Abstract
Voluntary exercise reduces the incidence of stress-related psychiatric disorders in humans and prevents serotonin-dependent behavioral consequences of stress in rodents. Evidence reviewed herein is consistent with the hypothesis that exercise increases stress resistance by producing neuroplasticity at multiple sites of the central serotonergic system, which all help to limit the behavioral impact of acute increases in serotonin during stressor exposure.
Keywords: wheel running, learned helplessness, anxiety, depression, prefrontal cortex, dorsal raphe nucleus
INTRODUCTION
Stress-related psychiatric disorders, including anxiety and depression, are currently the most common mental health disorders in the United States. By 2020, depression will have the second highest lifetime burden of disease, exceeded only by heart disease. Exposure to stressful life events is a primary causal factor in the development of depression and anxiety, which often are comorbid. Although stress can have an impact on mental health in a large number of individuals, not all individuals exposed to a traumatic event develop full-blown depression or anxiety. Identification of variables that influence the development of stress-related psychiatric disorders is of primary importance to gain insight into the neurobiological basis of these diseases and to develop more effective pharmacological treatments.
Exercise is one variable that can influence individuals’ responses to stressful events. The stress-buffering effects of exercise are demonstrable at many levels, including neuroendocrinological, immunological, and behavioral. For example, both the human and animal literatures indicate that exercise can constrain the activation of the sympathetic nervous system in response to stress (e.g., (10,18)), attenuate mild stressor-evoked increases in stress hormones (3), prevent stress-induced immunosuppression (10), and reduce the incidence and severity of stress-related psychiatric disorders, such as depression and anxiety. We will review our work in rodent models focusing on the neurobiological mechanisms by which exercise prevents the negative affective and cognitive consequences of stressor exposure.
Voluntary wheel running is a useful method of increasing physical activity status in rodents because it avoids the potential confounding effects of exposure to the chronic stress that forced exercise paradigms, such as treadmill training or forced swimming, could impose on the animals. Consistent with the protective effects of exercise in humans, we and others have observed that rats allowed to run voluntarily on in-cage running wheels display typical anxiolytic and antidepressant responses in several animal models and are protected against depressive- and anxiety-like behavioral consequences of exposure to a variety of stressors. Using the established learned helplessness (LH) paradigm, we have reported that wheel running blocks the exaggerated fear and deficits in instrumental escape learning produced by exposure to an acute uncontrollable stressor (6,16).
Accumulating data support the hypothesis that wheel running prevents the behavioral consequences of uncontrollable stress by producing resistance against the behavioral impact of excessive 5-hydroxytryptamine (5-HT). Hyper-activation and sensitization of 5-HT neurons in the dorsal raphe nucleus (DRN) are critical for the development and expression, respectively, of the behavioral consequences of uncontrollable stress. The neuroplasticity produced by exercise leading to resistance against the anxiogenic effects of 5-HT could occur in DRN-afferent regions that can modulate DRN 5-HT activity during stressor exposure; the DRN itself, such as a facilitation of 5-HT1A inhibitory autoreceptor-mediated inhibition of 5-HT neurons; and/or DRN projection sites, such as reduced expression or sensitivity of postsynaptic 5-HT receptors in brain regions critical for the expression of stress-induced behaviors. The current article will discuss each facet of this hypothesis and conclude that voluntary exercise produces plasticity at multiple sites within the central 5-HT system that converge to facilitate stress resistance. The hypothesis supported herein has important clinical implications and predictive value.
STRESS-RELATED BEHAVIORS AND LEARNED HELPLESSNESS
Stressor exposure is an important causal factor in the development of anxiety and depressive disorders, including posttraumatic stress disorder, generalized anxiety, and major depression. Accordingly, symptoms of both anxiety and depressive disorders include behaviors that can be produced by stressor exposure, including social avoidance, inappropriate or exaggerated fear, and alterations in learning and memory processes. Stressors that are repeated over a long duration, severe in nature, unpredictable, or uncontrollable often are the most detrimental to mental and physical health. Because of the critical role for stress in the development of depression and anxiety, animal models using exposure to stressors can help to identify potential neurobiological mechanisms of depression and anxiety.
Exposure to an acute uncontrollable stressor produces behaviors in rodents that resemble symptoms of human anxiety and depression. Rats exposed to a series of uncontrollable shocks over a 2-h period later display a constellation of behaviors including a reduction in social exploratory behavior, exaggerated conditioned fear, and interference with instrumental escape behavior (see (28)). Importantly, these behavioral outcomes depend on the uncontrollability of the stressor; they do not occur if the stressor is controllable.
The behavioral consequences of stressor exposure that depend on the controllability of the stressor have been called LH (see (13,29) for reviews of LH). The acquisition, or development, of LH typically is produced by exposing rats to a series of uncontrollable tail shocks. The expression of LH is observed during behavioral testing at a later time point, typically 24 h later. The behavioral consequences of uncontrollable stress are transsituational (i.e., they occur in environments different from the one in which stressor exposure occurred), are sensitive to pharmacological therapeutics, and have been argued to represent animal analogs of human anxiety and depression. Because of the close resemblance of LH behaviors to symptoms of human anxiety and depression, we have used uncontrollable stress and LH to begin to elucidate factors potentially involved in the protective effect of exercise against stress-related psychiatric disorders.
EXERCISE AND STRESS RESISTANCE
Using the LH model, we have reported that voluntary wheel running can create resistance against the anxiety- and depression-like behavioral consequences of stressor exposure. Rats allowed voluntary access to running wheels are protected against the social avoidance (Greenwood B., unpublished observation, 2011), exaggerated freezing, and the escape deficit produced by uncontrollable stress (13,16,21). The protective effect of wheel running against LH does not seem to be dependent on running distance. We and others typically observe no significant correlation between running distance and anxiety-related behavioral outcomes. Although running distance may not be related directly to the effect of exercise on emotional behavior, distance is correlated with exercise-induced enhancements in spatial learning and memory and related neuroplastic changes including the exercise-induced increase in brain-derived neurotrophic factor (BDNF) and neurogenesis. On the other hand, the stress-buffering effect of wheel running does depend on the duration of previous wheel running, whereby 6 wks of wheel access is sufficient to prevent LH behaviors, but 3 wks is not (14). This time course carries over to several of the neurobiological changes we have observed after wheel running as well (discussed below). In addition to preventing LH, we have observed that wheel running also reverses a long-lasting, modified version of LH (20). This observed therapeutic effect also is dependent on the duration of wheel access.
All of our experiments examining the impact of voluntary exercise on the stress responses to date have used single-housed animals. Single-housed animals, each with its own wheel, allow each animal’s running behavior to be analyzed and correlated with other dependent measures. However, rats are social creatures and single housing may be a less preferred housing environment compared with social housing. It is possible that the single housing conditions interact with exercise in such a way that only isolated rats will benefit from wheel running. For example, single housing conditions could facilitate the induction of LH in sedentary rats, and wheel running could buffer the long-term impact of isolated housing and not uncontrollable stressor exposure, per se.
To determine if housing conditions have an impact on the protective effects of wheel running against LH, male F344 rats were either single- or pair-housed upon arrival to the colony. Rats had access to either a locked (sedentary) or a freely mobile (run) running wheel (1 wheel/cage) for 6 wk. After 6 wks of the sedentary or run conditions, rats were either exposed to uncontrollable tailshock or were left in their home cages (n = 5 per group). Analysis of the running data revealed that average weekly running distance recorded by the wheels in the cages of the pair-housed rats was twice that recorded by the wheels in the cages of the single-housed rats (F1,11 = 17.6; P < 0.01; Fig. 1A), suggesting that pair-housed rats share equal access to the running wheel. Body weights are depicted in Figure 1B. Interestingly, single-housed rats gained less weight over time than their pair-housed counterparts, regardless of exercise condition (F1,29 = 19.08; P < 0.001), and wheel running did not affect body weight gain. The difference in weights between the single- and pair-housed rats could reflect an effect of chronic stress imposed by chronic single housing. Upon behavioral testing, we observed that both single- and pair-housed sedentary rats displayed exaggerated shock-elicited freezing (Fig. 1C) and escape deficits (Fig. 1D) after uncontrollable stress. Wheel running, regardless of housing conditions, prevented these LH behaviors. Analysis of variance revealed a main effect of stress (F1,25 = 13.3; P < 0.05) and an exercise by stress interaction (F1,25 = 13.1; P < 0.05) on average freezing behavior. The main effects of both exercise (F1,25 = 28.1; P < 0.0001) and stress (F1,25 = 36.7; P < 0.0001) and the exercise by stress interaction (F1,25 = 14.8; P < 0.01) on average escape latency were all significant. Interestingly, there was a trend (F1,25 = 3.1; P = 0.08) for a main effect of housing conditions on escape latency, suggesting that 6 wk of pair housing may improve active stress-coping behaviors. However, the effect of housing occurred regardless of exercise or stress condition, indicating that effects of exercise are independent from those of social housing. These data exemplify the powerful protective effect of voluntary wheel running against the behavioral consequences of uncontrollable stress.
Figure 1.
Effect of housing conditions on the protective effect of wheel running against learned helplessness (LH) behaviors. Male F344 rats (n = 5 per group) were either single (1 rat per cage) or pair (2 rats per cage) housed for 6 wk. Rats in the sedentary condition (sed) had locked wheels and rats in the run condition had voluntary access to wheels (1 running wheel per cage). After 6 wk of these housing conditions, rats were either left undisturbed in their home cages (no stress) or were exposed to uncontrollable tail shock (stressed). Testing for shock-elicited freezing and escape behaviors took place 24 h later in shuttle boxes. A. Average weekly running distance recorded by the wheels in the cages of the single- and pair-housed run rats. B. Body weight gain over the course of the experiment. C. Average freezing behavior (over the course of a 20-min period) elicited by two brief foot shocks (0.6 mA) in the shuttle box. D. Average latency to escape from 25 escapable foot shocks (fixed ratio-2; 0.6 mA) administered in the shuttle box immediately after the freezing period. Data represent means ± SEM. *P < 0.05 relative to groups noted.
Although the stress-buffering effects of exercise are clear, the underlying mechanisms remain unresolved. Identification of the mechanisms involved in the protective effects of exercise against stress-induced behaviors could reveal novel targets for the treatment and, perhaps more importantly, prevention of stress-related affective disorders.
THE ROLE OF SEROTONIN IN LEARNED HELPLESSNESS
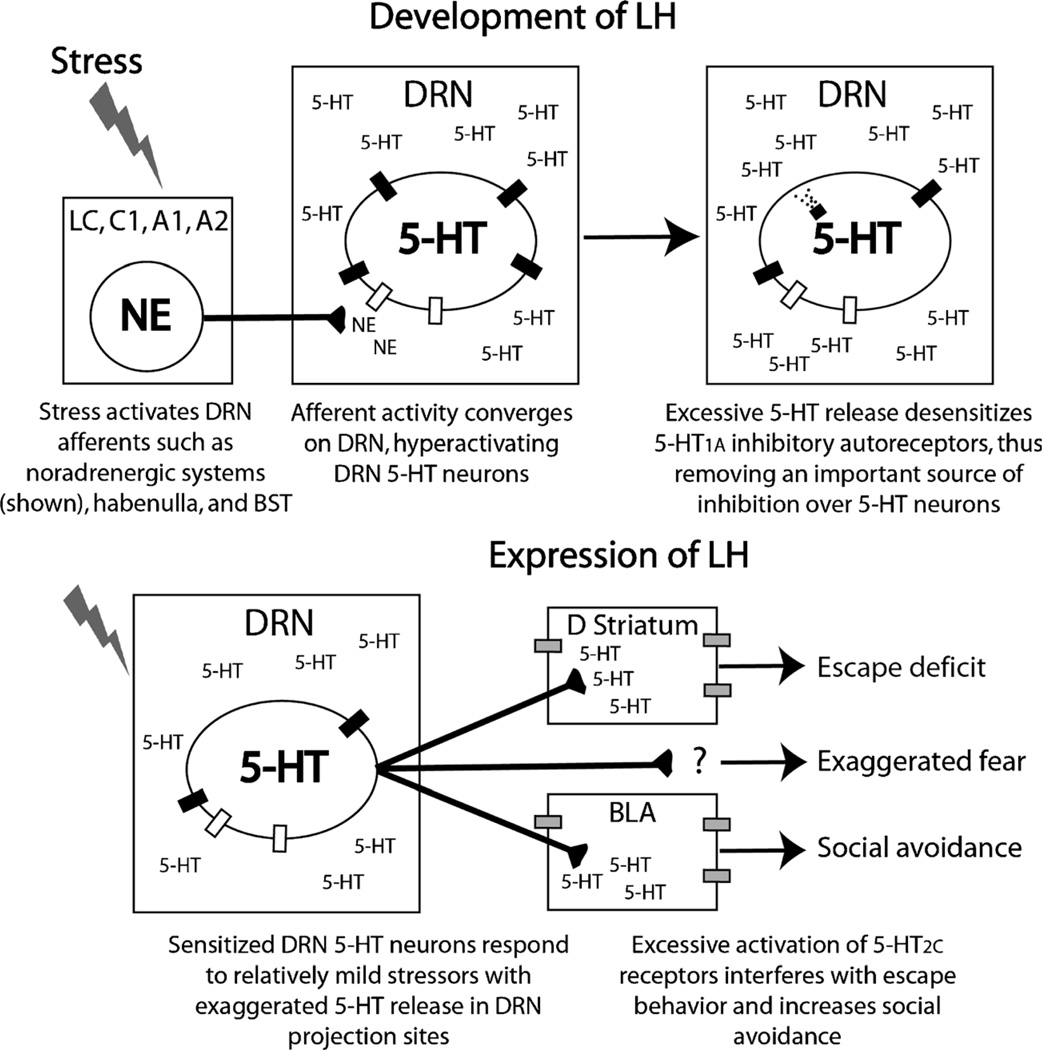
The central 5-HT system plays a pivotal role in the development and expression of LH behaviors. The DRN is a distinct brainstem structure that contains a high concentration of ascending 5-HT neurons. 5-HT neurons in the DRN have long been associated with depression, anxiety, and behavioral responses to stress. The efferent and afferent projections of the DRN contribute to its role in stress-related mood disorders. There are, for example, dense 5-HT projections from the DRN to structures involved in fear, anxiety, and depression, such as the prefrontal cortex (PFC), striatum, bed nucleus of the stria terminalis, amygdala, periaqueductal gray, and locus coeruleus (LC). The DRN also receives projections from these regions that can modulate the activity of DRN 5-HT neurons.
A growing body of evidence, led primarily by Maier et al. (see (29) for a review), suggests that the development of LH is produced by hyperactivation and sensitization of 5-HT neurons in the DRN. Immunohistochemical and microdialysis studies indicate that uncontrollable stress activates 5-HT neurons in the DRN more than controllable stress of a matched intensity and duration. This hyperactivation of 5-HT neurons during stressor exposure leads to the expression of LH by sensitizing DRN 5-HT neurons so that during later exposure to mildly stressful or typically emotionally neutral stimuli, sensitized DRN 5-HT neurons respond in an exaggerated manner with excessive 5-HT release in brain regions important for the expression of anxiety. The mechanism underlying how hyper-activation of 5-HT neurons leads to sensitization of 5-HT neurons is not yet clear but could involve desensitization of 5-HT1A inhibitory autoreceptors that are particularly sensitive to ligand-induced internalization (discussed later). What is clear is that, for a period of time after uncontrollable stress, relatively harmless situations will elicit a large 5-HT response and will manifest themselves as anxiety- or depression-like behaviors.
Hyperactivation and sensitization of DRN 5-HT neurons are clearly necessary and sufficient to produce LH. Hyperactivation of DRN 5-HT neurons is necessary for the development (i.e., during stress) of LH. Lesions of the DRN, as well as pharmacological inhibition of the DRN during uncontrollable stress, block the behavioral consequences of uncontrollable stress. On the other hand, sensitization of the DRN is necessary for the expression (i.e., during behavioral testing) of LH. Manipulations that inhibit DRN 5-HT neurons, such as intra-DRN injections of the 5-HT1A agonist 8-hydroxy-2-(di-N-propylamino) tetralin (8-OH-DPAT), prevent the expression of the behavioral effects of uncontrollable stress including the reduction in social exploration, exaggerated fear conditioning, and escape deficits (4). Similarly, acute increases in extracellular 5-HT or manipulations that activate DRN 5-HT neurons produce behaviors that resemble LH in the absence of stress (13,19).
In the last few years, we have made significant progress toward identifying both the brain regions and the specific 5-HT receptors subserving the expression of specific LH behaviors (summarized in Fig. 2). Although the location and the receptor at which 5-HT acts to produce exaggerated freezing still eludes us, 5-HT2C receptors (5-HT2CR) in the basolateral nucleus of the amygdala (BLA) contribute to the social avoidance produced by uncontrollable stress (5), whereas recent unpublished data from our laboratory suggest that 5-HT2CR activation in the dorsal striatum contributes to the deficit in shuttle-box escape learning produced by uncontrollable stress. The role of the 5-HT2CR in LH behaviors is consistent with the therapeutic effects of 5-HT2CR antagonists, such as agomelatine, in ongoing clinical trials involving patients with major depression, and with known involvement of the 5-HT2CR in other anxiety-like behaviors in rodents.
Figure 2.
Proposed role of 5-hydroxytryptamine (5-HT) in the development and expression of learned helplessness (LH) behaviors. ■ indicates 5-HT1A autoreceptor; □, α1b-adrenergic receptor;  , 5-HT2C receptor; BLA, basolateral nucleus of the amygdala; BST, bed nucleus of the stria terminalis; NE, norepinephrine.
, 5-HT2C receptor; BLA, basolateral nucleus of the amygdala; BST, bed nucleus of the stria terminalis; NE, norepinephrine.
It may seem counterintuitive that an increase in 5-HT release can lead to LH behaviors, especially considering that an increase in 5-HT neurotransmission seems to be important for the therapeutic effects of antidepressant drugs, such as selective 5-HT reuptake inhibitors (SSRIs). However, SSRIs increase extracellular 5-HT immediately, yet the therapeutic benefits of SSRIs are not realized for weeks. In fact, an increase in anxiogenic and depressive symptoms often are reported during the onset of antidepressant treatment. It is possible that the rapid rise in extracellular 5-HT contributes to the exacerbation of clinical symptoms during the onset of pharmacotherapy, whereas consequences of a long-term elevation of 5-HT, such as downregulation of postsynaptic receptors or enhanced neural plasticity, contributes to the therapeutic effects of chronic antidepressant treatment.
EXERCISE AND CENTRAL SEROTONIN SYSTEMS
Examination of Figure 2 reveals several sites at which exercise-induced plastic changes in the central 5-HT system could help prevent the development or expression of LH. Plasticity within the DRN itself could help constrain activation of DRN 5-HT neurons or produce resistance against given increases in extracellular 5-HT. Wheel running also could reduce activation of DRN afferents that contribute to DRN hyperactivation during stressor exposure. Alternatively, wheel running could facilitate activation during stress of other DRN afferents that could inhibit DRN activity. Finally, wheel running could prevent the expression of LH behaviors by producing plasticity in DRN projection sites important for the expression of various LH behaviors, thus limiting the effects of 5-HT in sites efferent to the DRN.
Intra-DRN Plasticity and 5-HT1A Autoreceptors
Although clear progress has been made toward understanding the role of 5-HT in stress-related affective disorders and LH, less is known about the impact of exercise on central 5-HT systems. Motor activity stimulates 5-HT neurons (24), although it is difficult to determine if the increase in 5-HT activity reported in previous work reflects motor activity per se or the perceived stress of the forced motor task used. For this reason, it is particularly important to focus on effects of voluntary exercise on the 5-HT system. We have investigated the effects of voluntary wheel running on gene expression of several factors capable of influencing 5-HT neurotransmission within the DRN.
Two factors that can modulate central 5-HT neuro-transmission are 5-HT1B autoreceptors and 5-HT transporters (SERT). The 5-HT1B autoreceptors are terminal autoreceptors that can inhibit the release of 5-HT from 5-HT neurons, both in 5-HT cell body regions and in terminal regions. The SERT is responsible for the reuptake of 5-HT from the extracellular space back into 5-HT neurons and is a primary target of SSRIs. Using in situ hybridization, we have observed that wheel running reduces levels of messenger ribonucleic acid (mRNA) coding for 5-HT1B autoreceptors and SERT in the DRN (15). Because 5-HT1B autoreceptors and SERT can be expressed within the DRN or transported to DRN terminal regions, it is not yet clear how these changes impact 5-HT neurotransmission. It also is unclear how these changes could contribute to the resistance against LH produced by wheel running. The reduction in 5-HT1B receptor and SERT mRNA in the DRN is present after 3 wk of wheel running or less (15), but the protective effect of wheel running against LH is not observed until 6 wks of wheel running has elapsed (14).
In contrast to the rapid changes in 5-HT1B and SERT mRNA, we have observed a delayed increase in 5-HT1A auto-receptor mRNA in the DRN after wheel running (15,16). In fact, the increase in 5-HT1A mRNA follows a time course similar to that observed for the behavioral effects of exercise; whereby 6 wks, but not 3 wks of wheel running prevents LH behaviors and increases 5-HT1A mRNA (15).
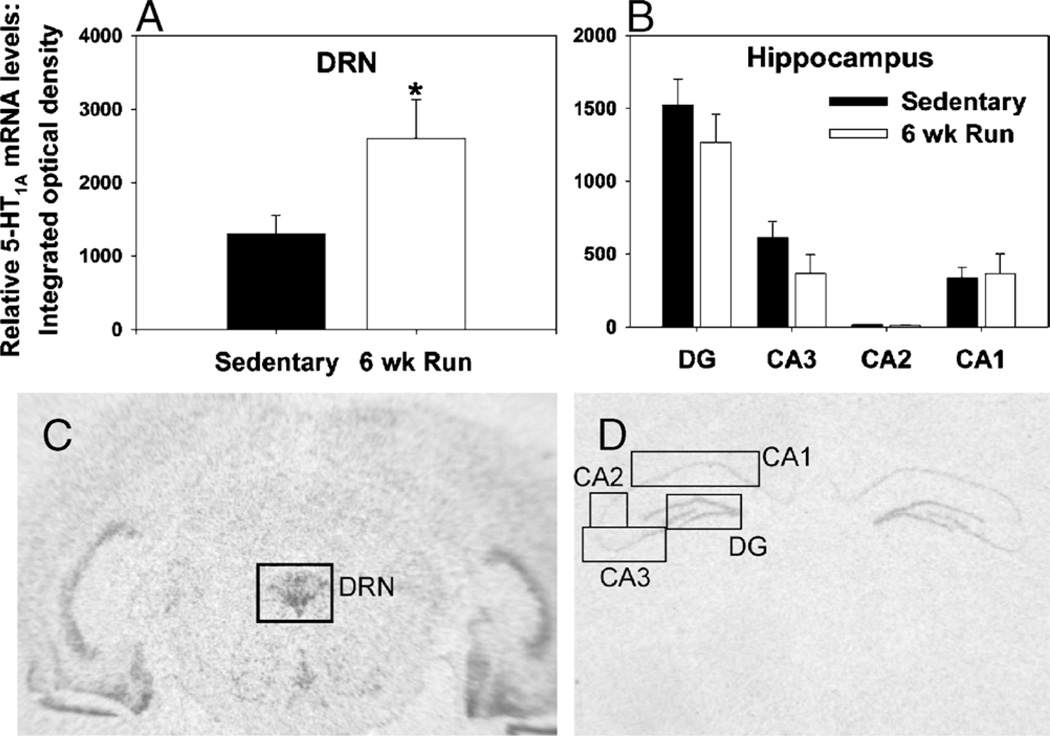
The 5-HT1A receptor has received much attention in the literature regarding its potential role in the etiology and treatment of depression and anxiety. The 5-HT1A receptors are expressed on non5-HT neurons as postsynaptic hetero-receptors and on 5-HT neurons throughout the raphe complex as presynaptic somatodendritic autoreceptors. The 5-HT1A autoreceptors are Go/Gi-protein linked receptors, activation of which opens inwardly rectifying K+ channels, inhibits the opening of voltage-gated calcium (Ca2+) channels, and inhibits both the firing rate of 5-HT neurons and the synthesis of 5-HT. Interestingly, the effect of wheel running on 5-HT1A mRNA seems to be selective for autoreceptors. The increase can be observed in both the DRN (Figs. 3A, C) and median raphe (15) but not in the hippocampus (Figs. 3B, D), an area rich in 5-HT1A heteroreceptor mRNA. A microdialysis study revealed that the increase in 5-HT1A autoreceptor mRNA is accompanied by a reduction in baseline levels of extracellular 5-HT in the DRN of physically active, relative to sedentary, rats (Greenwood B., unpublished observation, 2010). This could reflect enhanced 5-HT1A-mediated autoinhibition of DRN 5-HT neurons during resting conditions after 6 wks of voluntary exercise.
Figure 3.
Effect of voluntary exercise on 5-hydroxytryptamine (5-HT)1A messenger ribonucleic acid (mRNA) levels in the dorsal raphe nucleus (DRN) and hippocampus. Single-housed male F344 rats (n = 8 per group) either remained sedentary with locked wheels (Sedentary) or were allowed voluntary access to running wheels for 6 wk (6-wk run). Rats were killed by rapid decapitation, and brains were processed with in situ hybridization for 5-HT1A mRNA. A. 5-HT1A mRNA levels in the DRN, *P < 0.05. B. 5-HT1A mRNA levels in the subfields of the hippocampus. Representative autoradiographs show in situ hybridization for 5-HT1A mRNA in the DRN (C) and hippocampus (D) of a sedentary rat. Data represent mean ± SEM.
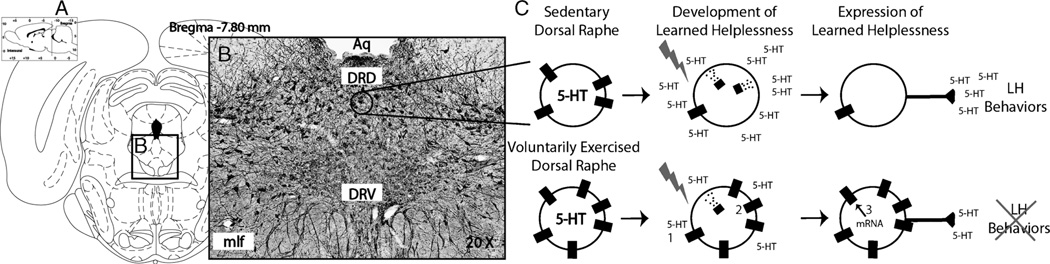
An increase in 5-HT1A autoreceptors in the DRN of physically active rats could contribute to the protective effects of exercise against LH by limiting the ability of stress to sensitize DRN 5-HT neurons. A greater number of 5-HT1A autoreceptors could accomplish this effect through several mechanisms, summarized in Figure 4. The first is a simple increase in receptor numbers that could produce resistance against 5-HT1A desensitization in the face of a given increase in intra-DRN 5-HT. Somatodendritic 5-HT1A autoreceptors are extremely sensitive to ligand-induced desensitization. Desensitization refers to a decrease in response of the receptor to its endogenous ligand. Desensitization can be achieved through pretranslational or posttranslational mechanisms. Both stress and single administrations of either the 5-HT1A agonist 8-OH-DPAT (33) or the SSRI fluoxetine (33) can rapidly (within 1 h) desensitize, via receptor internalization, DRN 5-HT1A autoreceptors but not heteroreceptors. These data suggest that the acute rise in extracellular 5-HT in the DRN during uncontrollable stress could be enough to desensitize DRN 5-HT1A autoreceptors. Although it is well established that stress and 5-HT can desensitize 5-HT1A autoreceptors, whether this effect is modulated by stressor controllability remains a critical piece of missing data. If 5-HT1A autoreceptor desensitization contributes to stress-induced sensitization of DRN 5-HT neurons as hypothesized, then an increase in 5-HT1A autoreceptor number in the DRN after exercise could help resist DRN sensitization by simply providing a greater number of autoreceptors.
Figure 4.
Intra-dorsal raphe nucleus (DRN) plasticity produced by 6 wk of voluntary exercise potentially contributing to exercise-induced stress resistance. A. Graphical reconstruction of the region of the DRN in the rat brain (adapted from Paxinos and Watson (31)). B. Photomicrograph of a coronal section through the region of the rat DRN immunohistochemically labeled with an antibody against rat 5-hydroxytryptamine (5-HT). C. Cartoon representation of the effect of uncontrollable stress on DRN 5-HT neurons in sedentary (top) and physically active (bottom) rats. Numbers 1–3 represent potential sites of intra-DRN plasticity elicited by voluntary exercise. 1) Higher levels of 5-HT1A messenger ribonucleic acid (mRNA) in the DRN of physically active rats could lead to enhanced 5-HT1A-mediated autoinhibitory control over the activity of DRN 5-HT neurons, thereby preventing hyperactivation of the DRN during stressor exposure and limiting 5-HT release within the DRN. 2) Constraint over the activation of DRN 5-HT neurons, or a simple increase in 5-HT1A autoreceptor numbers, could limit potential 5-HT1A internalization, thus preventing DRN sensitization after stress. 3) Facilitated transcriptional recovery of 5-HT1A autoreceptors in the DRN of physically active rats could reverse stress-induced sensitization of DRN 5-HT neurons by restoring 5-HT1A autoreceptor-mediated inhibition of 5-HT neurons during behavioral testing. Aq indicates cerebral aqueduct; DRD, dorsal aspect of the DRN; DRV, ventral aspect of the DRN; mlf, medial longitudinal fasciculus; LH, learned helplessness.
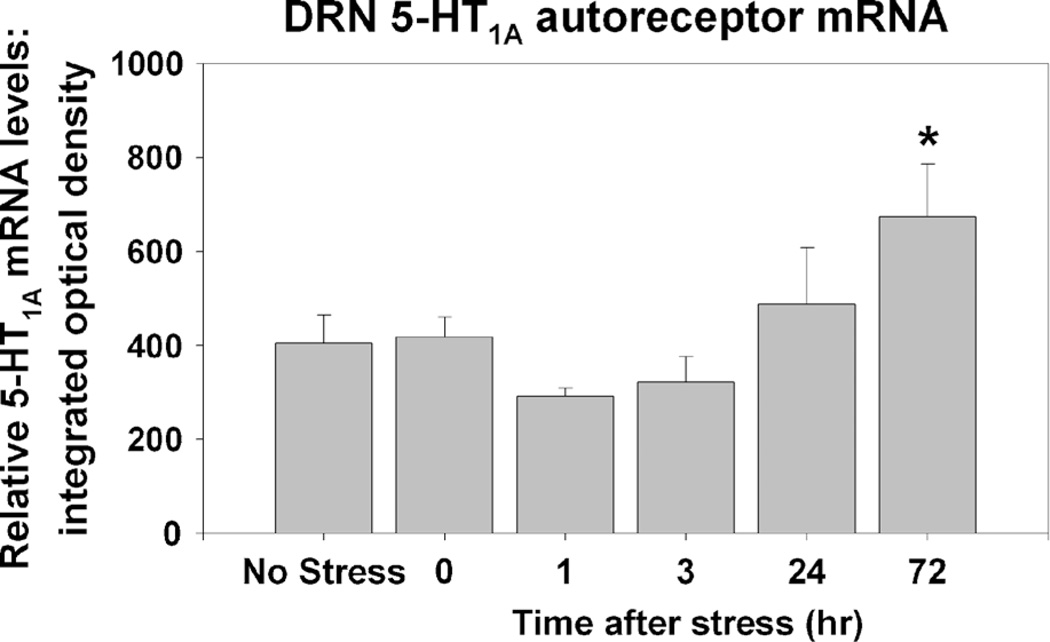
A second mechanism by which a greater capacity to transcribe 5-HT1A autoreceptors could help prevent DRN sensitization and LH is by facilitating transcriptional recovery of 5-HT1A autoreceptors. Pharmacological inactivation of DRN 5-HT1A autoreceptors with the alkylating agent N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline produces a compensatory increase in transcription of the 5-HT1A gene, which corresponds to the recovery of 5-HT1A protein expression (32). We have evidence that a similar mechanism underlies recovery of 5-HT1A autoreceptors after uncontrollable stress. Figure 5 depicts levels of 5-HT1A mRNA in the DRN assessed with in situ hybridization. Sedentary rats were either left in their home cages (no stress) or were exposed to uncontrollable tail shock stress and killed at various time points later. We observed a slight (nonsignificant) decrease in 5-HT1A mRNA in the DRN 1 and 3 h after uncontrollable stress. It is unlikely that this slight reduction in 5-HT1A gene transcription contributes to the behavioral consequences of uncontrollable stress because the behaviors are present rapidly after stressor termination, and 5-HT1A receptor internalization would have much more rapid functional effects in terms of the inability of 5-HT1A autoreceptors to inhibit 5-HT neuronal activity. However, of interest is the observed rate of recovery of 5-HT1A gene transcription. Notice that levels of 5-HT1A mRNA 72 h after stress are greater than those of nonstressed rats. This increase in 5-HT1A mRNA levels could represent a compensatory mechanism controlling the recovery of 5-HT1A protein expression. In fact, the elevation of DRN 5-HT1A mRNA shown in Figure 5 occurs in a time course consistent with the disappearance of LH behaviors after uncontrollable stress, which are absent by 72 h (27). Because wheel running affects 5-HT1A gene transcription, it is possible that wheel running could facilitate the transcriptional recovery of 5-HT1A autoreceptors after stress, thereby restoring 5-HT1A-mediated autoinhibition of DRN 5-HT neurons during behavioral testing and preventing the expression of LH behaviors.
Figure 5.
Effect of uncontrollable tail shock stress on levels of 5-hydroxytryptamine (5-HT)1A messenger ribonucleic acid (mRNA) in the dorsal raphe nucleus (DRN). Adult, male F344 rats (n = 8 per group) were either left undisturbed in their home cages (no stress) or were exposed to uncontrollable tail shock and were killed immediately (0), 1, 3, 24, or 72 h after stress. In situ hybridization was used to visualize levels of 5-HT1A mRNA in DRN slices. Data represent means ± SEM. *P < 0.05 relative to no-stress, 0-, 1-, and 3-h groups.
A third mechanism by which a potential increase in 5-HT1A autoreceptors could prevent desensitization of DRN 5-HT neurons is by helping to constrain the activity of DRN 5-HT neurons during stress, thereby limiting the release of 5-HT into the DRN and reducing 5-HT1A internalization. Indeed, wheel running does constrain activation of DRN 5-HT neurons during uncontrollable stress. Using c-Fos as a marker of neuronal activation, we observed that 6 wk, but not 3 wk, of wheel running reduced the number of double c-Fos/5-HT-positive cells in the DRN elicited by uncontrollable stress (14,16). Importantly, the greatest stress-buffering effect of wheel running on DRN 5-HT neuronal activity occurs in the dorsal aspect of the rostral-mid DRN (16), the same region of the DRN where 6 wk of wheel running has the greatest effect of 5-HT1A mRNA (15).
Supporting the ability of enhanced autoinhibition of DRN 5-HT neurons to contribute to stress resistance, Kaiyala et al. (25) have performed a series of experiments indicating that higher levels of terminal 5-HT1B autoreceptors are associated with stress resistance and anxiolytic effects in animal models. Moreover, positron emission tomography studies have revealed that human depression could be associated with a decrease in 5-HT1A autoreceptor binding potential in the raphe (8). Especially interesting is that while we propose that an increase in DRN 5-HT1A autoreceptors could be important for the protective effect of exercise against stress-related psychiatric disorders, a decrease in 5-HT1A autoreceptors has been proposed as a mechanism underlying the therapeutic effects of SSRIs (1). Although exercise and chronic SSRI treatment may have opposite effects on the 5-HT1A autoreceptor, they may similarly reduce the expression or sensitivity of specific postsynaptic 5-HT receptors. Understanding which of these changes is critical for the beneficial effects of the manipulations remains an important challenge. Regardless of the specific role of 5-HT1A autoreceptors, it is possible that constraint over activation of the 5-HT system is an adaptive feature of the stress response that is dysregulated in stress-related psychiatric disorders and facilitated by physical activity.
DRN Afferents: the Locus Coeruleus and Medial Prefrontal Cortex
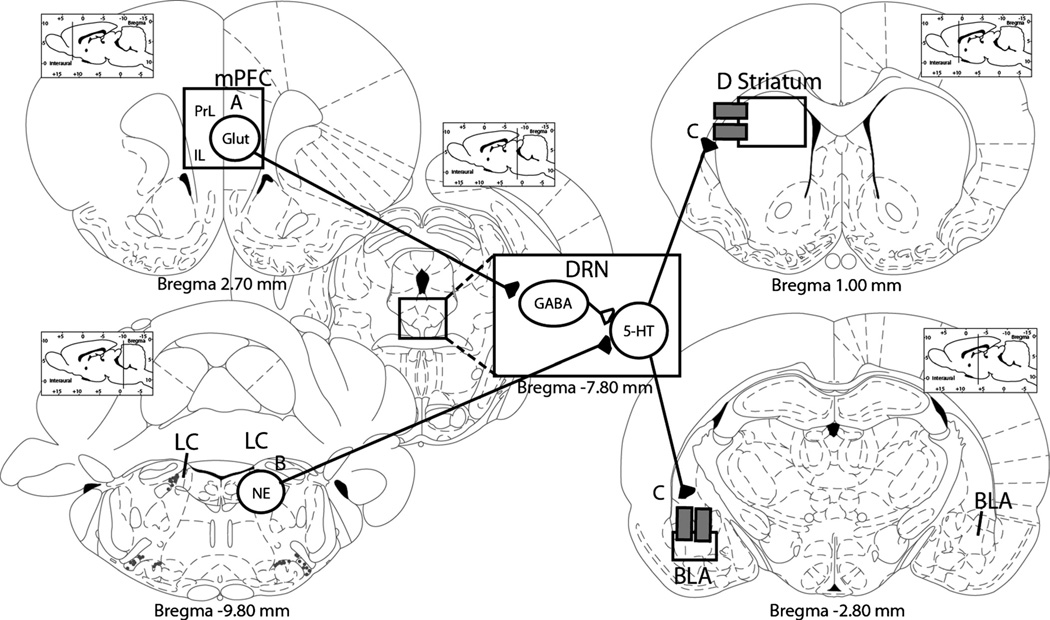
Exercise-induced plasticity within the DRN, such as increased numbers of 5-HT1A autoreceptors, could contribute to the constraint over activation of DRN 5-HT neurons during stress and the prevention of LH. Changes in regions afferent to the DRN that can modulate activity of DRN 5-HT neurons also could contribute to 5-HT constraint. Wheel running could decrease activation during stress of excitatory inputs to the DRN or increase activation of inhibitory inputs to the DRN. Any of these scenarios (depicted in Fig. 6) could potentially reduce activation of 5-HT neurons in the DRN during stress, thus preventing DRN sensitization. These scenarios are not necessarily mutually exclusive. In fact, there is evidence that both occur in the brains of voluntarily exercised rats during exposure to uncontrollable stress.
Figure 6.
Plasticity produced by 6 wk of voluntary exercise in dorsal raphe nucleus (DRN) afferents and projection sites potentially contributing to exercise-induced stress resistance. A. Increased activation of the medial prefrontal cortex (mPFC) during stressor exposure could actively inhibit DRN 5-hydroxytryptamine (5-HT) neurons. B. Constraint of norepinephrine (NE) neurons in adrenergic regions, such as the locus coeruleus (LC), could reduce excitation of DRN 5-HT neurons during stressor exposure. C. A reduction in expression or sensitivity of 5-HT2C receptors in DRN projection sites critical for the expression of learned helplessness (LH) behaviors could reduce the behavioral impact of increases in 5-HT in these regions during behavioral testing. BLA, basolateral nucleus of the amygdala; D striatum, dorsal striatum; Glut, glutamate; IL, infralimbic cortex; PrL, prelimbic cortex. Brain diagrams are graphical reconstructions of coronal slices through the rat brain adapted from Paxinos and Watson (31).
Of note among the regions contributing to DRN hyper-activation during uncontrollable stress is the noradrenergic LC (Fig. 2). Although the DRN receives NE input from many regions other than the LC, including the C1, A1, and A2, the LC provides the majority of input and long has been implicated in stress-related behaviors, including LH. The NE neurons in the LC project to the DRN and can stimulate 5-HT neurons through a mechanism involving α1b-ADRs (Fig. 2). The contribution of LC NE to stress-induced activity of DRN 5-HT neurons is exemplified by the observations that LH behaviors are sensitive to manipulations of the LC and NE activity in the DRN (12,35).
The contribution of the LC to stress-induced hyperactivation of DRN 5-HT neurons is especially relevant for exercise research because the NE system and the LC are particularly sensitive to physical activity. Noradrenergic systems, such as the LC and other brainstem catecholaminergic regions, are activated by exercise (7), likely reflecting the increase in sympathetic nervous system activity that is required to maintain activity. Increased NE activity has been suggested as a mechanism for several of the beneficial effects of exercise, including improved hippocampus-dependent learning and memory and increased memory-related gene expression such as BDNF (23). Wheel running also increases NE content in several brain regions including brainstem raphe and catecholaminergic regions (6,7). These data suggest that the NE system is indeed sensitive to exercise and could provide the necessary signal to increase growth factors that could support some of the synaptic and neurocircuitry changes that are unique to exercise.
Although speculative, a mechanism exists whereby repeated activation of DRN-projecting NE neurons in the LC also could contribute to the observed increase in 5-HT1A gene transcription produced by wheel running. The 5-HT1A gene expression is regulated by a complex balance of gene promoters and repressors, including the promoters nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and specificity protein 1, and the repressors nuclear-deformed epidermal autoregulatory factor 1-related protein and 5’ repressor element under dual repression binding protein-1 (Freud-1; (30)). Because of its relative specificity for the 5-HT1A gene and its powerful control over constitutive levels of 5-HT1A expression in 5-HT neurons, Freud-1 is an attractive candidate for exercise modulation. Increased intra-cellular Ca2+ and subsequent activation of calcium/calmodulin-dependent protein kinase type IV has been reported to inhibit Freud-1 activity posttranslationally, thereby derepressing 5-HT1A gene expression (30). It is therefore possible that an increase in intracellular Ca2+ in DRN 5-HT neurons elicited by repeated activation of Gq-linked α1b-ADR could enhance 5-HT1A transcription by inhibition of the 5-HT1A repressor Freud-1.
In addition to repeatedly activating the NE system, we (18), as well as Dishman et al. (6,7) and Dun et al. (9), have observed that physical activity produces an adaptation in the LC that helps to constrain LC activity during exposure to nonexercise stressors, such as uncontrollable shock. Constraint over stress-induced activity of LC NE neurons that project to the DRN could help prevent LH behaviors by limiting α1b-ADR-mediated excitation of DRN 5-HT neurons (Fig. 6). Importantly, the anatomical localization of the effect of exercise on DRN 5-HT neuronal constraint coincides with the relative NE innervation of the DRN, such that both are greater in the rostral-mid DRN relative to other DRN subregions. The “cross-stressor habituation” that could occur in the NE system after exercise is intriguing because it is opposite to the sensitization of the stress response that typically occurs when exposure to a novel stressor follows habituation to a different, repeated one.
Although constraint over activity of LC NE neurons during stress certainly could contribute to the protective effect of wheel running against LH and exercise-induced stress resistance, it is unlikely to be the only mechanism. Many stressors activate the LC, but repeated exposure to stress certainly does not prevent LH and increase stress resistance. Cross-stressor habituation might be one feature making exercise unique from other physiological stressors and contributing to exercise-induced stress resistance. Another feature that could make exercise unique is that it might recruit other neural systems, such as higher order executive function circuits or reward systems; plasticity in which could interact with the 5-HT system in such a way as to constrain its activity during stressor exposure. Indeed, wheel running is a voluntary and rewarding experience, thus exercise-induced plasticity in circuits sensitive to behavioral control and reward might be expected.
A recent report from our laboratory discusses the plasticity in the reward system after voluntary exercise and the relevance of these changes to stress resistance (17). Less data directly implicate circuits sensitive to behavioral control in exercise-induced stress resistance. However, supporting the involvement of behavioral control is the fact that, compared with other stressors, exercise typically is voluntary and controllable. In fact, the majority of data (e.g., (2,26)) suggest that only controllable (i.e., voluntary) exercise will prevent depression- and anxiety-like behavioral effects of stress, such as LH, thereby implicating neural circuits involved in the perception of behavioral control in the mechanisms by which exercise produces stress resistance. The medial PFC (mPFC) is one region particularly sensitive to behavioral control (28).
The mPFC has been described as an “executive control” center that functions to guide appropriate behavioral responses to stimuli based on integrated information from multiple sensory modalities. The prelimbic (PL) and infra-limbic (IL) regions of the ventral mPFC project to limbic, midbrain, and brainstem structures involved in integrative aspects of the stress response. These projections are important for the reported role of the mPFC in the regulation of neuroendocrine, autonomic, and behavioral responses to a variety of stressors. Although there are inconsistencies in the literature, the mPFC is thought to dampen, or constrain, many aspects of the stress response. Accordingly, damage to the mPFC is implicated in stress-related psychiatric disorders. Based on these data, the mPFC has immerged as a region central in the production of stress resistance.
It makes intuitive sense that the mPFC would be involved in the stress-buffering effects of exercise that extend beyond 5-HT. Because of the unique ability of the mPFC to constrain many aspects of the stress response, it is possible that involvement of the mPFC is a bridge between the many stress-buffering effects of exercise. Although very little work thus far has examined the effects of exercise on the mPFC specifically, available data are consistent with exercise improving mPFC function in humans (22).
The anatomy supports the ability of the ventral portion of the mPFC (mPFCv) to modulate DRN 5-HT activity. The IL and PL regions of the mPFCv provide the major cortical input to the DRN. Glutamate neurons in these regions project to and synapse on predominantly γ-aminobutyric acid (GABA)-containing neurons in the DRN and thus inhibit the activity of 5-HT neurons. Indeed, electrical stimulation of the mPFCv inhibits activity of DRN 5-HT neurons. Based on this anatomy, we propose that exercise-induced plasticity in the mPFC could result in the mPFC being more active during stressor exposure or behavioral testing, thereby inhibiting the activity of the DRN. Interestingly, established stress-buffering effects of exercise are consistent with increased activity of the mPFC during stress. Exercise constrains stress-induced activation of the sympathetic nervous system, attenuates HPA axis responses to acute mild stressors, and prevents LH behaviors. All of these stress-buffering effects might be expected in exercise increased mPFC-mediated inhibition of the stress response. Additionally, we have observed that 6 wk of wheel running initiated after contextual fear conditioning facilitates within-session extinction of fear during the first test of fear expression (20). Because the IL is involved in within-session fear extinction (34), the observed facilitation of fear extinction also is consistent with increased activation of the IL during stressful events, in this case, exposure to an environment previously paired with foot shocks.
Although the observations that forced exercise, such as treadmill training, often does not produce stress resistance (but instead can increase anxiety) are consistent with the idea that exercise controllability is an important factor, forced exercise studies are confounded by the external stressors imposed on the animals by the forced exercise paradigm. No studies to date have directly manipulated exercise controllability while keeping constant such factors as time of day and exercise apparatus, as well as exercise frequency, duration, and intensity. If such a study finds that the protective effect of wheel running against LH behaviors is indeed dependent on exercise controllability, where only controllable wheel running prevents LH, then neural substrates sensitive to behavioral control, such as the mPFC, would likely be critical to the development of exercise-induced stress resistance. However, this would not imply that effects of exercise per se, which are not dependent on exercise controllability, are not important for other beneficial effects of exercise. In fact, several effects of exercise, such as enhanced hippocampal neurogenesis, increased BDNF, and some hippocampus-dependent learning and memory benefits, appear after both voluntary wheel running and forced exercise (see (26)). Additionally, it is possible that plasticity in the “behavioral control” circuits and in neural circuits sensitive to exercise per se are both required for the unique stress resistance and resilience produced by exercise.
DRN Projection Sites and the 5-HT2C Receptor
An additional possibility for exercise-induced plasticity in the central 5-HT system could be changes in 5-HT2CR sensitivity in DRN projection sites important for the expression of the 5-HT-dependent behavioral consequences of uncontrollable stress. These regions include the BLA (social avoidance; (5)) and the dorsal striatum (escape deficit). Fox et al. (11) have reported that wheel running in mice reduces the behavioral effects of a nonselective 5-HT2R agonist in the acoustic startle paradigm. We have obtained similar data using micro-injection of selective 5-HT2CR compounds into the dorsal striatum. Injection of the selective 5-HT2CR agonist 2-[(3-chlorophenyl) methoxy]-6-(1-piperazinyl) pyrazine (CP-809101) into the dorsal striatum of sedentary rats interferes with shuttle box escape behavior in a dose-dependent manner. Six weeks of wheel running produces a rightward shift in the dose response curve; whereby a higher dose of CP-809101 is required to interfere with shuttle box escape in physically active rats (Greenwood B., unpublished observation, 2011). Together, these data suggest that reduced sensitivity of post-synaptic 5-HT receptors, in this case, the 5-HT2CR, could help resist the behavioral effects of acute increases in 5-HT and thus contribute to exercise-induced stress resistance (Fig. 6).
CONCLUSIONS
Understanding the neuroplastic changes elicited by exercise is useful because this knowledge can facilitate the formation of testable predictions regarding other beneficial effects of exercise. For example, although chronic treatment with SSRIs can reduce clinical symptoms of depression and anxiety, the onset of SSRI pharmacotherapy often is associated with an exacerbation of anxiety and cognitive symptoms consistent with a rapid increase in 5-HT neurotransmission. If wheel running produces resistance against the behavioral effects of acute increases in 5-HT, as hypothesized, then we would predict that wheel running should reduce the negative behavioral impact of acute SSRI administration. This is indeed what we have observed. Sedentary rats administered a single systemic dose of the SSRI fluoxetine demonstrate exaggerated freezing and deficits in shuttle box escape learning 1 h later, effects dependent on activation of the 5-HT2CR (19). In contrast, rats allowed access to running wheels for 6 wk before fluoxetine administration do not display the typical anxiety-like behavioral consequences of the SSRI (19). Although these preclinical data are consistent with our hypothesis, to our knowledge, this prediction has yet to be tested in a clinical setting. However, this prediction is important because it implies that exercise participation could be an effective adjunct therapy during the onset of SSRI treatment to reduce potential deleterious behavioral effects associated with acute SSRI administration, including anxiety and noncompliance.
In conclusion, exposure to uncontrollable stressors of sufficient intensity, relative to controllable or mild stressors, elicits behaviors in rodents that resemble symptoms of human stress-related affective disorders through hyperactivation and sensitization of DRN 5-HT neurons. Available data are consistent with the hypothesis that physical activity produces plasticity in neural circuitry that result in DRN 5-HT neuronal constraint and resistance against the behavioral impact of acute increases in 5-HT. Evidence suggests that plasticity at multiple sites within the central 5-HT system converge to facilitate stress resistance and resilience. These sites include the DRN itself, such as a facilitation of 5-HT1A inhibitory autoreceptor-mediated inhibition of 5-HT neurons; DRN afferent systems, such as the LC or mPFC, which can modulate DRN 5-HT activity during stressor exposure; and DRN projection sites, such as reduced expression or sensitivity of 5-HT2C receptors in brain regions critical for the expression of stress-induced behaviors. An increase in 5-HT1A autoreceptors and a reduction in sensitivity of 5-HT2CR are two examples of exercise-induced neuroplasticity. Determining whether exercise controllability is a critical factor in eliciting exercise-induced stress resistance will aid in understanding how the experience of exercise is communicated to the 5-HT system to result in these plastic changes.
Acknowledgments
The authors thank Paul V. Strong, Teresa E. Foley, Alice B. Loughridge, John P. Christianson, and Steven F. Maier for help in completing the experiments discussed in the current article. We also acknowledge the work of others discussed herein who could not be cited because of the reference limitations.
This study was supported by the American Foundation for Suicide Prevention, National Institutes of Health (MH068283 and MH086665), and National Alliance for Research on Schizophrenia and Depression.
References
- 1.Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53(3):193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 2.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain. Res. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 3.Campeau S, Nyhuis TJ, Sasse SK, et al. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J. Neuroendocrinal. 2010;22(8):872–888. doi: 10.1111/j.1365-2826.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson JP, Paul ED, Irani M, et al. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav. Brain Res. 2008;193(1):87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christianson JP, Ragole T, Amat J, et al. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol. Psychiatry. 2010;67(4):339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dishman RK, Renner KJ, Youngstedt SD, et al. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res. Bull. 1997;42(5):399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 7.Dishman RK, Warren JM, Youngstedt SD, et al. Brain monoamines, exercise, and behavioral stress: animal models. Med. Sci. Sports Exerc. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl. Med. Biol. 2007;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain monoamines and metabolites after treadmill training and wheel running in rats. Med. Sci. Sports Exerc. 1996;28(2):204–209. doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc. Sport Sci. Rev. 2005;33(3):120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behav. Neurosci. 2008;122(4):943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- 12.Grahn R, Hammack S, Will M, et al. Blockade of alphal adrenoreceptors in the dorsal raphe nucleus prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behav. Brain Res. 2002;134(1–2):387. doi: 10.1016/s0166-4328(02)00061-x. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10(2):81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033(2):164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood BN, Foley TE, Day HE, et al. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol. Psychiatry. 2005;57(5):559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Greenwood BN, Foley TE, Day HE, et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J. Neurosci. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwood BN, Foley TE, Le TV, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120(1):269–281. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 2008;199(2):209–222. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behav. Neurosci. 2007;121(5):992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144(4):1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillman CH, Belopolsky AV, Snook EM, Kramer AF, McAuley E. Physical activity and executive control: implications for increased cognitive health during older adulthood. Res. Q. Exerc. Sport. 2004;75(2):176–185. doi: 10.1080/02701367.2004.10609149. [DOI] [PubMed] [Google Scholar]
- 23.Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol. Biochem. Behav. 2003;75(1):81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2 Suppl):9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 25.Kaiyala KJ, Vincow ES, Sexton TJ, Neumaier JF. 5-HT1B receptor mRNA levels in dorsal raphe nucleus: inverse association with anxiety behavior in the elevated plus maze. Pharmacol. Biochem. Behav. 2003;75(4):769–776. doi: 10.1016/s0091-3057(03)00152-7. [DOI] [PubMed] [Google Scholar]
- 26.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 27.Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol. Psychiatry. 2001;49(9):763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- 28.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29(4–5):829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Ou XM, Lemonde S, Jafar-Nejad H, et al. Freud-1: a neuronal calcium-regulated repressor of the 5-HT1A receptor gene. J. Neurosci. 2003;23(19):7415–7425. doi: 10.1523/JNEUROSCI.23-19-07415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. New York (NY): Academic Press; 1998. [Google Scholar]
- 32.Raghupathi RK, Brousseau DA, McGonigle P. Time-course of recovery of 5-HT1A receptors and changes in 5-HT1A receptor mRNA after irreversible inactivation with EEDQ. Brain Res. Mol. Brain Res. 1996;38(2):233–242. doi: 10.1016/0169-328x(95)00311-f. [DOI] [PubMed] [Google Scholar]
- 33.Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A auto-receptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J. Neurosci. 2004;24(23):5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss JM, Simson PG. Depression in an animal model: focus on the locus ceruleus. Ciba Found Symp. 1986;123:191–215. doi: 10.1002/9780470513361.ch11. [DOI] [PubMed] [Google Scholar]