X | X | |||||
| IIEF (male) and SQOL (female) | X | X | X | X | X | |||||
| Pulmonary function (FEV1) | X | X(***) | X | X | X | X | ||||
| 48 hour ambulatory EKG | X(##) | X | X | X | X | X | X | X | ||
| Serum troponin & CK-MB(**) | X | X | ||||||||
| Hematology and clinical% chemistry, BNP, uric acid, and CRP immune monitoring(XX) | X, XX | X,XX | X | X,XX | X | X | X,XX | X | ||
| Retinal examination | X | X | X | |||||||
| Urinalysis | X | X | X | X | X | X | X | X | ||
| Serum or urine pregnancy test | X | X($$) | X | X | X | |||||
| HIV-1, 2 & Hepatitis B, C | X | |||||||||
| Donor screening tests | X | |||||||||
| Biomarkers assessment(%%) | X(Gp. 1) | X(Gp. 2) | ||||||||
| Adverse events | X | X | X | X | X | X | X | X | X | X |
All baseline visit tests will occur within 28 days or the final screening visit
If there is a sustained or short run of ventricular tachycardia on the 12-lead ECG or 48 hour ambulatory ECG obtained in the screening phase testing, the patient will be removed from the study
Serial Troponins and CK-MB laboratory assays will be performed every 12 hours of the first 48 hours post-cardiac catheterization
Unless patient is not capable, then 48 hours prior to discharge
- Hematological Tests: white blood cell count, platelet count, hemoglobin and hematocrit
- Liver Function Tests: albumim, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, prothrombin time, activated partial thromboplastin time, and bilirubin
- Renal Function Tests: creatinine, blood urea nitrogen, creatinine clearance, glomerular filtration rate, sodium, potassium, chloride, bicarbonate, glucose, serum uric acid, BNP, and C-reactive protein (CRP)
Following biomarkers will be analyzed:
Cell Surface markers: CXCR4, C-kit & Connexin 43
Trancriptomic/Proteome: RNA, miRNA, protein samples and telomerase, akt
Growth factor: Sdf-1, notch
Functional assays: cell growth rate and CFU assay
All subjects will undergo transthoracic echocardiogram assessment of overall and regional LV systolic function at baseline, day 2, and months 3, 6, and 12. There will be a transthoracic echocardiographic assessment immediately following the catheterization procedure, and 4–6 hours later.
A serum or urine pregnancy test will be completed within 36 hours prior to injection.
Immune monitoring for graft rejection. The following markers will be used for analysis to assess for activated T-cells based upon a CD3+CD25+ or CD3+CD69+ phenotype: CD3, CD25, CD69
Outcome measures for safety
The primary outcome measures for safety will include the incidence (at one month post catheterization) of any treatment emergent serious adverse events (TE-SAE), defined as the composite of death, non-fatal MI, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, sustained ventricular arrhythmias (characterized by ventricular arrhythmias lasting more than 15 seconds or with hemodynamic compromise), or any other potential late effects detected and corroborated by clinical presentation, laboratory investigations, imaging analyses and when necessary, with biopsy from suspected target sites in the body.
The secondary safety endpoints will be evaluated in this trial at the six month follow up period and at the final 12 month post injection time point. They will include treatment emergent adverse events, ectopic tissue formation (as identified from CT scans of chest, abdomen and pelvis), 48 hour ambulatory EKG recording, hematology, clinical chemistry and urinalysis values, Pulmonary Function (measured by forced expiratory volume in one second FEV1), serial troponin and CKMB values (every 12 hours for the first 48 hours post cardiac catheterization) and post cardiac catheterization echocardiogram (day 1 post catheterization).
Myocardial infarction (MI) will be defined by an adaptation of the diagnostic criteria for MI with coronary bypass graft surgery, as outlined in a recent consensus document that has become the authoritative standard for the definition of MI9. A procedure-related MI will be defined within the first 48 hours after study agent delivery if at least 2 of the following 3 criteria are met:
Typical ischemic cardiac pain lasting at least 30 minutes
Troponin I values more than 5 times the 99th percentile of the normal reference range or creatinine-kinase-MB levels more than 5 times of the 99th percentile of the normal reference range during the first 48 hours after transendocardial cell delivery
New pathological Q waves or new left bundle branch block in conjunction with the echocardiographic evidence of new loss of viable myocardium.
Myocardial perforation will be considered to have occurred if
there is a new pericardial effusion >1 cm thickness or
new ventricular septal defect is detected by Doppler echocardiography immediately after, 4–6 hours after, or on day 2 post catheter injection or
the tip or any part of the catheter system is observed under fluoroscopy to exit the left ventricular cavity across its myocardium, even if neither pericardial effusion nor ventricular septal defect results from the catheter exit.
Outcome measure for efficacy
Efficacy endpoints will be evaluated at the 6-month and 12-month follow up visits. These will include MRI, CT and echocardiographic measures of the left ventricular function difference between the baseline, 6 month (echocardiogram only) and 12 month, scar size10 (ISS) as determined by delayed contrast enhanced CT or MRI, difference between the baseline and 12 month regional left ventricular function at the site or autologous cell injection as determined by CT or MRI, difference between the baseline and 12 month regional left ventricular wall thickening as determined by CT or MRI. It would also include the differences between the baseline, 6-month (echocardiogram only) and 12 month left ventricular end diastolic wall thickness as determined by CT/MRI and echocardiogram, differences between the baseline, 6-month (echocardiogram only) and 12 month left ventricular ejection fraction, end diastolic and end systolic volumes as determined by CT/MRI and echocardiogram, and differences between the baseline and 12 month left ventricular regional myocardial perfusion as determined by CT/MRI.
In addition to the above the additional end points will include tissue perfusion measured by CT/MRI, Peak VO2, Six Minute Walk test, NYHA classification, Minnesota Living with Heart Failure (MLHF) questionnaire, and incidence of Major cardiac events (MACE) endpoint, defined as composite incidence of (1) death, (2) hospitalization for worsening heart failure or (3) non fatal recurrent MI.
Blinding/randomization
This will be a non-blinded full comparator randomized trial. Patients will be randomized to treatment strategy (Auto-hMSCs vs Allo-hMSCs) in a 1:1 ratio. The treatment assignment will be sent via email to the cell therapy laboratory.
Cell harvesting/processing
Bone marrow (BM) will be harvested from patients and normal volunteers with 60 ml aspirated from the posterior iliac crest under moderate sedation and local anesthesia by a hematologist. All the standard pre-procedure aseptic techniques will be applied. The BM aspiration will be done with a special needle attached to heparinized syringes. The mononuclear fraction (MNC) will be isolated using a density gradient with Lymphocyte Separation Medium (MediaTech Inc, Manasas, VA) (LSM; specific gravity 1.077). The low-density cells will be collected from the gradient and washed with Plasma-Lyte A (Baxter, Deerfield, IL) containing 1% human serum albumin (HAS). The washed cells will be sampled and viable cell counts performed to determine the total number of viable cells.
For Group 1 (auto-hMSCs), the required dose of MSCs will be generated using standard conditions. Preclinical validations studies have determined the reproducible generation of >200 million MSCs in 21–28 days of culture. The BM MNC will be seeded into 225 cm2 tissue culture flasks in α MEM containing 20% FBS. After 14 days of culture passage, zero (P0) cells are harvested by trypsin treatment and expanded into 60 flasks. These flasks are incubated for a further 7 to 10 days and the MSCs are harvested by trypsin treatment (P1 cells). The P1 cells are washed and viability counts determined8.
The MSCs will be resuspended in cryoprotectant consisting of Pentaspan (10% pentastarch in 0.9% sodium chloride) supplemented with 2% HSA and 5% dimethyl sulfoxide. The cells will then be frozen and stored in a liquid nitrogen freezer.
Upon request, the cells will be thawed in a 37°C water bath. In a biosafety cabinet, the cell suspension will be transferred to conical tubes and slowly diluted with a phosphate- buffered saline (PBS) supplemented with 1% HSA or Plasma-Lyte A supplemented with 1% HSA. The suspension will be centrifuged, and the cell pellet, resuspended in the dilution buffer. Viability counts will be performed, and the cells will be delivered to the catheterization laboratory. For group 2 (allo-hMSCs), the same above protocol is used.
Endomyocardial biopsy
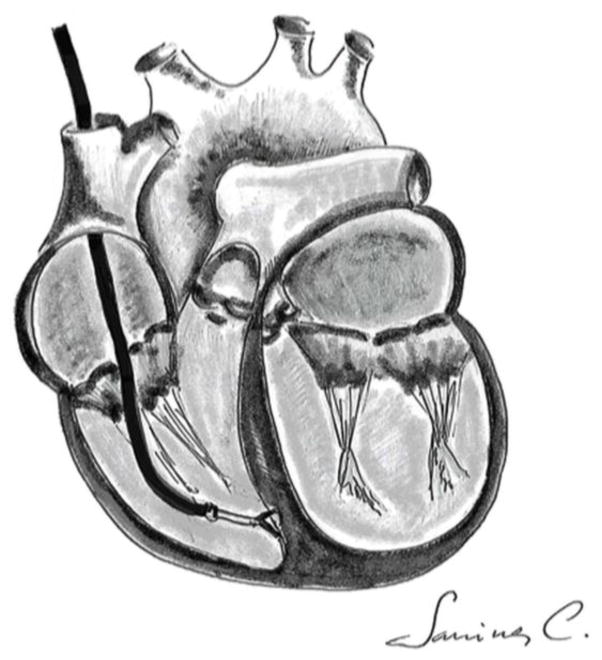
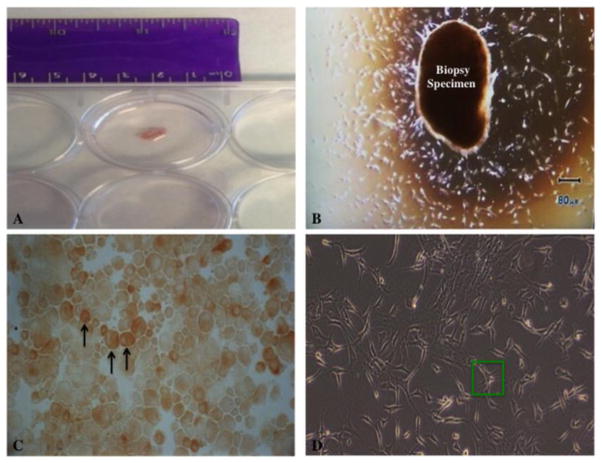
Patients will undergo a right heart catheterization preceding the investigational agent administration to obtain 2 to 4 heart biopsy samples. The right Internal Jugular vein (RIJ) will be accessed after the insertion of an introducer sheath using the Seldinger technique. A 5.5 French JAWZ-Endomyocardial biopsy forceps (Maxi-Curved 50 cm) will then be inserted into the sheath using fluoroscopic guidance. The catheter will be rotated 180 degrees at the border of right atrium and then advanced to right ventricle (RV). A PVC is noted when the catheter will touch the wall of right ventricle and approximately 2 to 4 biopsy samples will be taken (Figure 2). All samples will be identified so that they can be linked to individual patients. Heart tissue obtained from the biopsy will be cultured to obtain cardiac stem cells11 (Figure 3A & B). The c-Kit+ cells will be isolated with the help of magnetic selection12. These cells will be grown in cell culture and then they will be stored at P3 (Figures 3C & D). The other samples will be used for gene expression profiling13 and one sample will be send to the pathology lab for histological diagnosis. These samples may be stored indefinitely. Data presented in publication will not contain an individual patient’s gene expression or clinical characteristics or outcome; only aggregate data from the entire study will be disclosed.
Fig. 2.
Endomyocardial biopsy technique using the bioptome
Fig. 3. Cell harvesting and culture.
(A) and (B) show endomyocardial biopsy specimen in culture dish. Several days after culture, in (B), mixed cell population proliferate peripherally around specimen. Black arrows in (C) point to immunostained c-Kit+ cells post-magnetic selection. (D) shows isolated c-Kit+ cells at P3 stage, with an example of a single c-Kit+ cell in green rectangle.
Injection
Cardiac catheterization will be done by percutaneous femoral arterial access. An 8F vascular sheath will be inserted. Heparin (unfractionated heparin, 50 U/kg body weight) will be administered intravenously, and additional doses will be given as needed to achieve an activated clotting time (ACT) of ≥250 sec. The ACT will be re-checked every 30 minutes and heparin re-dosed as needed to maintain an ACT of ≥250 sec during the injection procedure.
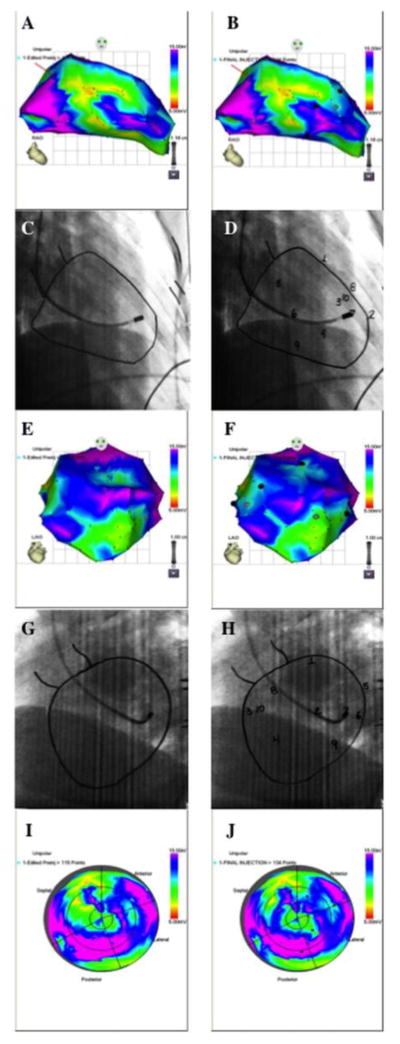
The NOGA XP Cardiac Navigation System will be used to create an electroanatomical map of the left ventricle to guide the transendocardial injections. A NOGA-STAR mapping catheter will be inserted into the femoral introducer and guided to the aortic root via fluoroscopy. The catheter tip will be placed in full flexion to cross the aortic valve. Once in the LV, the catheter tip will be straightened for mapping. The catheter tip will be placed at various points throughout the LV to acquire real-time electrical and anatomical positioning, which is displayed on a monitor in the catheterization laboratory. These points are acquired by sweeping the catheter from the apex to the basal aspect of the LV to include the septal, anterior, inferior, and lateral walls. As points are acquired, a 3-D electroanatomical map will be displayed on the NOGA-XP monitor (Figure 4). Once a satisfactory number of points (approximately 90) are acquired, the NOGA-STAR mapping catheter will be removed from the LV and withdrawn from the femoral introducer.
Fig. 4. NOGA XP Cardiac Navigation System.
NOGA mapping projections of the left ventricle in RAO views pre-injection (A) and post-injection (B). Corresponding fluoroscopic images with endocardial tracings are shown in (C) and (D). Numbers indicate injection sites. NOGA mapping projections of the left ventricle in LAO views pre-injection (E) and post-injection (F), and corresponding fluoroscopic images with endocardial tracings are displayed in (G) and (H). Regional, or “bull’s eye”, NOGA mapping views can be seen in (I) and (J) pre- and post-injection, respectively.
The NOGA-MYOSTAR injection catheter will then be inserted into the femoral introducer and guided to the aortic root under fluoroscopy. The tip of the catheter will be placed in full flexion to cross the aortic valve and the curve relaxed once in the LV chamber. Using the 3-D electroanatomical map, the location of the NOGA-MYOSTAR catheter’s tip will be displayed on the monitor. The catheter tip will then be guided by this map to the target areas for injection. No injections will be performed on areas of the LV in which the wall diameter is less than two times the depth of injection.
While catheterization is being accomplished, the MSC suspension will be readied on a sterile field in the catheterization laboratory. Cell suspension will be prepared in aliquots of [20M MSCs/ml] in individual 1cc syringes, and maintained at room temperature until administered. Syringes will be gently inverted to maintain cells in suspension prior to injection.
By adjusting catheter deflection, torque, and position, the NOGA-MYOSTAR catheter will be manipulated to engage the endocardium of the LV at the first point within the target zone. (Figures 1B & C) Using the NOGA electroanatomical map and fluoroscopic imaging in both the RAO and LAO projections (Figures 4A, C, E, G, & I), the cardiologist will confirm that the tip of the injection catheter is in the selected target territory by confirming a loop stability of less than 4 as shown on the NOGA-XP system before exteriorizing the needle tip into the endocardium. To confirm engagement of the needle tip with the myocardium, a premature ventricular contraction (PVC) will be seen on the ECG monitor. If no PVC is noted, the needle will be retracted into the catheter and the above steps repeated until a PVC is noted on ECG after exteriorizing the needle into the myocardium.
Injection of the cell suspension into the myocardium via the needle will be done by slow infusion of 0.5 ml from the aliquot syringe with a rate of 0.1mL per 15 seconds. The site of injection will be marked on the NOGA XP system (Figures 4B, F, & J) as well as the endocardial tracings (Figures 4D & H). Once the aliquot is injected, the needle will be retracted into the catheter. The cardiologist will then maneuver the catheter system into another unique endocardial position and repeat the process for the 2nd and subsequent injection sites.
A maximum of 0.5 ml per injection, and 5 ml total volume, will be injected into a goal of 10 sites. The pattern of these sites will be selected by the cardiologist, who will attempt to encompass a uniform distribution throughout the left ventricle area. Injection sites will be at least 5 mm (measured in two planes on the two plastic tracings of the endocardial border) distant from adjacent injection sites. The point of the apex of the LV will not be selected as an injection site.
Intramyocardial injections will be discontinued during the procedure if one or more of the following occur:
The patient complains of severe chest pain
The patient develops a sustained ventricular arrhythmia requiring cardioversion
The patient experiences a sustained drop in blood pressure exceeding 20mmHg
After the final injection and retraction of the needle into the catheter tip, the NOGA-MYOSTAR catheter will be relaxed to a straight configuration and withdrawn from the body. A final left ventricular cine angiogram will be repeated in the same projections as before.
Dose rationale
The dose for both auto-hMSC and allo-hMSC preparations in both arms of the study is the same: 1 x 108 cells (20 million cells/mL delivered in 10 injections of 0.5 ML each). In two preclinical studies using a porcine model14, 15, MSC therapy was safely administered via intramyocardial injections at a dose of up to 1 x 108 cells, thus supporting the use of this dose level for hMSC preparation in this clinical study. The hMSC doses were also chosen based on practical considerations and ability to grow this quantity of cells for more patients within an approximate 28-day time frame.
Statistical analysis
Analysis of the primary end point will be focused on characterizing the serious adverse events (SAE) portion in each treatment arm, whereas secondary efficacy end points will be used in the development of a larger efficacy study. Analyses of the 2 treatment groups will be conducted in comparison with each other. The per-treatment arm sample sizes were generated based on assumption of 30-day SAE proportion of 25%, which translates into between 4 and 5 patients out of 18 patients with events. The per-treatment arm sample sizes were generated based on an assumption of a 30 day SAE proportion of 25%. In this setting, the confidence interval (CI) length for a binominal proportion is 33%, ranging from 6% to 48%, with a probability of 59% to rule out an SAE proportion of 50%.
Bayesian-motivated safety stopping guidelines will be used to monitor adverse events including grades 3–5 central nervous system cerebrovascular ischemia, grades 4–5 prolonged arrhythmia, grades 3–5 pericardial effusion, or death as defined by the Common Terminology Criteria for Adverse Events version 4.0.
Descriptive analyses of all secondary end points will be performed using point and CI estimation. Treatment effects will be assessed using appropriate methods for continuous, dichotomous, or ordinal categorical data. Reported measurements analyses will be used for data collected at various time points.
Safety and monitoring
Interim analyses will be conducted by an independent Data and Safety Monitoring Board (DSMB), which will be notified each time an SAE occurs. The DSMB will evaluate AE data (including SAEs) in each Group (1 or 2) at pre-specified intervals. Monitoring of key safety endpoints will be conducted, and if rates significantly exceed preset thresholds, the DSMB Chair will be notified and information will be supplied to the DSMB.
Discussion
Dilated cardiomyopathy (DCM) accounts for approximately one quarter of the cases of congestive heart failure in the United States16. The majority of the other causes are due to either ischemic or hypertensive cardiomyopathies17, or non-systolic heart failure18. The hallmark of this disease is an enlarged, remodeled ventricle with increased end-diastolic and end-systolic volumes, reduced ejection fraction, impaired contractility, and diastolic dysfunction. The prognosis of DCM may be more variable then previously appreciated19. Some patients may have stable disease for several years, or even decades, whereas others may experience a completely different course. The period of stability has been linked to reverse remodeling, which could be spontaneous or in response to pharmacological or device therapy. Microarray transcriptomic analysis revealed a gene signature that predicts the long term event-free survival compared with poor prognosis manifesting as death or need for heart transplant within two years20, 21. While there is accumulating data supporting novel cell based therapies as providing benefit in the ischemic model of myocardial injury by improving cardiac function and causing reverse remodeling, there are substantially fewer trials examining stem cell therapy for the treatment of DCM6, 22.
The POSEIDON-DCM study is designed to study safety as well as efficacy as its primary end point; it will also explore several key issues in cell based therapy such as determining the optimal cell type, number of cells injected, and delivery method. This trial will also address the issue of autologous vs. allogeneic stem cell transplantation. We have recently shown the use of allogeneic stem cells for the treatment of ischemic cardiomyopathy4, 7. Mesenchymal stem cells are both immunoprivileged and immunosuppressive, and thus can be used as allografts. We also performed randomized comparisons of allografting vs. autologous therapy in patients with ischemic cardiomyopathy. Both of these cell types were safe and each type demonstrated potential regenerative bioactivity in patients with ischemic cardiomyopathy by reducing infarct size and improving ventricular remodeling, as measured by sphericity index7. The patients receiving allograft in the study did not mount increased panel-reactive antibodies in response to therapy. If we are able to replicate these earlier findings in this trial as well, it would support the use of allogeneic mesenchymal stems cells as the preferred cell type source due to avoidance of the need for bone marrow aspiration in the patient, as well as earlier time to treatment.
The other key issue to be addressed in this study is the delivery method, using the Biosense Webster MyoStar NOGA Injection catheter System for the transendocardial delivery of mesenchymal stems cells. The NOGA XP Cardiac Navigation System will be used to create an electroanatomical map of the left ventricle to guide the transendocardial injections.
The advantage of transendocardial delivery include the ability to directly target the injection area of the myocardium while avoiding the need for open heart surgery and the potential for microvascular obstruction that can complicate intracoronary delivery23. Animal studies that have been conducted in the past, comparing intracoronary vs. transendocardial delivery24, demonstrate greater cell retention and improvements in global LV ejection fraction with transendocardial delivery and less potential to engraft in other organs compared with intravenous vs. intracardiac routes23. The potential disadvantages of the transendocardial delivery system include technical complexity and myocardial perforation. Initial and follow up animal and human studies have shown the safety and efficacy of the NOGA catheter system25–31. These are encouraging studies, though more robust studies are needed to determine the best approach to optimize efficacy without compromising safety. The POSIDEON-DCM study will provide crucial data using a novel transendocardial delivery system.
A crucial aspect of this work will be to explore the underlying basis for beneficial effects. MSCs exert their anti-remodeling effects through and orchestration of scar reduction, neovascularization, and stimulation of endogenous repair32, 33. All of these effects could be operative in the NICM setting, which is associated with myocardial fibrosis and diminished tissue perfusion. Moreover, several laboratories have documented the presence of c-kit cells in the hearts of patients with NICM34, 35. To advance mechanistic insights, patients in POSEIDON-DCM will undergo endomyocardial biopsy for culture expansion of c-kit+ CSCs.
Conclusion
In conclusion, POSEIDON-DCM study is designed to shed more light on the critical questions of optimal cell type, dose, donor source, delivery method, patient population and timing of delivery. This would also ease the burden of heart transplantion needed in patients with dilated cardiomyopathy and will give us new direction for the treatment of this debilitating disease.
Acknowledgments
All authors have provided the corresponding author with written permission to be names in the manuscript. They all have reviewed and approved the final version of the manuscript. The authors are solely responsible for the design and conduct of the study, interpretation of the data, and the contents of the article. The authors would like to thank Dr. Cristina Sanina for her artistic contribution, Mark Martin and Jonathan Wong who are employees of Biologics Delivery Systems, Cordis Corporation (Irwindale, CA) for their figure contributions, and Dr. Mendizabal who is an employee of the EMMES Corporation.
No human or animal studies were carried out by the authors for this article.
Funding
This study has been funded by NIH grant 5R01HL110737 to J.M.H. and the Starr Foundation. This trial is listed at clinicaltrials.gov (NCT01392625).
Footnotes
Disclosures
Drs. Hare and Heldman report equity interest in Vestion Inc.. Dr. Hare is a consultant to Kardia.
Author Contributions:
Dr. Mushtaq: Manuscript writing
Ms. DiFede: Conception and design, manuscript writing
Dr. Golpanian: Manuscript writing, final approval of manuscript
Dr. Khan: Conception and design
Dr. Gomes: Conception and design
Dr. Mendizabal: Conception and design
Dr. Heldman: Conception and design
Dr. Hare: Conception and design, final approval of manuscript, financial support
References
- 1.Hare JM. The dilated, restrictive and infiltrative cardiomyopathies. In: Bonow ROMDL, Zipes DP, Libby P, editors. Braunwald’s heart disease: A textbook of cardiovascular medicine. Philadelphia, PA: Saunders Elsevier; 2011. [Google Scholar]
- 2.Hare JM, Walford GD, Hruban RH, Hutchins GM, Deckers JW, Baughman KL. Ischemic cardiomyopathy: Endomyocardial biopsy and ventriculographic evaluation of patients with congestive heart failure, dilated cardiomyopathy and coronary artery disease. Journal of the American College of Cardiology. 1992;20:1318–1325. doi: 10.1016/0735-1097(92)90243-g. [DOI] [PubMed] [Google Scholar]
- 3.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yousef M, Schannwell CM, Kostering M, Zeus T, Brehm M, Strauer BE. The balance study: Clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. Journal of the American College of Cardiology. 2009;53:2262–2269. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 6.Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, Socan A, Schrepfer S, Torre-Amione G, Haddad F, Wu JC. Effects of intracoronary cd34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circulation research. 2013;112:165–173. doi: 10.1161/CIRCRESAHA.112.276519. [DOI] [PubMed] [Google Scholar]
- 7.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Silva JS, Hare JM. Cell-based therapies for myocardial repair: Emerging role for bone marrow-derived mesenchymal stem cells (mscs) in the treatment of the chronically injured heart. Methods in molecular biology. 2013;1037:145–163. doi: 10.1007/978-1-62703-505-7_8. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Biomarker S, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Subcommittee ECG, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging S, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification S, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Intervention S, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Trials Registries S, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Trials Registries S, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Trials Registries S, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Trials Registries S, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document R, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force Members C, Guidelines ESCCfP. Third universal definition of myocardial infarction. Journal of the American College of Cardiology. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki T, Miller CF, Hansford R, Zipunnikov V, Zviman MM, Marine JE, Spragg D, Cheng A, Tandri H, Sinha S, Kolandaivelu A, Zimmerman SL, Bluemke DA, Tomaselli GF, Berger RD, Halperin HR, Calkins H, Nazarian S. Impact of nonischemic scar features on local ventricular electrograms and scar-related ventricular tachycardia circuits in patients with nonischemic cardiomyopathy. Circulation Arrhythmia and electrophysiology. 2013;6:1139–1147. doi: 10.1161/CIRCEP.113.000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, Welt FG, Givertz MM, Mitchell RN, Leri A, Kajstura J, Pfeffer MA, Anversa P. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circulation research. 2011;108:857–861. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, Hall J, Kittleson MM, Baughman KL, Hare JM. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. European heart journal. 2010;31:1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. Journal of the American College of Cardiology. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 16.Parakh K, Kittleson MM, Heidecker B, Wittstein IS, Judge DP, Champion HC, Barouch LA, Baughman KL, Russell SD, Kasper EK, Sitammagari KK, Hare JM. The variable natural history of idiopathic dilated cardiomyopathy. The Israel Medical Association journal: IMAJ. 2012;14:666–671. [PubMed] [Google Scholar]
- 17.Hare JM. The etiologic basis of congestive heart failure. In: Colucci WS, editor. Atlas of heart failure. Philadelphia: Springer; 2008. pp. 29–56. [Google Scholar]
- 18.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 19.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 20.Heidecker B, Kasper EK, Wittstein IS, Champion HC, Breton E, Russell SD, Kittleson MM, Baughman KL, Hare JM. Transcriptomic biomarkers for individual risk assessment in new-onset heart failure. Circulation. 2008;118:238–246. doi: 10.1161/CIRCULATIONAHA.107.756544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidecker B, Kittleson MM, Kasper EK, Wittstein IS, Champion HC, Russell SD, Hruban RH, Rodriguez ER, Baughman KL, Hare JM. Transcriptomic biomarkers for the accurate diagnosis of myocarditis. Circulation. 2011;123:1174–1184. doi: 10.1161/CIRCULATIONAHA.110.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martino HF, Oliveira PS, Souza FC, Costa PC, Assuncao ESE, Villela R, Gaze M, Weitzel LH, Oliveira A, Jr, Muccillo FB, Arvelo SN, Sa R, Guimaraes TC, Tura BR, Campos de Carvalho AC. A safety and feasibility study of cell therapy in dilated cardiomyopathy. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica … [et al.] 2010;43:989–995. doi: 10.1590/s0100-879x2010007500093. [DOI] [PubMed] [Google Scholar]
- 23.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. European heart journal. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 24.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. Journal of molecular and cellular cardiology. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Perin EC, Silva GV, Fernandes MR, Munger T, Pandey A, Sehra R, Talcott M, Bichard CJ, Creed J, Wong JW, Oliveira EM, Zheng Y, Canales J, Cardoso CO, Patterson MS, Serruys PW. First experience with remote left ventricular mapping and transendocardial cell injection with a novel integrated magnetic navigation-guided electromechanical mapping system. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2007;3:142–148. [PubMed] [Google Scholar]
- 26.Mathiasen AB, Jorgensen E, Qayyum AA, Haack-Sorensen M, Ekblond A, Kastrup J. Rationale and design of the first randomized, double-blind, placebo-controlled trial of intramyocardial injection of autologous bone-marrow derived mesenchymal stromal cells in chronic ischemic heart failure (msc-hf trial) American heart journal. 2012;164:285–291. doi: 10.1016/j.ahj.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Duran JM, Taghavi S, Berretta RM, Makarewich CA, Sharp T, III, Starosta T, Udeshi F, George JC, Kubo H, Houser SR. A characterization and targeting of the infarct border zone in a swine model of myocardial infarction. Clinical and translational science. 2012;5:416–421. doi: 10.1111/j.1752-8062.2012.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharlamov AN, Duckers HJ, van Beusekom HM, Smits PC, Perin EC, Serruys PW. Do we have a future with transcatheter adventitial delivery of stem cells? International journal of cardiology. 2013;165:217–221. doi: 10.1016/j.ijcard.2012.11.063. [DOI] [PubMed] [Google Scholar]
- 29.Dib N, Dinsmore J, Lababidi Z, White B, Moravec S, Campbell A, Rosenbaum A, Seyedmadani K, Jaber WA, Rizenhour CS, Diethrich E. One-year follow-up of feasibility and safety of the first u.S., randomized, controlled study using 3-dimensional guided catheter-based delivery of autologous skeletal myoblasts for ischemic cardiomyopathy (causmic study) JACC Cardiovascular interventions. 2009;2:9–16. doi: 10.1016/j.jcin.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Smits PC, Nienaber C, Colombo A, Ince H, Carlino M, Theuns DA, Biagini E, Valgimigli M, Onderwater EE, Steendijk P, Peters NS, Goedhart DM, Serruys PW. Myocardial repair by percutaneous cell transplantation of autologous skeletal myoblast as a stand alone procedure in post myocardial infarction chronic heart failure patients. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2006;1:417–424. [PubMed] [Google Scholar]
- 31.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with six-month follow-up. Journal of the American College of Cardiology. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Williams AR, Hare JM. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation research. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singla DK, Abdelli LS. Embryonic stem cells and released factors stimulate c-kit/flk-1 progenitor cells and promote neovascularization in doxorubicin-induced cardiomyopathy. Cell Transplant. 2014 doi: 10.3727/096368914X679219. [DOI] [PubMed] [Google Scholar]
- 35.Rupp S, Bauer J, von Gerlach S, Fichtlscherer S, Zeiher AM, Dimmeler S, Schranz D. Pressure overload leads to an increase of cardiac resident stem cells. Basic Res Cardiol. 2012;107:252. doi: 10.1007/s00395-012-0252-x. [DOI] [PubMed] [Google Scholar]