Abstract
Background and Purpose
Carotid atherosclerosis is a risk factor for cerebrovascular disease in older adults. Although age-related cognitive decline has been associated with cerebrovascular disease, not much is known about the consequences of carotid atherosclerosis on longitudinal cognitive function. This study examines the longitudinal relationship between atherosclerosis and cognition in a sample of non-demented older subjects using baseline measurements of carotid intima media thickness (CIMT) and annual cognitive measures of executive function (EXEC) and verbal memory (MEM).
Methods
Baseline measurements included CIMT derived from B-mode carotid artery ultrasound, structural T1-weighted images of white matter hypointensities (WMH), white matter lesions (WML) and cerebral infarct. Hypertension, low-density lipoprotein (LDL), diabetes and waist to hip ratios (WHR) were included as covariates in our models to control for cerebrovascular risks and central adiposity. Annual composite scores of EXEC and MEM functions were derived from item response theory. Linear mixed models were used to model longitudinal cognitive change.
Results
A significant inverse relationship was found between baseline CIMT and annual EXEC score, but not annual MEM score. Subjects included in the highest 4th quartile of CIMT showed a rate of annual decline in EXEC score that was significant relative to subjects in lower quartile groups (p<0.01). The relationship between the 4th quartile of CIMT and annual EXEC score remained significant after independently adjusting for imaging measures of white matter injury and cerebral infarct.
Conclusions
Older adult subjects with the highest index of CIMT showed an annual decline in EXEC scores that was significant relative to subjects with lower quartile measurements of CIMT, independent of our measures of white matter injury and cerebral infarct. Our findings suggest elevated measures of CIMT may mark an atherosclerotic state, resulting in decline in executive function and not memory in non-demented older adults.
Approved search terms: Aging, Longitudinal Cohort Study, Carotid Intima-Media Thickness, Cognition, Magnetic Resonance Imaging
Introduction
Carotid intima-media thickness (CIMT), a marker of atherosclerosis1, has been reported to predict longitudinal decrements in memory, processing speed and executive functioning23. Results from current studies have been mixed, however, with no consensus regarding which cognitive domains are most affected. Furthermore, the mechanisms underlying the relationship between atherosclerosis and cognitive decline are poorly understood. Ischemic white matter injury has been proposed as the cause of cognitive decline in elderly persons with atherosclerosis4, but the role of white matter damage in predicting longitudinal cognitive decline in the presence of elevated CIMT has not been empirically established.
The current study aims to address two issues – (1) To determine whether CIMT predicts longitudinal change of memory or executive functioning; (2) To determine whether baseline measurements of white matter injury or cerebral infarct predict the longitudinal CIMT-cognitive relationship.
Methods
Participants
Two hundred fifty-one, non-demented, community dwelling older adults with a mean age of 78 (SD=6.4; Range: 62–94) years were recruited to participate in a longitudinal multi-center study “Aging Brain: Vasculature, Ischemia and Behavior”. Eighty-three participants were recruited at the University of California, San Francisco (UCSF), 101 from the University of California, Davis (UCD) and 67 from the University of Southern California (USC). Participants were followed over a span of 3 years. Eligible subjects were fluent English speakers over the age of 60 with either normal cognition or mild cognitive impairment, represented by a Clinical Dementia Rating (CDR) score5 of 0 or 0.5, respectively. Exclusion criteria included a current diagnosis of Major Depressive Disorder, history of schizophrenia, bipolar disorder, or neurological disease (e.g., seizures, Parkinson’s disease, multiple sclerosis, head injury with loss of consciousness over 15 minutes, dementia), current drug or alcohol abuse, significant hematologic or metabolic illness, and the use of medications that affect cognition (e.g., prescription-level pain medications). Annual neuropsychological assessment, and B-mode carotid artery ultrasounds and MRI scan were performed on each participant at baseline visit. Written informed consent was obtained from all participants at each participating institution following the protocols approved by the institutional review boards.
Cognitive Assessment
Psychometrically matched measures of executive function, and verbal and non-verbal memory were created using item response theory, described in detail in a previous publication by Mungas et al6. In brief, scales were created based on linear measurements of 400 older individuals with a diverse range of cognitive ability. The scales were designed to account for individual deficits and differential sensitivity of neuropsychological assessments to subtle and severe deficits. The executive functioning measure (EXEC) is a composite derived from the Dementia Rating Scale Initiation–Perseveration subscale7, the Wechsler Memory Scale—Revised digit span backward and visual span backward8, and a controlled oral word fluency task (F-A-S)9. The memory measure (MEM) was derived using total recall on Trials 2–6 on the Word List Learning Test of the Memory Assessment Scales (MAS)10, as well as the short and long delayed free recall from the same test. The average time between repeated visits for cognitive assessment was 1.52 (SD=0.58; range=0.67–3.32) years.
Carotid Artery Intima-Media Thickness
Carotid artery intima-media thickness (CIMT) was assessed at the far wall of the right and left distal common carotid arteries using high-resolution B-mode ultrasound10, 11. Sonographers at each site were trained to interrogate the arterial wall with standardized acquisition techniques along with the electrocardiogram signal (patents 2005, 2006, 2011) and were certified by the Atherosclerosis Research Unit (ARU) Core Imaging and Reading Center (CIRC) at the University of Southern California. Briefly, the jugular vein and carotid artery were imaged longitudinally with the former stacked above the latter, and all examinations were recorded with the electrocardiogram signal. CIMT was measured centrally at the ARU CIRC with an automated computerized edge detection algorithm (Prowin, patents 2005, 2006, 2011) using methods specifically designed to assess CIMT longitudinally11,12. Ultrasound images were analyzed blinded to cognitive status and other characteristics of study participants. CIMT was determined as the average of 70 to 100 measurements between the intima-lumen and media-adventitia interfaces along a 1 cm length just distal to the carotid artery bulb at the same point of the cardiac cycle. The coefficient of variation of these methods is <3%. The mean values of the right and left CIMT were used in subsequent statistical analyses. Twenty-seven participants only had a measure of the right or left carotid artery at baseline visit, in which case either value was used for analyses rather than the mean.
Medical History
Information pertaining to medical health history was provided by means of self-report. Health variables included hypertension (yes/no) and type-2 diabetes (yes/no). Hypertension and diabetes were then quantified with 0 indicating no hypertension (or diabetes), and 1 indicating presence of hypertension (or diabetes). Waist and hip circumferences were collected using a flexible tape measure. Waist circumference was defined as the smallest abdominal area at the midpoint between the ribcage and the edge of the pelvic crest. Hip circumference was defined as the maximal protrusion point of the gluteal muscles and the symphysis pubis. Both measurements were then used to compute the WHR.
Laboratory Measures
Plasma and serum blood specimens were collected by means of separator tubes. Specimens were then left to clot at room temperature for 30–60 minutes and placed into EDTA plasma tubes. Blood was then centrifuged at 2500 rpm at room temperature for 15 minutes. The plasma and serum was then stored at −80 °C until the samples were analyzed. Low-density lipoprotein (LDL) was used as a biologic marker of hypercholesterolemia. LDL samples were assessed in the University of California, Davis Medical Center Clinical Laboratory.
MRI Acquisition and Processing
Structural images were obtained using a 3T or 4T MRI system. Sixty-seven participants were scanned at the USC using a 3T General Electric Signal HDx system with an 8-channel head coil. One hundred-one participants were scanned at the UCD research center using a 3T Siemens Magnetom Trio Syngo system with an 8-channel head coil and a 3T Siemens Magnetom TrioTim system with an 8-channel head coil. Thirty-nine participants were scanned at the San Francisco Veterans Administration Medical Center using a 4T Siemens MedSpec Syngo System with an 8-channel head coil. Forty-four participants were scanned at the UCSF Neuroscience Imaging Center using a 3T Siemens Magnetom TrioTim system with a 12-channel head coil. Inter- and intra-reliability tests were run with preliminary experimental subjects on all scanners involved. These subjects were evaluated on each machine twice and their data were processed and analyzed to ensure that the scanners were harmonized across and within sites.
White matter hypointensity and lesion quantification
Quantitative measures of white matter hypointensities (WMH) were derived using FreeSurfer (v5.1) segmentation of T1-weighted images13. Reconstructed cortical surface models for each participant were manually inspected to ensure segmentation accuracy. Scans with gross estimation errors were edited and re-run through the segmentation program. Final quality check for image artifact and processing errors was performed before the study.
White matter lesions (WML) was acquired by performing a basic skull strip of the fluid-attenuated inversion recovery (FLAIR) image using MRIcro’s Brain Extraction Tool (BET) with a value of 0.5. A FLAIR mask was created by constraining parameters on the skull-stripped FLAIR to exclude any connective tissue, meninges, dura or skull. Each subject went through a WML bias correction and segmentation program14,15. Outputs were visually inspected to ensure that segmentations included at least 80% of observed WMLs. After 80% threshold had been reached, WMLs were binarized and volumed using FSL scripts. This process was only performed on subjects with WMLs that appeared in three consecutive tissue slices in three orientations, or on subjects with WMLs appearing in at least five consecutive slices in the area where periventricular WML capping occurs. Subjects who fell below threshold for WML quantification were assigned a value equal to half of the threshold for measurement (13.5 mm3).
Cerebral Infarct Classification
A vascular neurologist blind to participant data identified cerebral infarcts using the T1-weighted, T2-weighted, and FLAIR MRIs. Subjects were assigned a value of 1 in the presence of 1 or more infarcts and a value of 2 in the presence of no infarcts.
Statistical Analysis
Mean CIMT was categorized into quartiles to examine the threshold effect of CIMT with cognitive measures. Linear mixed effects models were used to model change in EXEC (or MEM) performance over time for each CIMT quartile group. EXEC and MEM cognitive composite scores were longitudinal dependent variables modeled separately. Independent variables included baseline mean CIMT quartiles (the main effect of interest) and follow-up time of each cognitive assessment measured as years since the baseline visit. Age at baseline, sex, education, CDR score and hypertension were included in our main model. Additional cerebrovascular risk factors (LDL, diabetes) and WHR were included as covariates in subsequent models. CIMT quartiles were modeled as a class variable; an interaction term of CIMT-by-years of follow-up tested whether annual rates of change in EXEC (or MEM) differed by CIMT quartile. Random effects were specified to model subject-specific intercepts (baseline EXEC or MEM).
We repeated the mixed effects analyses detailed above to address our second question, adjusting for MRI measures. These measures were included as independent covariates to evaluate whether estimates of yearly rates of change in cognitive score for each CIMT quartile were cerebrovascular mediated. A 2-sided p<0.05 was considered statistically significant. Analyses were performed using Statistical Package for the Social Sciences (SPSS® V.20, IBM, Chicago, Illinois), Statistical Analysis System (SAS® V9.2, SAS Institute Inc., Cary, N.C., USA) and GraphPad Prism version 6 (GraphPad Software, San Diego, CA).
Results
Sample Characteristics
Our sample (n=251) included 54% male subjects with a mean age of 78 (SD=6.4; Range: 62–94) years, and a mean education of 15.6 (SD=2.9; Range: 8–23) years. The mean CIMT at baseline was 0.883 mm (SD=0.130; Range: 0.62–1.27). A total of 926 EXEC and MEM measures were obtained between baseline and follow-up visits with a mean number of 1.61 (SD=0.731; Range: 1–3) longitudinal assessments per subject. The mean number of years from first to last cognitive assessment per subject was 2.3 (SD=0.373; Range: 1.69–3.32). Additional sample characteristics are displayed in Table 1.
Table 1.
Characteristics of Sample (n=251)
| Variable | Mean (SD) | Range |
|---|---|---|
| Age (years) | 78.0 (6.4) | 62 – 94 |
| Education (years) | 15.6 (2.9) | 8 – 23 |
| Sex (% male) | 57 | |
| Hypertension (%) | 82 | |
| Diabetes (%) | 29 | |
| LDL (mg/dl) | 93.7 (31.8) | 25 – 208 |
| WHR | 0.91 (0.08) | 0.70 – 1.13 |
| CDR score (% 0.5) | 40 | |
| EXEC Score | 93.5 (16.7) | 46.8 – 129.53 |
| MEM Score | 99.2 (19.8) | 39.89 – 141.08 |
| Mean CIMT (mm) | 0.88 (0.13) | 0.62 – 1.27 |
| Years between visits | 1.52 (0.58) | 0–3.32 |
CIMT on Longitudinal Cognitive Performance
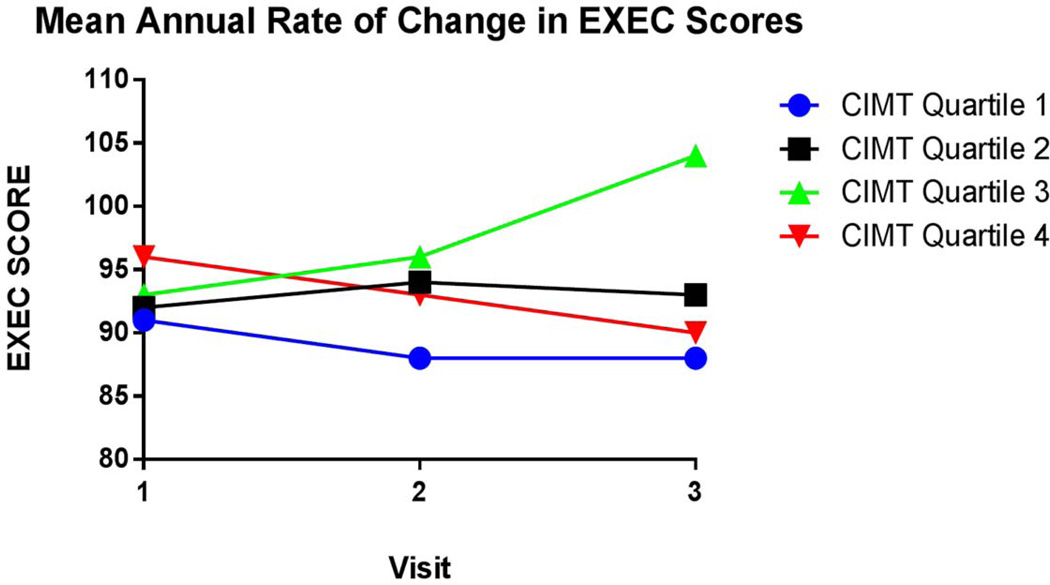
Adjusting for age, sex education and CDR, our analysis showed a significant association between CIMT and change in EXEC score (p < 0.01) (See Table 2). Participants in the 4th quartile of CIMT showed a mean rate of annual decline in executive function score that was significantly greater than persons in the 1st quartile of CIMT (mean difference in annualized rate from quartile 1 = −2.95 [SE = 0.77]; p-value for group difference < 0.01). Results are illustrated in Figure 1. Further adjusting our model for cerebrovascular risks (LDL and diabetes) and WHR showed participants in the 4th quartile continued to have a mean annual rate of decline in executive function score that was significantly greater relative to participants in the 1st quartile (mean difference in annualized rate from quartile 1 = −2.65 [SE=0.94]; p-value for group difference <0.01). The rate of change in EXEC scores did not significantly differ among the three lower quartile groups. A positive, but non-significant trend was noted between the second and third visit in the 3rd quartile group (mean difference in rate from quartile 1 = 1.47 [SE = 0.83]; p-value for group difference = 0.07). Separate analyses using the MEM score as the longitudinal dependent variable showed that annual change in MEM score did not significantly differ among CIMT quartile groups (p = 0.35 among CIMT quartile group; Figure 2).
Table 2.
Associations of EXEC and MEM Composite Score with CIMT Quartiles and Demographic Covariates (n=251)
| Effect | EXEC β Estimates (SE) | MEM β Estimates (SE) |
|---|---|---|
| Age (per year) | −0.243(0.14)* | −0.201(0.16) |
| Male Sex | −4.50(1.91)* | −10.74(2.18)** |
| Education (per year) | 1.67(0.31)** | 0.898(0.36)* |
| CDR 0.5 (compared to CDR 0) | −8.34(1.88)** | −10.31(2.14)** |
| Annual Rate of Cognitive Change (SE) by CIMT Quartile | 0.016(0.77) | 1.89(1.19) |
| CIMT Quartile 1 | 0.063 (0.77) | 1.89(1.19) |
| CIMT Quartile 2 | −0.007(0.92) | 2.71(1.34) |
| CIMT Quartile 3 | 1.46(0.83) | 1.56(1.18) |
| CIMT Quartile 4 | −2.95(0.77)** | −0.54(1.15) |
p-value<0.05
p-value<0.01 (p-value for CIMT quartile 4 relative to referent CIMT quartile 1)
p-value for difference in change rate among CIMT quartiles = 0.0064 (EXEC), 0.14 (MEM)
Figure 1. Mean Annual Change of EXEC Score.
Comparison of mean annual executive function score per CIMT quartile. The 4th quartile group significantly declined in EXEC score relative to the lower quartile groups (p-value for group difference <0.01).
Figure 2. Mean Annual Change of MEM Score.
Comparison of mean annual memory score per CIMT quartile. Quartile groups did not significantly differ in mean annual MEM score (p=0.35 among CIMT quartile group).
Longitudinal Mediation Analyses of CIMT and EXEC Score
Subsequent models evaluated the relationship between annual rates of change of EXEC score in each CIMT quartile with adjustment for baseline MRI measures. Separate analyses included WMH and WML as covariates. The independent adjustment of WMH (β=−3.09 [SE(β)=1.10]), WML (β=−2.96 [SE(β)=1.09]), or cerebral infarct (β=−2.94 [SE(β)=1.09]), relative to quartile 1 (p=0.001) did not attenuate the estimate of the mean rate of annual decline in EXEC scores in the 4th quartile of CIMT (See Table 3).
Table 3.
Estimates of CIMT Group Difference in Annual Rate of Change in EXEC (CIMT Quartile 4 versus CIMT Quartile 1) (n=251)
| Adjusted for: | β (SE) Quartile 4 |
|---|---|
| Main Model (n=251) | −3.01 (1.08)** |
| LDL, diabetes and WHR (n=207) | −2.65 (0.95)** |
| WMH (n=207) | −3.16 (1.43)* |
| WML (n=164) | −3.43 (1.45)* |
| Infarct (n=209) | −3.12 (1.42)* |
p-value <0.01
p-value <0.05
Discussion
The major findings of our study were (1) subjects with the highest baseline CIMT showed an average longitudinal decline in EXEC score that was significantly greater than subjects with the lowest baseline measure of CIMT, independent of demographic variables, CDR score, cerebrovascular risk and WHR; (2) the impact of CIMT was specific to executive function; no significant association was found between CIMT and MEM score; (3) the relationship between CIMT and decline in executive function persisted after independently controlling for baseline MRI measures of white matter injury and cerebral infarct. These findings demonstrate increased CIMT may be related to decline in executive function and may not correspond to baseline MRI brain scan.
Our finding that CIMT affected executive functioning and not memory is in contrast to Komulainen et al16, who reported longitudinal decline of memory scores in subjects with elevated CIMT. However, their 12-year study did not include a measure of executive functioning, and they had a longer follow-up interval. It is possible that memory may eventually be impacted by increased CIMT with a longer follow-up period.
Our results are most consistent with the Zhong et al.17 study, in which subjects with higher CIMT values showed poorer performance on a measure of executive function relative to a measure of verbal memory at 10 years follow-up. The authors have speculated advanced atherosclerosis may result in hypoperfusion and subsequent ischemic injury to frontostriatal connections involved in executive function18,19.
While the suggestion of focal ischemic lesions affecting prefrontal-subcortical loops has been widely accepted in the presence of atherosclerotic processes, distinctions between focal, diffuse or generalized ischemic disease are not clearly understood with regard to cognition in non-demented groups20. Moreover, focal ischemic lesions, regardless of location, may interrupt the functioning of frontal structures through reduced metabolic activity of distant, but interconnected brain regions21, resulting in related executive dysfunction. Intact functioning of frontal systems depends not only on cortical integrity but on integrity of the white matter tracts that connect frontal structures to the cognitive systems that they help regulate22. This is reflected in the frequently replicated finding that WMLs are associated with executive deficits in elderly persons23, and that this effect is seen regardless of the specific location of the WML24. Episodic memory, in contrast, is critically dependent on the function of medial temporal structures; while frontal systems contribute to memory encoding and retrieval, the effects are more subtle and memory changes are not typically a consequence of white matter change, at least in cognitively normal adults. We tested the role of vascular brain injury by determining whether white matter injury or infarcts altered the CIMT-EXEC relationship, and found no mediating effect in our model, contrary to previous reported findings of an association between carotid atherosclerosis and cerebral white matter abnormalities25.
CIMT is an imperfect measure of atherosclerosis. It is particularly sensitive to the early steps in the atherosclerotic process and does not capture later events such as plaque or calcification well, nor is it a measure of stenosis26. It does, however, systemically capture preclincal atherosclerosis, as well as non-atherosclerotic processes associated with compensatory remodeling of the arterial vessel walls1. It may be that the highest quartile of CIMT marks a state in which endothelial damage is occurring at a microstructural level causing pathological changes to arteries that penetrate cerebral white matter tissue. It is much less likely that elevated CIMT is directly associated with hypoperfusion or ischemic damage, consistent with the lack of evidence that WMH, WML or infarcts mediated the CIMT effect in our models. This is further confirmed by the finding that atherothromboemboli may confer minimal risk in the development of WML27.
Strengths and Limitations
The present study applied psychometrically-matched measures to assess cognitive change. The construction of such cognitive domain scores ensures that the scores reflect an individual’s abilities rather than psychometric characteristics of the test itself and allows detection of subtle differences in performance by eliminating ceiling effects in high functioning populations. The composite scores also ensure linearity of measurement over the range of cognitive function. A second strength in our study was the use of linear mixed-effects models in our analyses. Employing a sophisticated statistical procedure provided estimates of cognitive changes within each subject by pooling information across subjects. Also, white matter pathology was quantified using carefully calculated WMH and WML such that we were able to include multiple continuous MRI measurements in our analyses.
The limitations of our study include a high mean level of education, which may limit the generalizability of the results. We used one-time measurement of CIMT to relate to longitudinal cognitive change. Previous studies documenting the longitudinal assessment of carotid atherosclerosis and WMH using repeated measures of CIMT and plaque have documented robust associations in large cohorts28. Using follow-up CIMT measures may have been a stronger predictor of cognitive performance. Furthermore, we may have seen a mediating effect of CIMT on longitudinal cognitive measure when applying measures of white matter injury in subsequent models. While single-measures of MRI data were used to test the CIMT-EXEC association, we found no mediating effect in our models. It may be that one-time measurement of WMH, WML or infarct may not be sufficient to explain longitudinal cognitive change. Thus, repeated imaging measures may have also been more effective in our analysis.
Cognitive functioning is known to change by loss of vasomotor reactivity, which could result in small or large vessel changes29. It is possible our sample included subjects with internal carotid artery stenosis, which may explain cognitive decline in the absence of overt stroke and white matter change on MRI. Transcranial Doppler measurements would have been helpful to determine the integrity of intracerebral hemodynamics in our cohort of subjects. It is also important to note, our study did not include other cerebrovascular risk factors, such as smoking history or parental history of cardiovascular disease. Nor did our study include measures of carotid plaque. We acknowledge that including these measures in our own analyses may have significantly benefited our findings.
Conclusions
These findings may offer some insight regarding longitudinal cognitive assessment and the impact of elevated CIMT. The results are clinically relevant as CIMT can be measured using quick, noninvasive means and is useful for identifying general atherosclerosis. In addition, CIMT screening can be beneficial to older adults who may take preventative measures to reduce atherosclerosis and decrease their risk of cognitive impairment. Future studies should include repeated measures of CIMT and imaging.
ACKNOWLEDGMENTS
Steven Krieger and Ling Zhang, Ph.D. helped with acquiring imaging data. Nerses Sanossian, MD reviewed MRI scans for infarcts.
FUNDING/SUPPORT: This work was supported by National Institutes of Aging, Bethesda, MD (AG123435, AG10129) and by the California Department of Health Services Alzheimer’s Disease Program, contract 94-20354, 94-20355, 98-14970, and 98-14971.
Footnotes
DISCLOSURES: The authors have no financial or any other personal conflicts with this paper.
Contributor Information
Darvis T. Frazier, Email: dfrazier@memory.ucsf.edu.
Talia Seider, Email: tseider@phhp.ufl.edu.
Brianne M. Bettcher, Email: bbettcher@memory.ucsf.edu.
Wendy J. Mack, Email: wmack@usc.edu.
Laura Jastrzab, Email: ljastrzab@memory.ucsf.edu.
Linda Chao, Email: linda.chao@ucsf.edu.
Michael W. Weiner, Email: michael.weiner@ucsf.edu.
Charles DeCarli, Email: cdecarli@ucdavis.edu.
Bruce R. Reed, Email: brreed@ucdavis.edu.
Dan Mungas, Email: dmungas@ucdavis.edu.
Helena C. Chui, Email: helena.chui@med.usc.edu.
Joel H. Kramer, Email: jkramer@memory.ucsf.edu.
References
- 1.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004–2006) Cerebrovascular Diseases. 2012;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 2.Gatto NM, Henderson VW, St. John JA, McCleary C, Detrano R, Hodis HN, et al. Subclinical atherosclerosis is weakly associated with lower cognitive function in healthy hyperhomocysteinemic adults without cardiovascular disease. Int J Geriatr Psychiatry. 2009;24:390–399. doi: 10.1002/gps.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sander K, Bickel H, Förstl H, Etgen T, Briesenick C, Poppert H, et al. Carotid-intima media thickness is independently associated with cognitive decline. The INVADE study. Int J Geriatr Psychiatry. 2010;25:389–394. doi: 10.1002/gps.2351. [DOI] [PubMed] [Google Scholar]
- 4.Arntzen KA, Schirmer H, Johnsen SH, Wilsgaard T, Mathiesen EB. Carotid Atherosclerosis Predicts Lower Cognitive Test Results: A 7-Year Follow-Up Study of 4,371 Stroke-Free Subjects–The Tromsø Study. Cerebrovascular Diseases. 2012;33:159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics. 1997;9(S1):173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 6.Mungas D, Reed B, Kramer J. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 7.Mattis S. Dementia rating scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 8.Wechsler D. WMS-R: Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 9.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- 10.Williams JM. Memory assessment scales. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 11.Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 12.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis: A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 13.Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 14.Hadjidemetriou S, Studholme C, Mueller S, Weiner M, Schuff N. Restoration of MRI data for intensity non-uniformities using local high order intensity statistics. Medical image analysis. 2009;13:36–48. doi: 10.1016/j.media.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjidemetriou S, Lorenzen P, Schuff N, Mueller S, Weiner M. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2008. Springer Berlin Heidelberg: 2008. Computational atlases of severity of white matter lesions in elderly subjects with MRI; pp. 450–458. [DOI] [PubMed] [Google Scholar]
- 16.Komulainen P, Kivipelto M, Lakka TA, Hassinen M, Helkala EL, Patja K, et al. Carotid intima-media thickness and cognitive function in elderly women: A population-based study. Neuroepidemiology. 2007;28:207–213. doi: 10.1159/000108112. [DOI] [PubMed] [Google Scholar]
- 17.Zhong W, Cruickshanks KJ, Schubert CR, Acher CW, Carlsson CM, Klein BE, Chappell RJ. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224:506–510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen RA, Poppas A, Forman DE, Hoth KF, Haley AP, Gunstad J, et al. Vascular and cognitive functions associated with cardiovascular disease in the elderly. Journal of clinical and experimental neuropsychology. 2009;31:96–110. doi: 10.1080/13803390802014594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith PJ, Blumenthal JA, Babyak MA, Hoffman BM, Doraiswamy PM, Waugh R, et al. Cerebrovascular risk factors, vascular disease, and neuropsychological outcomes in adults with major depression. Psychosomatic medicine. 2007;69:578–586. doi: 10.1097/PSY.0b013e31812f7b8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emery VOB, Gillie EX, Smith JA. Noninfarct Vascular Dementia. In: Emery VOB, Oxman TE, editors. Dementia: Presentations, Differential Diagnosis, and Nosology. 2nd Ed. Baltimore, MD: Johns Hopkins University Press; 2003. pp. 263–253. [Google Scholar]
- 21.Luria AR. Higher Cortical Functions in Man. 2nd Ed. New York, NY: Basic Books; 1998. [Google Scholar]
- 22.Cummings JL. Vascular subcortical dementias: clinical aspects. Dementia and Geriatric Cognitive Disorders. 1994;5:177–180. doi: 10.1159/000106718. [DOI] [PubMed] [Google Scholar]
- 23.Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR, Harvey J, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;62:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Leeuw FE, De Groot JC, Bots ML, Witteman JCM, Oudkerk M, Hofman A, et al. Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. Journal of neurology. 2000;247:291–296. doi: 10.1007/s004150050586. [DOI] [PubMed] [Google Scholar]
- 26.Spence JD, Hegele RA. Noninvasive phenotypes of atherosclerosis similar windows but different views. Stroke. 2004;35:649–653. doi: 10.1161/01.STR.0000116103.19029.DB. [DOI] [PubMed] [Google Scholar]
- 27.Potter GM, Doubal FN, Jackson CA, Sudlow CL, Dennis MS, Wardlaw JM. Lack of association of white matter lesions with ipsilateral carotid artery stenosis. Cerebrovascular Diseases. 2012;33:378–384. doi: 10.1159/000336762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pico F, Dufouil C, Levy C, Besancon V, de Kersaint-Gilly A, Bonithon-Kopp, et al. Longitudinal study of carotid atherosclerosis and white matter hyperintensities: the EVA-MRI cohort. Cerebrovascular Diseases. 2002;14:109–115. doi: 10.1159/000064741. [DOI] [PubMed] [Google Scholar]
- 29.Marshall RS, Lazar RM. Pumps, Aqueducts, and Drought Management Vascular Physiology in Vascular Cognitive Impairment. Stroke. 2011;42:221–226. doi: 10.1161/STROKEAHA.110.595645. [DOI] [PMC free article] [PubMed] [Google Scholar]