Abstract
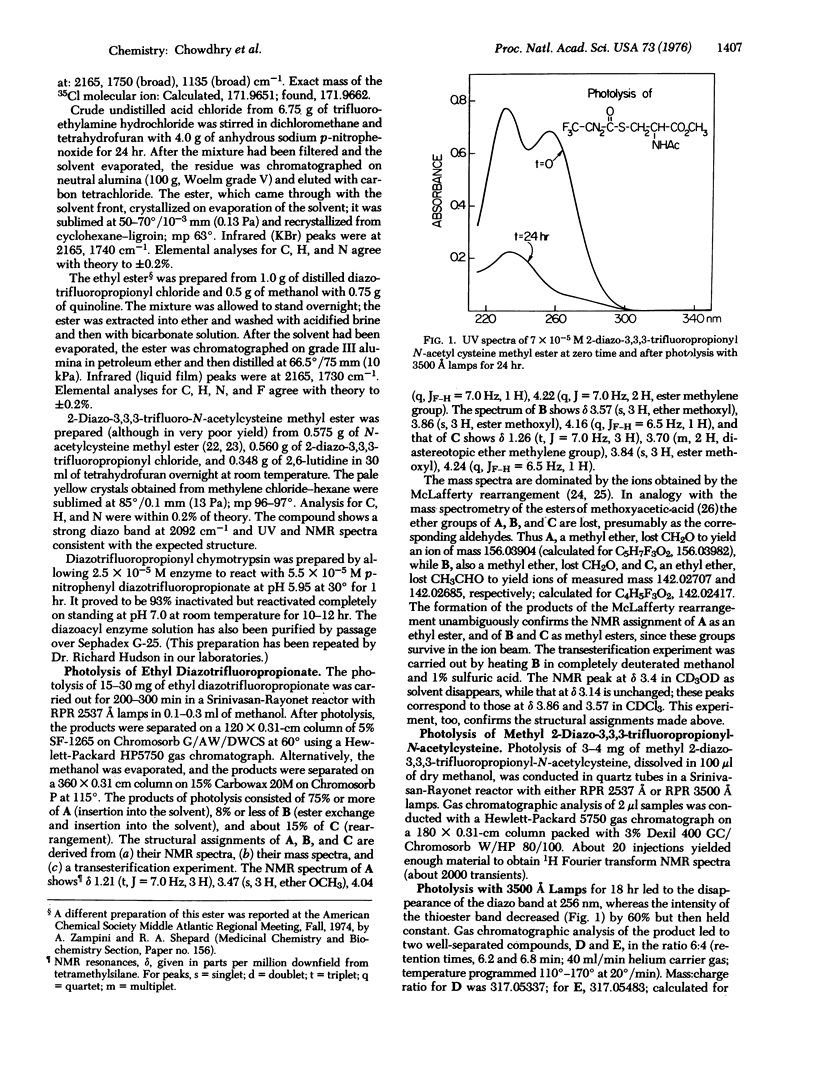
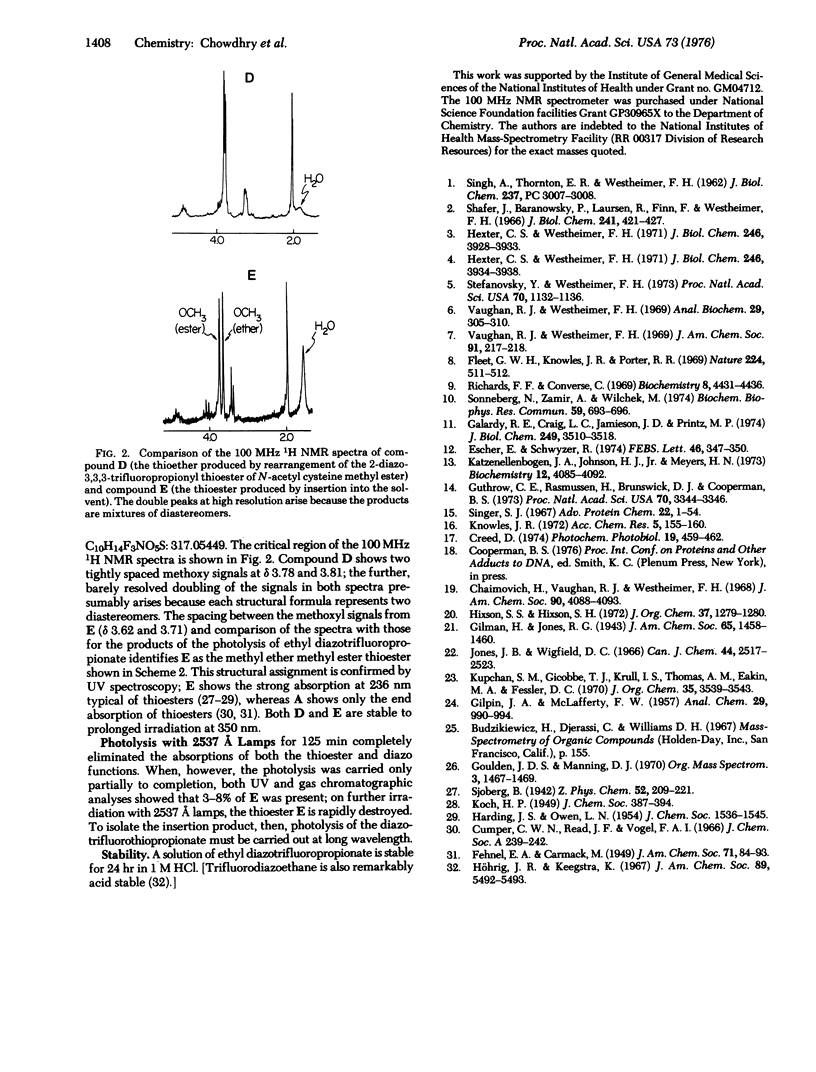
2-Diazo-3,3,3-trifluoropropionyl chloride has been synthesized from trifluorodiazoethane and phosgene. Its derivatives are acid stable, can be used to label enzymes, and undergo photolysis with substantially less rearrangement than do derivatives of other known diazoacyl reagents designed for photoaffinity labeling. In particular, the diazotrifluoropropionyl thioester of methyl N-acetylcysteine undergoes photolysis in methanol with about 40% insertion into the - OH bond of the solvent; by contrast, photolysis of other diazoacyl thioesters gives substantially quantitative Wolff rearrangement. The trifluoro compounds hold promise for the photoaffinity labeling of thiols.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Converse C. A., Richards F. F. Two-stage photosensitive label for antibody combining sites. Biochemistry. 1969 Nov;8(11):4431–4436. doi: 10.1021/bi00839a031. [DOI] [PubMed] [Google Scholar]
- Creed D. Photochemical probes for biological interactions. Photochem Photobiol. 1974 Jun;19(6):459–462. doi: 10.1111/j.1751-1097.1974.tb06538.x. [DOI] [PubMed] [Google Scholar]
- Escher E., Schwyzer R. p-Nitrophenylalanine, p-azidophenylalanine, m-azidophenylalanine, and o-nitro-p-azido-phenylalanine as photoaffinity labels. FEBS Lett. 1974 Sep 15;46(1):347–350. doi: 10.1016/0014-5793(74)80403-5. [DOI] [PubMed] [Google Scholar]
- Galardy R. E., Craig L. C., Jamieson J. D., Printz M. P. Photoaffinity labeling of peptide hormone binding sites. J Biol Chem. 1974 Jun 10;249(11):3510–3518. [PubMed] [Google Scholar]
- Guthrow C. E., Rasmussen H., Brunswick D. J., Cooperman B. S. Specific photoaffinity labeling of the adenosine 3':5'-cyclic monophosphate receptor in intact ghosts from human erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3344–3346. doi: 10.1073/pnas.70.12.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexter C. S., Westheimer F. H. Intermolecular reaction during photolysis of diazoacetyl -chymotrypsin. J Biol Chem. 1971 Jun 25;246(12):3928–3933. [PubMed] [Google Scholar]
- Hexter C. S., Westheimer F. H. S-carboxymethylcysteine from the photolysis of diazoacyl trypsin and chymotrypsin. J Biol Chem. 1971 Jun 25;246(12):3934–3938. [PubMed] [Google Scholar]
- Katzenellenbogen J. A., Johnson H. J., Jr, Myers H. N. Photoaffinity labels for estrogen binding proteins of rat uterus. Biochemistry. 1973 Oct 9;12(21):4085–4092. doi: 10.1021/bi00745a010. [DOI] [PubMed] [Google Scholar]
- SINGH A., THORNTON E. R., WESTHEIMER F. H. The photolysis of diazoacetylchymotrypsin. J Biol Chem. 1962 Sep;237:3006–3008. [PubMed] [Google Scholar]
- Shafer J., Baronowsky P., Laursen R., Finn F., Westheimer F. H. Products from the photolysis of diazoacetyl chymotrypsin. J Biol Chem. 1966 Jan 25;241(2):421–427. [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Zamir A., Wilchek M. A photo-induced reaction of chloramphenicol with E. coli ribosomes: covalent binding of the antibiotic and inactivation of peptidyl transferase. Biochem Biophys Res Commun. 1974 Jul 24;59(2):693–696. doi: 10.1016/s0006-291x(74)80035-5. [DOI] [PubMed] [Google Scholar]
- Stefanovsky Y., Westheimer F. H. Diazoacetyl subtilisin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1132–1136. doi: 10.1073/pnas.70.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan R. J., Westheimer F. H. A method for marking the hydrophobic binding sites of enzymes. An insertion into the methyl group of an alanine residue of trypsin. J Am Chem Soc. 1969 Jan 1;91(1):217–218. doi: 10.1021/ja01029a055. [DOI] [PubMed] [Google Scholar]
- Vaughan R. J., Westheimer F. H. A titration method for bovine trypsin. Anal Biochem. 1969 May;29(2):305–310. doi: 10.1016/0003-2697(69)90314-5. [DOI] [PubMed] [Google Scholar]



