Abstract
Short exposure to low concentrations of digitalis drugs like ouabain protects the rat heart against ischemia/reperfusion injury through the activation of the Na/K-ATPase/c-Src receptor complex and subsequent stimulation of key intracellular cardioprotective signals. Rat Na/K-ATPase, however, is relatively insensitive to digitalis, and it is not known if similar results could be obtained in species with higher sensitivity. Thus, to determine whether ouabain pretreatment protects against ischemic injury and activates the Na/K-ATPase signaling cascade in a species with a cardiac glycoside sensitivity comparable to humans, the present study was conducted in the rabbit model. In Langendorff-perfused rabbit hearts, 20 min exposure to 500 nM ouabain resulted in positive inotropy as evidenced by a significant increase in +dP/dt, and this increase was accompanied by the activation of several well-characterized downstream mediators of the cardiac Na/K-ATPase receptor pathway, including Src, Akt, ERK1/2, and PKCε. A short (4 min) administration of a sub-inotropic dose of ouabain (100 nM) followed by an 8 min washout before 30 min of global ischemia and 120 min of reperfusion resulted in protection against cell death, as evidenced by a significant decrease in infarct size. These data indicate that ouabain administration activates the Na/K-ATPase signaling cascade and protects against ischemic injury in a species with high cardiac Na/K-ATPase sensitivity.
Keywords: Cardiac inotropy, Preconditioning, Ouabain, Rabbit, Heart, Na/K-ATPase
INTRODUCTION
The ubiquitous Na/K-ATPase is classically known for the active transport of Na+ and K+ across the plasma membrane. The well-known positive inotropic effect of ouabain and other related cardiac glycosides is generally accepted to result from the partial inhibition of the cardiac sarcolemmal Na/K-ATPase, which results in the accumulation of intracellular Na+ and subsequent modulation of the Na+/Ca2+ exchanger. The resulting increase in intracellular Ca2+, in turn, augments contractile force (1). An increasing number of studies have shown that cardiac glycoside binding to the Na/K-ATPase also activates several signal transduction pathways. In the heart, ouabain activates multiple kinase activities including those of EGFR, c-Src, PKCs, ERK1/2, and Akt (8, 9, 15–17, 20, 23, 24, 32–34). We are just beginning to understand the physiologic role of the signaling function of Na/K-ATPases, but several studies have clearly shown its importance for the regulation of myocardial structure and function, including contractility, hypertrophy, and resistance to ischemia-reperfusion injury (5, 7, 11, 17, 22, 24, 28, 29, 31, 34). In the latter, the cardiac glycoside signal is transmitted from the sarcolemmal Na/K-ATPase to the mitochondria, and key mediators of this cardioprotective signaling pathway identified in Langendorff-perfused rat hearts include Src kinase, PKCε, mitoKATP and reactive oxygen species (ROS) (22, 24, 25). While much has been learned about the Na/K-ATPase mediated signal transduction pathways and their physiologic roles, it is important to note that the majority of the studies characterizing ouabain signaling and all of the studies on digitalis preconditioning were performed in rat hearts. Arguably, this restricts the implication of these findings, as myocardial physiology and vulnerability to ischemic injury are known to vary from one species to another (10, 14, 27, 30, 36). Further, the concentration of digitalis required to produce a positive cardiac inotropic effect is markedly higher in rat than in other species (6, 21).
Accordingly, in order to establish whether the cardioprotective digitalis signaling pathway is likely to be of clinical relevance, we conducted the present study in isolated perfused rabbit hearts. In this model, the expression of Na/K-ATPase and kinetics of digitalis association/dissociation are significantly more like that of human than is the rat (3, 26). The goals of the study were to determine 1) whether an ouabain induced increase in cardiac inotropy is accompanied by activation of the cardiac Na/K-ATPase signaling cascade, and 2) if sub-inotropic doses of ouabain protects against ischemic injury in the rabbit heart, a species with high cardiac Na/K-ATPase sensitivity.
MATERIALS AND METHODS
Chemicals and Reagents
Ouabain and all other reagents were purchased from Sigma-Aldrich. Antibodies against total and phosphorylated ERK1/2, total and phosphorylated Akt, total PKCε, and all secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The polyclonal anti-Src pY418 antibody was obtained from Invitrogen (Carlsbad, CA).
Langendorff Perfusion
Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Heath, and all protocols were approved by the Institutional Animal Care and Use Committee at the University of Toledo, College of Medicine. Male New Zealand White rabbits (2.2–2.8 kg) were anesthetized with pentobarbital sodium (50mg/kg, i.v.) and heparinized (1000 U/kg). The perfusion protocol was adapted from Kilgore et al, and Pierre et al (13, 24). Briefly, hearts were rapidly removed and mounted on a non-recirculating Langendorff apparatus and perfused with Krebs–Henseleit (KH) solution containing (in mmol/L): NaCl (118.0), KCl (4.0), CaCl2 (1.65), KH2PO4 (1.3), MgSO4 (1.2), Ethylene glycolbis (2-aminoethylether)-N, N, N′, N′-tetraacetic acid (0.3), NaHCO3 (25), D-glucose (11). The buffer was continuously oxygenated with a mixture of O2 (95%) and CO2 (5%), resulting in a pO2 of 618 mmHg, a pCO2 of 30 mmHg and a pH of 7.4. The KH buffer was delivered at a constant flow rate of 20–25 mL/minute, and coronary perfusion pressure (CPP) was monitored with a pressure transducer connected to a side arm of the aortic cannula. The temperature of the buffer and isolated heart was maintained at 37°C with a temperature-controlled circulating water bath. A drainage cannula was inserted into the apex of the left ventricular cavity to vent the Thebesian flow. End diastolic pressure (EDP), isovolumic left ventricular developed pressure (LVDP, the difference between systolic and diastolic left ventricle pressures) and the index of left ventricular contractile function during systole (+dP/dtmax) were measured via a water-filled latex balloon inserted in the left ventricle and secured with a purse string suture. The balloon was connected to a P23XL Becton Dickinson pressure transducer, and the pressure signal was augmented via a Grass CP122 AC/DC strain gage amplifier and recorded using a PowerLab data acquisition system (2/26, ADInstruments). LVDP and +dP/dtmax, were continuously monitored during the experiment and quantified afterwards using LabChart software (ADInstruments). Hearts were paced at 3 Hz (2 ms duration, 4V) with bipolar electrodes attached to the left ventricle using a Grass SD9 stimulator, and the pacing was maintained throughout the experiment. The EDP was adjusted initially to 6 mmHg. After 20 minutes of equilibration, one of two protocols (described below) was begun.
Perfusion Protocols
Protocol A
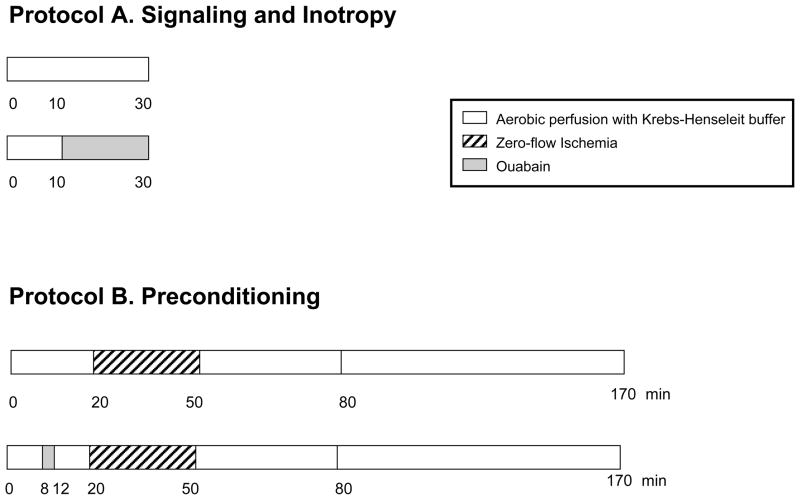
To determine the effect of ouabain exposure on contractility, rabbit hearts were perfused for a total of 30 minutes with either KH alone or with 10 minutes of KH followed by 20 minutes of 100nM or 500nM ouabain (Figure 1, Protocol A). To determine if signaling accompanied ouabain induced inotropy, (as evaluated by phosphorylation of Src, ERK1/2and Akt and PKCε translocation), rabbit hearts perfused with KH or with 500nM ouabain were quick-frozen in liquid nitrogen and powdered for immunoblotting as described below.
Figure 1. Experimental protocols.
Protocol A was used for all inotropy and signaling studies. Ouabain was perfused at 100 or 500 nM for 20 min. Protocol B was used for preconditioning studies. Ouabain was given for 4 min at the dose of 100 nM, as indicated. After ischemia, all hearts were reperfused with standard Krebs-Henseleit buffer for 2 hours.
Protocol B
To examine the effect of ouabain pretreatment on ischemic injury, hearts were perfused with either KH for 20 minutes or KH for 8 minutes followed by 100nM ouabain for 4 minutes followed by KH for 8 minutes (Figure 1, Protocol B). Both groups were then subjected to 30 minutes of normothermic global ischemia followed by 120 minutes of reperfusion. At the end of the experiment, the hearts were stained for the determination of infarct size as described below.
Tissue Preparation, SDS-Page and Immunoblotting
Rabbit hearts perfused with KH or with 500nM ouabain (Figure 1, Protocol A) were quick-frozen in liquid nitrogen. One hundred mg of powdered left ventricle was placed into an ice-cold buffer containing 30 mM histidine, 250 mM sucrose, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM NaF, 10 nM okadaic acid, 10 μg/ml aprotinin, 10 μg/ml leupeptin and then homogenized in a 30 ml homogenizer by repeating 5 times a series of 8 up-and-down strokes separated by 30 s intervals. Lysates were then centrifuged at 16,000×g for 15 minutes, and supernatants (50 μg) were probed for total and phosphorylated Src, Akt, and ERK1/2 levels as described previously (17, 24). To assay PKCε translocation from cytosolic to particulate fraction, the same homogenates were centrifuged at 100,000 ×g for 1 hour at 4°C. The supernatant designated as the cytosolic fraction was removed and saved. The pellet was homogenized in the solution containing 1% Triton and the particulate fraction was prepared as we previously described (21, 24).
Infarct Size
Rabbit hearts undergoing ischemia/reperfusion (Figure 1, Protocol B) were sliced into approximately 2 mm thick transverse sections and incubated in triphenyltetrazolium chloride solution (TTC, 1% in phosphate buffer, pH 7.4) at 37°C for 20 minutes, then further incubated for 20 minutes in formalin. TTC stains the viable tissue in a bright-red color, which allows the discrimination between viable (red) and nonviable (pale yellow) tissue. Slices were subsequently scanned and analyzed using Image J software (National Institutes of Health). Infarct size was expressed as a percentage of the area at risk (equivalent to total cardiac muscle mass), as reported previously (24, 37).
Statistical Analysis
Results are expressed as mean ± SEM. All data sets were normally distributed and compared using unpaired bilateral Student’s t test or one-way ANOVA followed by Dunnet’s post-hoc analysis. P<0.05 was considered statistically significant.
RESULTS
Ouabain-Induced Inotropy and Signaling
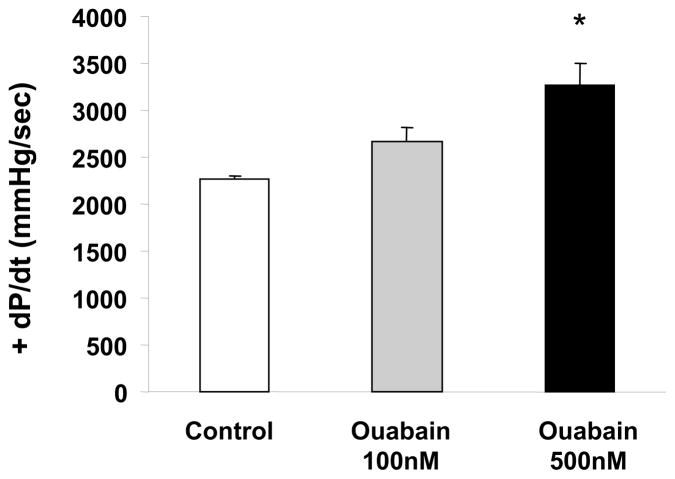
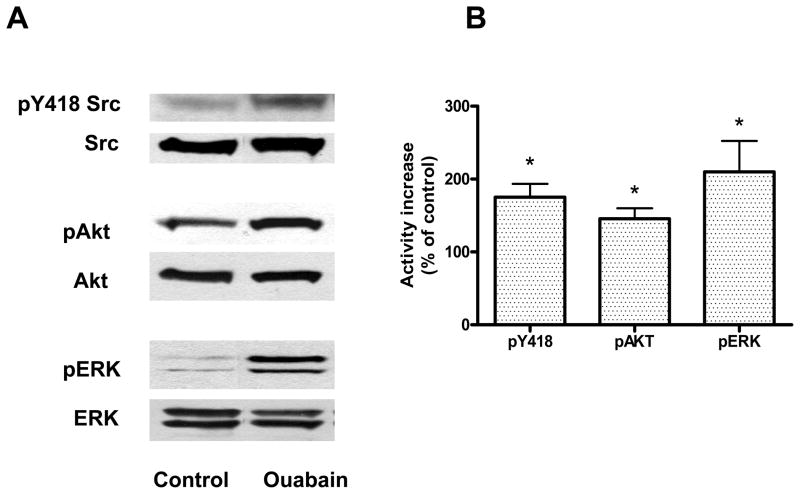
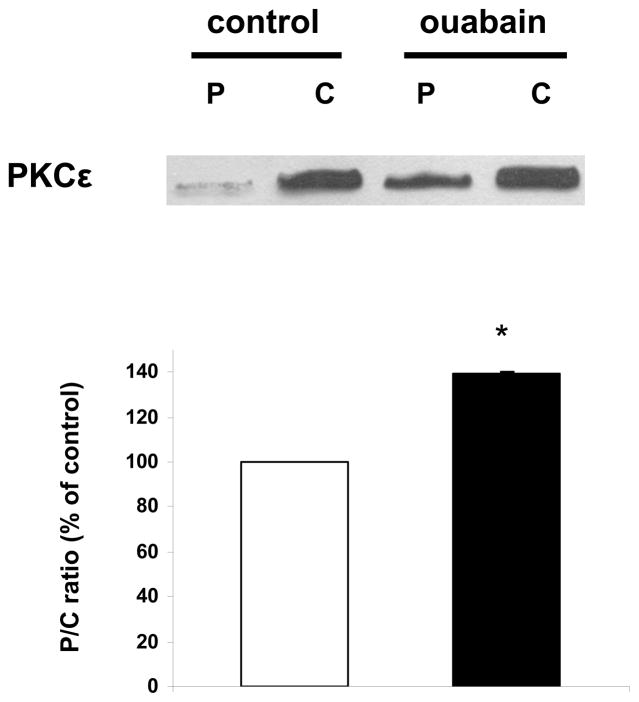
We first determined the effect of ouabain exposure on contractility (Figure 1, Protocol A). As shown in Figure 2, control +dP/dtmax was 2270 ± 30 mmHg/sec., and while 20 minutes of perfusion with 100 nM ouabain did not induce a significant increase in +dP/dtmax (2670± 150 mmHg/sec, N.S.), 500 nM ouabain resulted in a significant positive inotropic effect of about 40% (3270 ± 230, P<0.05). To determine whether ouabain induced signaling accompanies the positive inotropic action of 500 nM ouabain in the isolated rabbit heart, we examined the activation of Src, Akt, and ERK1/2 at the end of the 20 minute exposure to ouabain. The results presented in Figure 3 show that ouabain caused a significant activation of Src, Akt, and ERK1/2. Additionally, as shown in Figure 4, 500 nM ouabain resulted in a significant increase in the PKCε translocation to the particulate fraction of the Langendorff-perfused rabbit heart lysates. Taken together, these data demonstrate that the positive inotropic effect of ouabain in isolated rabbit hearts is accompanied by activation of Na/K-ATPase signaling cascade.
Figure 2. Dose Effect of Ouabain Exposure on contractility.
Rabbit hearts were Langendorff-perfused for 20 min in the presence or absence of 100 or 500 nM ouabain. Values are means ± SEM of 9 separate experiments. * P < 0.05 vs control.
Figure 3. Activation of Src, Akt and ERK 1/2 by ouabain in rabbit heart.
Rabbit hearts were Langendorff-perfused for 20 min in the presence or absence of 500 nM ouabain. Lysates were assayed for the total and phosphorylated forms of the kinases. A. Representative immunoblots. B. Activation quantified as ratio of phosphorylated to total form for each kinase. Shown are means ± SEM from 3 independent experiments. * P < 0.05 vs. control.
Figure 4. Effect of ouabain on PKCε activation.
Rabbit hearts were perfused for 20 min in the presence or absence of ouabain 500 nM (protocol A). Cytosolic (C) and particulate (P) fractions obtained from tissue lysates were then assayed and compared for their contents in PKCε. Upper panel: representative western blot. Lower panel: means ± SEM of 3 separate experiments. * P < 0.01 vs control.
Ouabain Preconditioning
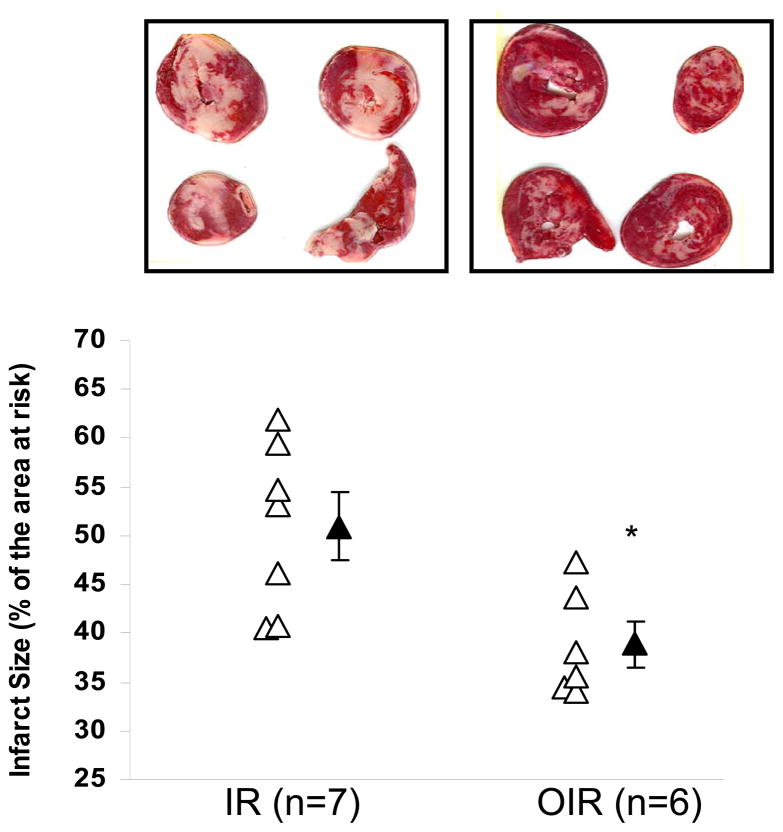
The functional study using protocol A revealed that a ouabain dose of 100 nM did not produce a significant inotropic effect in the rabbit, and we wished to determine if this sub-inotropic dose was capable of providing cardioprotection against ischemia/reperfusion injury in the rabbit similar to that observed in the rat (21). Cardiac baseline parameters were set equally for both the ouabain pretreated (OIR) and untreated (IR) groups (Table, Time = 0), consistent with values reported by others for Langendorff-perfused rabbit heart preparation (12, 13, 35). As expected, hemodynamics remained stable over the course of the pre-ischemic perfusion period in the untreated group (Table, IR at T=0 and 20). Furthermore, 4 minute infusion of 100 nM ouabain did not result in any significant change in CPP, EDP or LVDP (Table, OIR at Time = 0 vs 20 min). Ischemia resulted in cardiac arrest in all groups. Cardiac function resumed at reperfusion, but was significantly compromised in all groups. At the end of the 2 hour reperfusion period, the significantly decreased LVDP and the increased CPP and EDP (Table, time = 170 min vs. 0 or 20 min) indicated that cardiac function remained compromised to a comparable extent in hearts with or without ouabain pre-treatment. On the other hand, as shown in Figure 5, hearts pretreated with ouabain had significantly reduced infarct size compared to that of untreated hearts (39 ± 2% vs. 51 ± 4% of the area at risk, P < 0.05). Taken together, these data indicate that ouabain preconditioning in the Langendorff-perfused rabbit heart protects against ischemia-reperfusion-induced cell death but does not result in a significant improvement in functional recovery within two hours of reperfusion.
Table.
Hemodynamic variables at baseline, at the end of the pre-ischemic period, and at the end of 120min of reperfusion.
| Functional Parameter | Group | Time = 0 (baseline) | Time = 20 min (end of pre-ischemic perfusion) | Time = 170 min (120 min of reperfusion) |
|---|---|---|---|---|
| CPP (mmHg) | IR | 36 ± 2 | 36 ± 2 | 81 ± 8*** |
| OIR | 40 ± 4 | 45 ± 5 | 93 ± 9*** | |
| EDP (mmHg) | IR | 6 ± 1 | 6 ± 1 | 53 ± 8*** |
| OIR | 5 ± 1 | 6 ± 2 | 42 ± 4*** | |
| LVDP (mmHg) | IR | 61 ± 2 | 61 ± 2 | 18 ± 5*** |
| OIR | 66 ± 3 | 67 ± 4 | 16 ± 2*** |
IR, untreated ischemia/reperfusion; OIR, oubain (100nM) pretreatment ischemia/reperfusion; CPP, coronary perfusion pressure; EDP, end diastolic pressure; LVDP, left ventricular developed pressure. Values are mean ±SEM from 9 independent experiments.
P<0.001 vs. respective baselines (value at time=0).
Figure 5. Ouabain (100nM) pretreatment reduces infarct size after ischemia-reperfusion.
Top: representative picture of staining with TTC (viable tissue is red). Bottom: plot of infarct size as a percentage of the area at risk (equivalent to whole myocardium) for control (IR) and ouabain-treated heart (OIR). Open symbols depict individual values while solid symbols depict group means ± SEM of 7 independent experiments for each group. * P<0.05 vs. IR
DISCUSSION
The aim of this work was to investigate whether Na/K-ATPase signaling accompanies an increase in inotropy upon ouabain administration and to determine if sub-inotropic doses of ouabain protect against ischemic injury in an animal model that mimics human Na/K-ATPase expression and digitalis association/dissociation kinetics.
Signaling Accompanies Inotropy
These studies in the rabbit heart recapitulate previous findings in other animal models that demonstrate that the positive inotropic effect of ouabain in isolated hearts is accompanied by activation of the Na/K-ATPase signaling cascade (24). Specifically, the results presented in Figure 3 show that ouabain caused a significant activation of Src, Akt, and ERK1/2, and these findings are comparable to those observed in cardiac cells and isolated hearts from guinea pig and rat (24). In addition, as shown in Figure 4, 500 nM ouabain resulted in a significant increase in PKCε translocation to the particulate fraction of the Langendorff-perfused rabbit heart lysates, consistent with previous findings in the rat (24). Thus, the present study shows that cardiac Na/K-ATPase mediated signal transduction pathways described in other animal models are also present in the more human-like rabbit heart.
Ouabain Preconditioning in Rabbit vs. Rat Heart
The reperfusion protocol described in Figure 1 was chosen because it is one of the most commonly used protocols to study ischemia-reperfusion injury in Langendorff-perfused hearts (13). It is also the protocol that we and others have previously used to study the protective effect of digitalis in rat (5, 24). The functional study using protocol A revealed that a dose of 100 nM ouabain did not produce any significant inotropic effect in our conditions, comparable to the effect produced by 10 μM in the rat model we have previously reported (24). This finding is also in good agreement with the known difference in ouabain sensitivity between the two species (6, 19). Thus, we used this concentration in protocol B to test whether ouabain preconditioning occurs in the rabbit myocardium, which has a digitalis-sensitivity comparable to human. The present study demonstrates that pretreatment of isolated perfused rabbit heart with 100nM ouabain significantly reduces infarct size (Figure 5), consistent with previous studies conducted in isolated rat heart (5, 24). Unlike studies conducted in rats, however, we did not find any improvement in LVDP, CPP or EDP after 120 minutes of reperfusion when the hearts were pretreated with ouabain (Table). Apparent discrepancies between functional recovery at early reperfusion and infarction, which are the two most commonly used endpoints to assess the extent of ischemia-reperfusion injury and protection, have been well documented (4, 12, 36). While increased functional recovery is an important outcome of cardiac preconditioning, infarct size is considered a more reliable end point, as its measurement is independent of “stunning” phenomena that may accompany reperfusion (4, 18). In fact, many consider the measurement of infarct size the “gold standard” for determining the degree of cardioprotection provided by a given intervention (18, 36). Accordingly, the protective effect of ouabain on post-ischemic infarct in the rabbit heart is an indication of a preconditioning effect. Furthermore, like ouabain, other forms of preconditioning have shown a significant effect on post-ischemic function in rat but not rabbit hearts, and this difference has been attributed to differences in the ratio of stunning and infarction produced by ischemia in these species (12). Thus, the species-specificity in post-ischemic recovery is likely to be a factor in the apparent discrepancy between earlier reports in rats and the present study. Previous studies in the rat showed a clear association between the activation of the Na/K-ATPase signaling cascade and ouabain induced cardioprotection (24), suggesting that activation of the Na/K-ATPase cascade may also play a role in the ouabain induced cardioprotection observed in the rabbit. Although the evidence presented here that ouabain also induces activation of the cardioprotective PKCε in the rabbit heart (fig. 4) further suggests that Na,K-ATPase signaling cascade is involved, other mechanisms of action of cardiac glycosides have been proposed (2). Hence, additional studies designed to address this question are required to firmly establish the association between ouabain induced cardioprotection and activation Na/K-ATPase signaling cascade in rabbit heart.
Conclusion
In conclusion, the results of this work clearly demonstrate that in the rabbit heart, a species with high Na/K-ATPase affinity for ouabain, ouabain-induced increases in inotropy are accompanied by the activation of the Na/K-ATPase signaling cascade, and preconditioning the heart with sub-inotropic doses of ouabain protects against ischemia-reperfusion injury. The results of these studies provide strong support for a bridge between the findings in rat heart and the more human-like rabbit, and validate the rabbit heart as a more human-like model in which to examine the potential therapeutic value of ouabain preconditioning.
Acknowledgments
We would like to thank the staff of University of Toledo Department of Laboratory Animal Resources that assisted with this research project. This study was supported by National Heart, Lung, and Blood Institute Grant HL-36573.
References
- 1.Akera T, Brody TM. The role of Na+,K+-ATPase in the inotropic action of digitalis. Pharmacol Rev. 1977;29:187–220. [PubMed] [Google Scholar]
- 2.Arispe N, Diaz JC, Simakova O, Pollard HB. Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proc Natl Acad Sci U S A. 2008;105:2610–2615. doi: 10.1073/pnas.0712270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbey O, Gerbi A, Robert K, Mayol V, Pierre S, Paganelli F, Maixent JM. Immunological identification of Na,K-ATPase isoforms in nonfailing and failing myocardium. Ann N Y Acad Sci. 1997;834:656–657. doi: 10.1111/j.1749-6632.1997.tb52342.x. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 5.D’Urso G, Frascarelli S, Zucchi R, Biver T, Montali U. Cardioprotection by ouabain and digoxin in perfused rat hearts. J Cardiovasc Pharmacol. 2008;52:333–337. doi: 10.1097/FJC.0b013e3181884448. [DOI] [PubMed] [Google Scholar]
- 6.Ezzaher A, Mougenot R, Baggioni A, el Ouazzani T, Crozatier B, Maixent JM. Inotropic effects of ouabain and Na, K-ATPase activity in failing rabbit hearts. C R Acad Sci III. 1995;318:273–279. [PubMed] [Google Scholar]
- 7.Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem. 2004;279:33306–33314. doi: 10.1074/jbc.M402187200. [DOI] [PubMed] [Google Scholar]
- 8.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 9.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 10.Hearse DJ, Humphrey SM, Garlick PB. Species variation in myocardial anoxic enzyme release, glucose protection and reoxygenation damage. J Mol Cell Cardiol. 1976;8:329–339. doi: 10.1016/0022-2828(76)90007-9. [DOI] [PubMed] [Google Scholar]
- 11.Inserte J. Triggering of cardiac preconditioning through Na+/K+-ATPase. Cardiovasc Res. 2007;73:446–447. doi: 10.1016/j.cardiores.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DP, Pugsley WB, Yellon DM. Ischaemic preconditioning in a model of global ischaemia: infarct size limitation, but no reduction of stunning. J Mol Cell Cardiol. 1995;27:1623–1632. doi: 10.1016/s0022-2828(95)90590-1. [DOI] [PubMed] [Google Scholar]
- 13.Kilgore KS, Friedrichs GS, Johnson CR, Schasteen CS, Riley DP, Weiss RH, Ryan U, Lucchesi BR. Protective effects of the SOD-mimetic SC-52608 against ischemia/reperfusion damage in the rabbit isolated heart. J Mol Cell Cardiol. 1994;26:995–1006. doi: 10.1006/jmcc.1994.1120. [DOI] [PubMed] [Google Scholar]
- 14.Kim HD, Kim CH, Rah BJ, Chung HI, Shim TS. Quantitative study on the relation between structural and functional properties of the hearts from three different mammals. Anat Rec. 1994;238:199–206. doi: 10.1002/ar.1092380206. [DOI] [PubMed] [Google Scholar]
- 15.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol. 2007;293:C1489–1497. doi: 10.1152/ajpcell.00158.2007. [DOI] [PubMed] [Google Scholar]
- 18.Lochner A, Genade S, Moolman JA. Ischemic preconditioning: infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol. 2003;98:337–346. doi: 10.1007/s00395-003-0427-6. [DOI] [PubMed] [Google Scholar]
- 19.Maixent JM, Gerbi A, Barbey O, Lan C, Jamme I, Burnet H, Nouvelot A, Levy S, Cozzone PJ, Bernard M. Dietary fish oil promotes positive inotropy of ouabain in the rat heart. Am J Physiol. 1999;277:H2290–2297. doi: 10.1152/ajpheart.1999.277.6.H2290. [DOI] [PubMed] [Google Scholar]
- 20.Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276:42050–42056. doi: 10.1074/jbc.M107892200. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi K, Liu L, Tian J, Kometiani P, Xie Z, Askari A. Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. J Cardiovasc Pharmacol. 2003;41:609–614. doi: 10.1097/00005344-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Pasdois P, Quinlan CL, Rissa A, Tariosse L, Vinassa B, Costa AD, Pierre SV, Dos Santos P, Garlid KD. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving src kinase, mitoKATP, and ROS. Am J Physiol Heart Circ Physiol. 2007;292:H1470–1478. doi: 10.1152/ajpheart.00877.2006. [DOI] [PubMed] [Google Scholar]
- 23.Pierre SV, Xie Z. The Na,K-ATPase receptor complex: its organization and membership. Cell Biochem Biophys. 2006;46:303–316. doi: 10.1385/cbb:46:3:303. [DOI] [PubMed] [Google Scholar]
- 24.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas C, Garlid KD, Dos-Santos P, Xie Z. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73:488–496. doi: 10.1016/j.cardiores.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinlan CL, Costa AD, Costa CL, Pierre SV, Dos Santos P, Garlid KD. Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am J Physiol Heart Circ Physiol. 2008;295:H953–H961. doi: 10.1152/ajpheart.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamraj OI, Grupp IL, Grupp G, Melvin D, Gradoux N, Kremers W, Lingrel JB, De Pover A. Characterisation of Na/K-ATPase, its isoforms, and the inotropic response to ouabain in isolated failing human hearts. Cardiovasc Res. 1993;27:2229–2237. doi: 10.1093/cvr/27.12.2229. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Namekata I, Nouchi H, Shigenobu K, Kawanishi T, Takahara A. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: diversity in the excitation-contraction mechanisms of the heart. J Pharmacol Sci. 2009;109:327–333. doi: 10.1254/jphs.08r22fm. [DOI] [PubMed] [Google Scholar]
- 28.Tian J, Gong X, Xie Z. Signal-transducing function of Na+-K+-ATPase is essential for ouabain’s effect on [Ca2+]i in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;281:H1899–1907. doi: 10.1152/ajpheart.2001.281.5.H1899. [DOI] [PubMed] [Google Scholar]
- 29.Tian J, Liu J, Garlid KD, Shapiro JI, Xie Z. Involvement of mitogen-activated protein kinases and reactive oxygen species in the inotropic action of ouabain on cardiac myocytes. A potential role for mitochondrial K(ATP) channels. Mol Cell Biochem. 2003;242:181–187. [PubMed] [Google Scholar]
- 30.Vidavalur R, Swarnakar S, Thirunavukkarasu M, Samuel SM, Maulik N. Ex vivo and in vivo approaches to study mechanisms of cardioprotection targeting ischemia/reperfusion (i/r) injury: useful techniques for cardiovascular drug discovery. Curr Drug Discov Technol. 2008;5:269–278. doi: 10.2174/157016308786733555. [DOI] [PubMed] [Google Scholar]
- 31.Wasserstrom JA, Aistrup GL. Digitalis: new actions for an old drug. Am J Physiol Heart Circ Physiol. 2005;289:H1781–1793. doi: 10.1152/ajpheart.00707.2004. [DOI] [PubMed] [Google Scholar]
- 32.Xie Z. Ouabain interaction with cardiac Na/K-ATPase reveals that the enzyme can act as a pump and as a signal transducer. Cell Mol Biol (Noisy-le-grand) 2001;47:383–390. [PubMed] [Google Scholar]
- 33.Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 34.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 35.Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: upregulation by ischemia and reperfusion. Circ Res. 1998;82:1224–1230. doi: 10.1161/01.res.82.11.1224. [DOI] [PubMed] [Google Scholar]
- 36.Ytrehus K. The ischemic heart--experimental models. Pharmacol Res. 2000;42:193–203. doi: 10.1006/phrs.2000.0669. [DOI] [PubMed] [Google Scholar]
- 37.Ytrehus K, Liu Y, Tsuchida A, Miura T, Liu GS, Yang XM, Herbert D, Cohen MV, Downey JM. Rat and rabbit heart infarction: effects of anesthesia, perfusate, risk zone, and method of infarct sizing. Am J Physiol. 1994;267:H2383–2390. doi: 10.1152/ajpheart.1994.267.6.H2383. [DOI] [PubMed] [Google Scholar]