Abstract
The purpose of this study was to utilize a novel imaging biomarker to assess the associations between physical activity (PA), body mass index (BMI), and brain structure in normal aging, mild cognitive impairment (MCI) and Alzheimer's dementia (AD). We studied 963 participants (mean age: 74.1 ± 4.4) from the multi-site Cardiovascular Health Study including healthy controls (n=724), AD (n=104), and MCI (n=135). Volumetric brain images were processed using tensor-based morphometry for analyzing regional brain volumes. We regressed the local brain tissue volume on reported PA and computed BMI, and performed conjunction analyses using both variables. Covariates included age, sex and study site. PA was independently associated with greater whole brain and regional brain volumes, and reduced ventricular dilation. People with higher BMI had lower whole brain and regional brain volumes. A PA-BMI conjunction analysis showed brain preservation with PA and volume loss with increased BMI in overlapping brain regions. In one of the largest voxel-based cross-sectional studies to date, PA and lower BMI may be beneficial to the brain across the spectrum of aging and neurodegeneration.
Keywords: physical activity, body mass index, Alzheimer's, tensor based morphometry
1. Introduction
Alzheimer's Disease (AD) is the most common cause of dementia and the number of persons predicted to have the disease in the United States alone will increase to 13.5 million from 2.2 million by the year 2050 (Sperling, et al., 2011). Currently, about 34 million people worldwide have the disease, and lifestyle factors that are modifiable in principle, such as physical inactivity and obesity, are associated with a heightened risk for AD. If these associations were related to the risk of expressing clinical dementia, then increasing physical activity and decreasing the prevalence of obesity may reduce the number of AD cases by an estimated 50% (Barnes and Yaffe, 2011). These estimates are the foundation for the development of prevention strategies, which are becoming particularly important given the relatively poor efficacy of current drug treatments for AD.
Lack of physical activity (PA) may be the most important modifiable risk factor for AD in the United States, and the third most important worldwide (after low education and smoking) (Barnes and Yaffe, 2011). Mid-life obesity also contributes to a substantial proportion of cases worldwide and in the US (Barnes and Yaffe, 2011). Thus, the risk of AD might be reduced by systematically increasing PA (Chang, et al., 2010,Lautenschlager, et al., 2008,Rolland, et al., 2008,van Gelder, et al., 2004) and reducing obesity. We have previously shown that self-reported PA in healthy elderly people is associated with larger regional brain volumes and reduced risk for future conversion to AD, or its prodrome, mild cognitive impairment (MCI) (Erickson, et al., 2010,Petersen, et al., 1999). Higher body mass index (BMI) in midlife is associated with structural brain changes, cognitive decline, and an increased risk of AD in late life (Cronk, et al., 2010). This suggests that differences in brain structure are a useful intermediary in understanding the association between risk modifiers such as PA and BMI, and the clinical manifestations of neurodegeneration, in this case AD and MCI.
Here, we set out to assess the associations between self-reported PA, computed BMI and regional brain volumes in a large cohort including people with MCI and AD. We were especially interested in understanding whether potential effects of these variables were more easily detected in some parts of the brain relative to others, or if it was simply a pervasive association across the entire brain. To answer this, we used tensor-based morphometry (TBM), which creates detailed 3D maps pinpointing brain regions with the strongest statistical associations with PA and/or BMI, throughout the gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Finally, we examined how BMI influenced the association of PA with brain structure, as BMI is negatively associated with both PA and brain structure in aging, MCI and AD (Ho, et al., 2010a,Raji, et al., 2010a). We also examined whether PA explained associations between BMI and brain structure, and whether there were any common areas associated with both PA and BMI.
2. METHODS
2.1 Participants
The Cardiovascular Health Study (CHS) is a multisite, population-based longitudinal study of coronary heart disease and stroke in individuals 65 and older (Fried, et al., 1991). CHS recruitment was based on the Medicare eligibility lists in: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. In a first wave, 5,201 participants were recruited from 1989 to 1990. In a second assessment, 687 African-Americans were recruited from 1992 to 1993 leading to a cohort of 5,888 participants. The institutional review board at each site approved the study methods, and all participants gave written informed consent.
2.2 The CHS Memory Study
In 1991 and 1992, 3608 of the CHS enrollees participated in the CHS Memory Study (CHS-MS) and underwent a low-resolution MRI scan of the brain. In 1998 and 1999, a follow-up high-resolution MRI scan and neurobehavioral evaluations were completed for all available, living participants (n=2101) (Kuller, et al., 2003). Due to the late inclusion of the high-resolution spoiled-gradient echo (SPGR) sequence into the scanning protocol, not all participants had high-resolution anatomical imaging. Thus the present study includes only the data from the 963 CHS-CS participants who had an SPGR scan and whose MRI data met quality control standards. Prior CHS quality control measures included visual review of scans by a neuroradiologist, to ensure that no large space occupying lesions existed that could potentially hinder analysis (Bryan, et al., 1997,Raji, et al., 2009). We also performed our own visual assessment to ensure against any cropping of brain tissue from the scan field of view, or corruption of MR images in the TBM processing stream.
Participant demographics are shown in Table 1. A separate column identifies sites that were independently correlated with study variables, based upon ANOVA. Hagerstown and Pittsburgh were the sites most frequently correlated with the variables characterized in this study based upon the ANOVA weighting the correlation of these sites against the two other study locations (p < .05). Of the 963 participants included, APOE4 genotype was available in 894 and 221 (24.7%) were APOE4 positive. Full methods for obtaining the APOE4 genotypes in our study are described elsewhere (Kuller, et al., 1998).
Table 1.
Characteristics of CHS Memory Study Participants with MRI in 1998 and 1999 by CHS site
| Winston-Salem | Sacramento | Hagerstown | Pittsburgh | Total sample | Study Site As a Main Effect (t, p) | |
|---|---|---|---|---|---|---|
| Number of MRI Scans Analyzed | 18 | 315 | 192 | 438 | 963 | |
| Agea | 75.6 (4.4) | 74.8 (4.4) | 73.5 (4.1) | 73.8 (4.3) | 74.1 (4.3) | Hagerstown (−2.3, .02) |
| Sex, male, % (n) | 61 (11) | 41 (130) | 44 (84) | 40 (176) | 42 (401) | No study site was predictive |
| Race, White, % (n) | 100 (18) | 92 (289) | 99 (190) | 80 (348) | 88 (845) | Hagerstown (−2.7, .008); Pittsburgh, (4.6, .001) |
| Years of Educationa | 13 (2.1) | 13.4 (2.4) | 11.6 (3.1) | 13.8 (2.7) | 13.2 (2.8) | Pittsburgh (2.4, .02) |
| BMIa | 23.9 (3.4) | 26.4 (4.3) | 27 (4.5) | 26.6 (4.2) | 26.6 (4.3) | Hagerstown (−2.3, .02) |
| Blocks Walkeda | 97.3 (87.4) | 34.5 (56.1) | 33.1 (50.8) | 35.3 (45.2) | 35.6 (51.4) | Hagerstown (4.7, .001) |
| Time taken to walk 15 feeta | 4.8 (0.9) | 6 (3.3) | 5.6 (4.6) | 5.6 (2.2) | 5.7 (3.2) | No study site was predictive |
| Number of infarctsa | 0.39 (0.7) | 0.59 (1.0) | 0.53 (0.99) | 0.48 (0.89) | 0.52 (0.96) | No study site was predictive |
| Sulcal grade (0 to 9, worst)a | 4.11 (1.3) | 3.9 (1.4) | 3.89 (1.7) | 4 (1.6) | 3.96 (1.6) | Pittsburgh (4.2, <.001); Hagerstown (−2.3, .02) |
| Ventricular grade (0 to 9, worst)a | 3.72 (0.83) | 3.86 (1.4) | 3.48 (1.2) | 3.71 (1.4) | 3.72 (1.4) | Hagerstown (−2.7, .007) |
| White matter grade (0 to 9, worst)a | 3 (1.9) | 2.88 (1.7) | 2.34 (1.5) | 2.45 (1.6) | 2.58 (1.6) | Hagerstown (−3.7, <.001); Pittsburgh (−3.6,<.001) |
| Infarcts, % (n) | 28 (5) | 34 (107) | 30 (58) | 30 (130) | 31 (300) | No site was predictive |
mean (SD)
Neurobehavioral evaluations were assessed to determine the presence of any disorder that could affect cognition. Participants were classified as having normal cognition, MCI, or AD (Lopez, et al., 2003b). The diagnosis of dementia was based on deficits in performance in two or more cognitive domains that were sufficiently severe to affect activities of daily living and their history of normal intellectual function before the onset of cognitive abnormalities; a memory deficit was not required for the diagnosis of dementia (Lopez, et al., 2012). The Adjudication Committee consisted of experts in dementia who had access to the historical CHS cognitive test scores, primarily the 3MSE (and subscales), Benton Visual Retention Test, and the DSST, as well as the CES-D scores. The committee also reviewed data from vision and hearing tests, history of alcohol intake, activities of daily living questionnaire, IQ-CODE, Dementia Questionnaire, vital status, date of death where relevant, history of hospitalizations, medications to treat dementia, findings from MRI scans, results of neuropsychological assessments, and hospital records (Lopez, et al., 2003a).
2.3 Assessment of Physical Activity and Body Mass Index
PA was assessed at baseline and updated 10 years later, within one year, on average, of the high-resolution MRI scan. PA was assessed by the modified Minnesota Leisure-Time Activities Questionnaire (Elosua, et al., 1994,Folsom, et al., 1986), which evaluates the duration and frequency of PA. We selected “blocks walked over one week” as a main measure of PA, as it is the simplest form of physical activity so most individuals in our study would be likely to do it. In addition, we previously reported that the average number of blocks walked in one week is associated with variation in gray matter volume (Erickson, et al., 2010) with twelve city blocks equal to approximately one mile. On average, people with MCI and AD had lower levels of physical activity than did those with normal cognition (t = 3.6, p < .001). All participants had weight (pounds or kilograms) and height (inches or centimeters) measures taken during the physical exam, both at baseline and 9 years later, in the same year that the high-resolution MRI data were acquired; BMI was computed as: weight (kg) x [height (m)]2, using a constant to account for the unit conversion. For these analyses, we used PA measurements from year 10 of the study, and BMI measurements from year 9 of the study.
2.4 Structural MRI
MRI using the SPGR sequence was completed at each of the 4 sites using 1.5 Tesla scanners, as detailed elsewhere (Bryan, et al., 1994). The scanning protocol used in 1998 and 1999 included a sagittal T1-weighted localizer sequence, an axial T1 weighted, spin-density, and T2 weighted images. The axial images were 5 mm thick without interslice gaps. White matter hyperintensities, an imaging marker of small vessel ischemic disease, were visually determined using a standardized semi-quantitative white matter grade (WMG) that is fully described in prior work (Raji, et al., 2012).
For the specific TBM methods used to process the brain images, please refer to Supplementary Data 1s.
2.5. Voxel-wise linear regressions
At each point in the brain, a linear regression model (Calabrese A, 2011,Chu A, 2009a,Chu A, 2009b) was fitted to model relationships between regional brain volumes and our factors of interest. Predictors included: the site of data acquisition (represented by three ‘dummy variables’: B1, B2, B3), the age of the participant (B4), sex (B5), race (B6), years of education (B7), diagnosis (B8), BMI – year 9 (B9), and Physical Activity – year 10 (B10). This led to the following regression model:
We used these voxel-wise (at each point in the brain) multiple regressions to compute regression coefficients and assess whether the covariates of interest predicted volumetric differences anywhere in the brain. Statistical or “p-value” maps were generated to visualize the pattern of voxel-wise significance. To control for false positives, we enforced a standard false discovery rate (FDR) correction for multiple statistical comparisons across voxels in the whole brain and inside each brain lobe, using the conventionally accepted false-positive rate of 5% (q=0.05) (Benjamini Y, 1995,Chu A, 2009b). Regions associated with PA and BMI were visualized using unstandardized beta maps to indicate the direction of change (expansion or contraction).
To perform the PA-BMI, PA-Dx, and BMI-Dx conjunction analyses, we used a script to combine the “p-value” maps from the two covariates of interest (taking the maximum or least significant P value from the two maps being conjoined) and then enforced FDR correction to determine the overall significance of the map, in the same way as above. For exploratory analyses restricted to either cognitively normal or cognitively impaired subjects, we used the original regression model but with a limited population. For exploratory analyses involving statistical interactions, we used the entire subject population but added a term to the original regression model, involving the multiplication of two covariates of interest. Each covariate was “mean centered” prior to multiplication, when modeling the statistical interaction.
3. Results
3.1 Influences on Brain Structure
We found that higher physical activity levels were associated with significantly higher whole brain (FDR q = 0.05, critical uncorrected P = 0.0008) and parietal lobe volume, with reduced ventricular dilation. As found before (Ho, et al., 2010a,Raji, et al., 2010a), subjects with higher BMI had significantly lower whole brain (N = 963, FDR q = 0.05, critical uncorrected P = 0.0398) and regional volumes in frontal, temporal, parietal and occipital lobes with strongest associations in the frontal and occipital lobes. More specifically, higher BMI was related to volumetric brain tissue reductions in the orbitofrontal cortex and anterior cingulate gyrus. The PA-BMI conjunction analysis showed that physical activity and higher BMI had overlapping associations with volume of the orbitofrontal cortex, posterior cingulate gyrus and posterior hippocampus.
AD/MCI was significantly correlated with lower brain volume across the whole brain, with pervasive associations revealing frontal lobe atrophy and ventricular dilation. We were unable to find significance with the PA-Dx conjunction; however, significant p-values in the BMI-Dx conjunction analysis show where BMI and diagnosis are both associated with lower regional volumes across the whole brain, particularly in the frontal lobe region.
Figures 1A and 1B present two orthogonal slices that show the independent correlation of BMI (upper panels) and of PA (middle panels), as well as the significant correlation resulting from the conjunction analysis (lower panels), at different locations in the brain. Figure 2 duplicates the orthogonal slice in Figure 1B and was sufficient to compare the significant correlation of BMI (upper panels) with the separate correlation of AD/MCI (middle panels), and to display the conjunctional analysis results of BMI and diagnosis (lower panel). As mentioned, the p-value map resulting from the PA-Dx analysis revealed no statistical significance in common brain regions and is consequently not shown as a figure.
Figure 1A. (Brain Location #1).
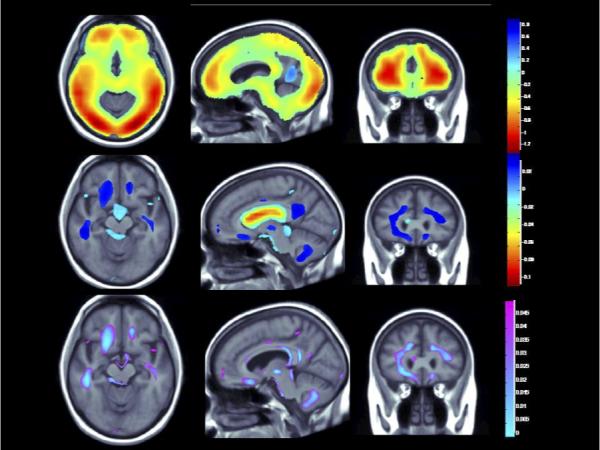
(Top panel) Whole brain 3D maps show areas where regional brain volumes correlated significantly with BMI after controlling for site, age, sex, race, educational level, and diagnosis, plus PA (N = 963; FDR q = 0.05, critical uncorrected P = 0.0398). (Middle panel) Maps show brain regions significantly associated with PA (blocks walked) after correcting for the same effects, plus BMI (N = 963; FDR q = 0.05, critical uncorrected P = 0.0008). Beta maps were significant after standard correction for multiple comparisons and represent the estimated degree of tissue excess or deficit at each voxel, as a percentage, for each unit gain in BMI and/or physical activity. (Lower panel) 3D maps show regions of significant brain volume differences from both higher BMI and physical activity using a conjunction analysis displayed over a study-specific template. P maps are corrected for multiple comparisons on the basis of FDR (FDR q-level = 0.05, critical uncorrected P = 0.0006). The same slices are shown in each panel and images are displayed in radiological convention (left side of the brain shown on the right).
Figure 1B. (Brain Location #2).
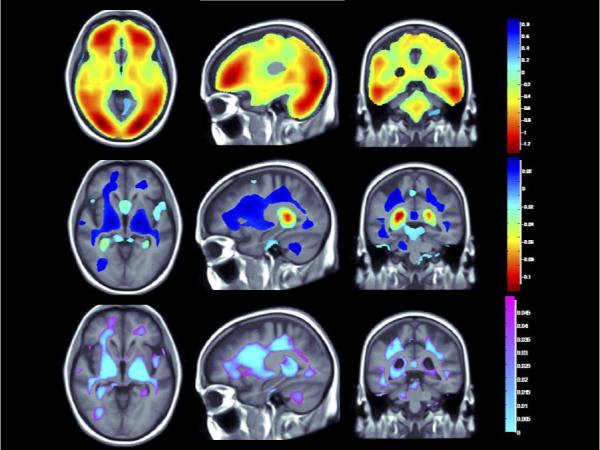
(Top panel) Whole brain 3D maps show areas where regional brain volumes correlated significantly with BMI after controlling for site, age, sex, race, educational level, and diagnosis, plus PA (N = 963; FDR q = 0.05, critical uncorrected P = 0.0398). (Middle panel) Maps show brain regions significantly associated with PA (blocks walked) after correcting for the same effects, plus BMI (N = 963; FDR q = 0.05, critical uncorrected P = 0.0008). Beta maps were significant after standard correction for multiple comparisons and represent the estimated degree of tissue excess or deficit at each voxel, as a percentage, for each unit gain in BMI and/or physical activity. (Lower panel) 3D maps show regions of significant brain volume differences from both higher BMI and PA using a conjunction analysis displayed over a study-specific template. P maps are corrected for multiple comparisons on the basis of FDR (FDR q-level = 0.05, critical uncorrected P = 0.0006). The same slices are shown in each panel and images are displayed in radiological convention (left side of the brain shown on the right).
Figure 2. (Brain Location #2).
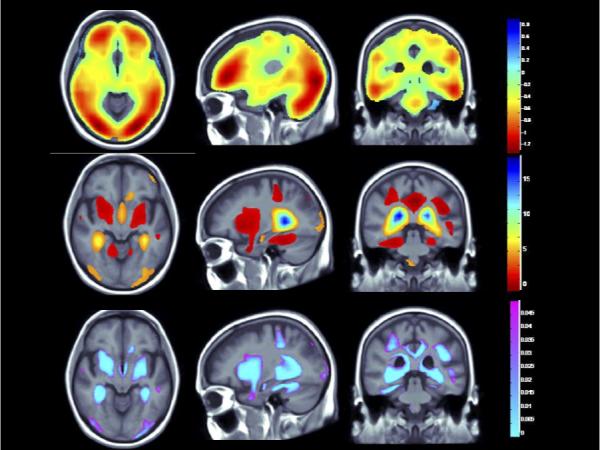
(Top panel) Whole brain 3D maps show areas where regional brain volumes correlated significantly with BMI after controlling for site, age, sex, race, educational level, and diagnosis, plus PA (N = 963; FDR q = 0.05, critical uncorrected P = 0.0398). (Middle panel) Maps show brain regions significantly associated with diagnosis (AD/MCI v. Controls) after correcting for the same effects, plus BMI (N = 963; FDR q = 0.05, critical uncorrected P = 0.0047). Beta maps were significant after standard correction for multiple comparisons and represent the estimated degree of tissue excess or deficit at each voxel, as a percentage, for each unit gain in BMI and/or positive diagnosis (AD/MCI). (Lower panel) 3D maps show regions of significant brain volume differences from both higher BMI and the positive diagnosis (AD/MCI) using a conjunction analysis displayed over a study-specific template. P maps are corrected for multiple comparisons on the basis of FDR (FDR q-level = 0.05, critical uncorrected P = 0.0008). The same slices are shown in each panel and images are displayed in radiological convention (left side of the brain shown on the right).
3.2 Exploratory Analyses
In an exploratory analysis restricted to the 724 cognitively normal participants, we found that BMI and PA remained strong predictors of brain structure. The inverse relationship with BMI resulted in a critical p = 0.0379 across the whole brain. PA did not reach significance across the whole brain, but the volume of the parietal lobe was significantly associated with PA (critical p = 0.0021). Again, all the same covariates were used in the ‘controls only’ model. This analysis confirmed that the association between PA and brain volume was significant in normal controls. In the same fashion, we analyzed data from the 239 cognitively impaired participants and BMI maintained a significant association with brain volume (critical p = 0.0250), but PA was no longer significant in any area. An analysis was also run to test for statistical interactions between PA and diagnosis (CTL=0; MCI/Dementia=1). This was added to the model to test whether the association between PA and brain volume was influenced by diagnosis. There was no statistically significant interaction between PA and Dx (p > 0.05); there was also no change in the significance of PA and BMI when this term was included (BMI: critical p = 0.0396; PA: critical p = 0.0003). This suggests that the association between PA and brain volume did not depend on whether or not a participant was cognitively impaired, or that we were underpowered to detect such a dependency.
In addition to confirming that cognitive impairment did not significantly moderate the association between PA and brain volume, the confounding influence of mobility on PA and its relationship with brain structure was explored via a statistical interaction between PA and mobility. It is important to understand any effect of mobility, as a less mobile person may be less able to be physically active. When included in the regression model, mobility, as defined as the time to walk 15 feet, was not statistically significant. Also, the significance of PA and BMI were maintained after including this variable in the model. Therefore, the relationship of PA with brain structure was not influenced by mobility restrictions.
Another statistical analysis was run to model the relationship of small vessel ischemic disease as measured by white matter grade (WMG) (Longstreth, et al., 1996,Raji, et al., 2012) with brain volume. In Longstreth et al. (1996) examples of single slices from complete scans used by board certified neuroradiologists to grade white matter are presented. Grading ranges from one to eight and consists of descriptions of the periventricular rim, levels of subcortical disease, and identification of periventricular lesions and confluence. Studies with no white matter findings were graded 0, and those with findings more remarkable than the highest grade (grade 8) were scored 9. Here we included a statistical interaction term between PA and WMG. Our analysis using this measure showed a significant correlation in the occipital lobe (critical p = 0.0004), which suggests a dependency of the PA-brain structure relationship on WMG.
Finally, an interaction between PA and APOE4 was modeled to determine whether the association of PA with brain volume was moderated by genotype, but this term was not statistically significant (p < 0.05). Therefore, the potential effect of PA on brain structure did not vary as a function of APOE4 status.
4. Discussion
The imaging method in our study, TBM, is used as a biomarker of atrophy, and is reasonably novel compared to traditional morphometry methods such as tracing structures on scans. It has been used before but the biological results can be considered evidence of the method's value in its sensitivity to biological factors that affect regional brain volumes. In other words, the results can be seen as biomarkers of biological processes. The influence of measurable lifestyle factors such as PA and BMI is revealed via direct association with this biomarker, and may present itself via effects on the brain that influence structure and also risk of disease, such as AD. Conjunction analyses revealed areas associated with both PA and BMI as well as BMI and dementia. By showing areas that shrink with BMI and are at risk for neurodegeneration in AD, our results support evidence that increased BMI is a potent risk factor which is associated with increased atrophy in areas at risk for AD. We were unable to detect a significant conjunction between areas affected by PA and AD. This would be consistent with the lack of PA significance in the AD-only analysis and may be due to low statistical power and/or less physical activity in subjects with AD.
Taken together with prior work, this study has several key implications. First, PA, as measured by the amount a person walks, is related to brain structure in late life, as is BMI. Greater amounts of walking are associated with larger relative brain volumes, especially in the parietal lobe. This finding was strong enough to survive after controlling for BMI, a standard measure of obesity, which we were unable to achieve in earlier human studies using PA and BMI (Ho, et al., 2010a). Given the frequently pervasive association of BMI with brain volume as shown in such human studies (Ho, et al., 2010a,Ho, et al., 2010b), this is novel and may be due to greater statistical power for detecting this relationship with a larger sample size. The association between BMI and brain structure is also strong even when accounting for PA; however, this is more in line with expectations due to the strong correlation of BMI. These results suggest that the potential benefit of PA on brain volume might be due to more than simply a lower BMI; likewise, the negative correlation of BMI with brain volume is likely due to more than just a lack of PA.
The imaging method in our study, TBM, is used as a biomarker of atrophy, and is reasonably novel compared to traditional morphometry methods such as tracing structures on scans. It has been used before but the biological results can be considered evidence of the method's value in its sensitivity to biological factors that affect regional brain volumes. In other words, the results can be seen as biomarkers of biological processes. The influence of measurable lifestyle factors such as PA and BMI is revealed via direct association with this biomarker, and may present itself via effects on the brain that influence structure and also risk of disease, such as AD. Conjunction analyses revealed areas associated with both PA and BMI as well as BMI and dementia. By showing areas that shrink with BMI and are at risk for neurodegeneration in AD, our results support evidence that increased BMI is a potent risk factor which is associated with increased atrophy in areas at risk for AD. We were unable to detect a significant conjunction between areas affected by PA and AD. This would be consistent with the lack of PA significance in the AD-only analysis and may be due to low statistical power and/or less physical activity in subjects with AD.
These data build on our prior work in a subset of this sample (N=299) showing that PA (i.e., blocks walked) at baseline was related to greater brain volumes measured 9 years later in a human cohort (Erickson, et al., 2010), independent of BMI. Collectively, this work is consistent with the hypothesis that increasing PA later in life could improve brain health. This assertion is supported by one randomized controlled trial showing that hippocampal volume can respond positively to exercise training over the course of a year in a randomized trial of cognitively normal individuals (Erickson, et al., 2011). We have also shown that hippocampal volume may be higher in people with lower BMI in a prior study of persons with AD (Ho, et al., 2011).
Our study further reveals that associations between PA, BMI and brain structure may be present across a range of cognitive status. This is consistent with our prior work with BMI in human imaging studies (Ho, et al., 2010a,Ho, et al., 2011). PA did not vary as a function of diagnostic classification, which is important as prior work on PA and brain structure has focused on cognitively normal individuals; (however, see (Honea, et al., 2009,Vidoni, et al., 2012). If our observation is confirmed, it may be reasonable to test PA as an adjuvant therapy for AD as shown in prior human investigation (Li, et al., 2012).
Multiple RCTs show the positive benefits of PA on both brain structure and function in human studies (Colcombe, et al., 2003,Colcombe, et al., 2006,Pereira, et al., 2007). While our study cannot establish causal link between PA and brain structure, other work suggests that there is such a link between aerobic PA and improved preserved regional brain volumes in cognitively normal (Burdette, et al., 2010,Hotting, et al., 2012,Ruscheweyh, et al., 2011) and AD populations (Burns, et al., 2008). The overall relationship between PA and brain structure has also been reported in numerous epidemiological and observational studies. According to one review, 20 of 24 longitudinal human epidemiologic studies found that PA was associated with a reduced risk for cognitive decline and dementia (Rolland, et al., 2008). More recently, a study of over 4,761 elderly human subjects found that midlife PA was associated with reduced prevalence of dementia and cognitive decline 26 years later (Chang, et al., 2010). Also, a meta-analysis of 29 RCTs examining the influence of PA on cognitive function showed that aerobic PA confers improvements in human attention and processing speed, executive function, and memory (Smith, et al., 2010).
RCTs have explored the effects of exercise in people with dementia. One RCT investigated the effectiveness of exercise programs at five nursing homes containing 134 ambulatory patients with mild to severe AD, and concluded that a simple exercise program leads to a slower decline in the ability to perform ADLs (activities of daily living) (Rolland, et al., 2007). Another RCT investigated the effects of intense and long-term exercise in 210 home-dwelling patients with AD, showing beneficial effects on physical functioning and mobility (Pitkala, et al., 2010). Comprehensive reviews of such RCTs have produced mixed results. One review of 16 studies found that physical activity was beneficial in all stages of dementia, and that multicomponent interventions involving endurance, strength and balance can improve physical functioning and basic activities of daily living in elderly human subjects with dementia (Blankevoort, et al., 2010). A second review of 13 human RCT's confirmed this, citing evidence that physical activity interventions improve physical function in older people with dementia (Potter, et al., 2011). However, a review of two human RCTs and one meta-analysis concluded that there was insufficient evidence that physical activity programs are effective in managing or improving cognition in people with dementia (Forbes, et al., 2008). This review was later updated to include sixteen trials and conclude there is promising evidence that exercise programs can have a significant impact in improving ability to perform ADLs and possibly in improving cognition in people with dementia (Forbes, et al., 2013).
Studies associating BMI and brain structure show mixed results. We have mentioned several studies where our group as well as others have shown that higher BMI or mid-life obesity is linked with cognitive decline in humans including brain volume deficits and increased risk of dementia (Barnes and Yaffe, 2011,Ho, et al., 2010a,Raji, et al., 2010a). However, these same studies cite support that suggests lower BMI and rapid weight loss in later life is associated with dementia. This has been appropriately labeled the ‘obesity paradox’. Prior longitudinal studies have suggested the possibility of a U-shape association whereby there was a significant curvilinear association between BMI and cognitive function scores at baseline, concluding that BMI in the elderly is not predictive of cognitive decline in a normal community human population (Sturman, et al., 2008). BMI is typically analyzed as a categorical variable comprised of underweight, normal, overweight and obese labels. The U-shape association suggests that volume loss is seen with both underweight and obese categories. To test this hypothesis with our data, we performed an ANOVA with a polynomial contrast using these categories to test for a u-shaped association against total gray matter volume as a proportion of total intracranial volume derived from voxel based analyses previously conducted. Covariates included age gender, race, AD, MCI, site and white matter grade, and was done in all 963 CHS subjects. Our results showed that the estimated means from the ANOVA is not U-shaped but linear. Thus our BMI analysis clearly shows an inverse relationship between BMI and brain volume whereby brain volume steadily decreases as BMI increases in the elderly.
PA may influence brain structure and reduce AD risk through multiple physiologic mechanisms. They may be broadly grouped into three categories: (i) counteraction of AD pathology, such as anti-amyloid effects, (ii) influencing the levels of neurotrophic factors and neurotransmitters, that may optimize neuronal function, and (iii) reducing vascular risk factors that can independently compromise brain structure. With respect to the first category, people aged 55-88 have lower levels of amyloid plaque as imaged with Pittsburgh Compound B if they had at least 7.5 metabolic equivalent hours of PA per week (Liang, et al., 2010). Further, exercise reduces hippocampal tau, in a transgenic mouse model of AD when compared to sedentary mice (Belarbi, et al., 2009). PA can promote neurogenesis in the dentate gyrus of the hippocampus and can increase the expression of brain derived neurotrophic factor in animal and human studies (Intlekofer and Cotman, 2012,Li, et al., 2012). PA also reduces some vascular risk factors such as obesity and type 2 diabetes mellitus, that are independent risk factors for AD, in a recent review (Ahlskog, et al., 2011). Our exploratory analysis is consistent with the third mechanism, i.e., that PA may reduce small vessel disease as indicated by the presence of white matter hyperintensities. PA may preserve brain structure by counteracting downstream effects of vascular risk factors such as hypertension that promote white matter injury with subsequent brain atrophy in human studies (Raji, et al., 2012,Wiseman, et al., 2004).
The patients in our cohort who had AD were relatively early in their clinical course in human imaging studies (Raji, et al., 2009). PA may benefit people who are early in their course of MCI and AD either by modifying the underlying neurodegenerative process or by affecting brain reserve in human studies (Stern, et al., 1996,Stern, et al., 1995). PA can reduce amyloid metabolism in APOE4 positive human individuals (Head, et al., 2011), and between PA and white matter grade in the occipital lobe suggests that, at least in that region, the main effect of PA on brain structure is moderated by small vessel ischemic disease, as reflected by WMG.
The physiological mechanisms at work between BMI and brain structure have been widely explored and consist of several possibilities. Adiposity may contribute to a broader syndrome. The most commonly proposed mediators for the relationship between higher body tissue adiposity and brain structure include hypercortisolemia, reduced exercise, impaired respiratory function, inflammation, cardiovascular/hypertension/hyperlipidemia and type II diabetes mellitus (Raji, et al., 2010a). The co-occurrence of at least three of the following cardiovascular factors including large waist circumference (or adiposity), increased triglycerides, elevated blood pressure, and fasting hyperglycemia has been referred to as “the metabolic syndrome” (Yaffe, et al., 2007). Adiposity is also associated with insulin resistance and subsequent type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, degenerative joint disease, cancer and lung disease (Poirier, et al., 2006). Moreover, an elevated BMI is significantly correlated (p < 0.01) with a reduction in neuronal fiber bundle length (FBL), which is believed to contribute to brain atrophy. Finally, greater brain atrophy may occur in people with central leptin insufficiency, a marker of obesity. Leptin, a hormone produced by body fat tissue, acts on hypothalamic receptors in the brain to regulate appetite and energy expenditure, and on neurons in the arcuate nucleus to signal satiety following a meal in a recent ADNI human study (Rajagopalan, et al., 2013). The exact mechanisms through which obesity leads to brain atrophy and cognitive decline are complex and may involve a number of factors such as diabetes, genetic vulnerability, brain metabolites, and cytokines. Much research has revealed the degree to which these factors are linked with obesity, but further analysis is required to understand the way these elements interact with each other in their effects on the brain.
Our study has limitations. First, given the observational nature of the design, it is impossible to establish causality between PA, BMI, or conjunctions, and brain structure. People with larger brains may be more likely to exercise or maintain low BMI; alternatively, once a person becomes ill, they may be less able to exercise or maintain their weight. To overcome this limitation in our sample, an RCT would be needed in a population of people with normal cognition and with MCI or AD. Second, while “blocks walked” is a useful measure of PA, it is subjective and not as reliable (or accurate) as objective measures of PA or cardiorespiratory fitness in several human studies (Erickson, et al., 2013,Erickson, et al., 2012). Consequently, our study may underestimate the influence of PA on brain structure. It is also possible that self report of physical activity is biased by the cognitive impairment of those with memory disorders, although persons with AD and MCI in our study were generally early in their disease course and only 10% had been diagnosed at the time of this human study (Raji, et al., 2010b).
One intriguing question our study raises is this: does a critical treatment window exist during the lifespan in which physical activity and reduced BMI will be most effective as preventive strategies for dementia? One future approach would be to study a proximal load of PA close to the collection of imaging biomarkers. Future directions may focus on other forms of PA such as resistance training, as recent human study suggests this specific type of exercise may also have brain benefits (Nagamatsu, et al., 2012).
The strengths of our study include the fact that this is one of the largest voxel-based studies of human brain aging. The multi-site community cohort structure allows for a wider generalization of our findings. In addition, the availability of the longitudinal lifestyle variables, and the use of a sensitive morphometric analysis technique (i.e., TBM) are strengths of our project. Future intervention research is needed to determine whether PA and reduced BMI can improve brain health across the spectrum of normal aging and AD.
Supplementary Material
Acknowledgements
The research reported in this article was supported in part by funds from contract numbers N01-HC-80007, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional funds were provided by the National Institute on Aging to O.L.L. (AG020098), L.H.K. (AG15928), P.T. (EB008281), and the University of Pittsburgh (AG05133), and by subcontract (N01-HC-055222) to J.T.B. A full list of principal CHS investigators and institutions can be found at www.chs-nhlbi.org/pi.htm.
Footnotes
Disclosure Statement
The authors have no potential financial or personal conflicts of interest including relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence this work.
References
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86(9):876–84. doi: 10.4065/mcp.2011.0252. doi:S0025-6196(11)65219-1 [pii] 10.4065/mcp.2011.0252 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet. 2011;10(9):819–28. doi: 10.1016/S1474-4422(11)70072-2. doi:S1474-4422(11)70072-2 [pii] 10.1016/S1474-4422(11)70072-2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K, Schindowski K, Burnouf S, Caillierez R, Grosjean ME, Demeyer D, Hamdane M, Sergeant N, Blum D, Buee L. Early Tau pathology involving the septo hippocampal pathway in a Tau transgenic model: relevance to Alzheimer's disease. Curr Alzheimer Res. 2009;6(2):152–7. doi: 10.2174/156720509787602843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, H. Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Blankevoort CG, van Heuvelen MJ, Boersma F, Luning H, de Jong J, Scherder EJ. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dementia and geriatric cognitive disorders. 2010;30(5):392–402. doi: 10.1159/000321357. doi:10.1159/000321357. [DOI] [PubMed] [Google Scholar]
- Bryan RN, Manolio TA, Scertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. Am J Neuroradiol. 1994;15(9):1625–33. [PMC free article] [PubMed] [Google Scholar]
- Bryan RN, Wells SW, Miller TJ, Elster AD, Jungreis CA, Poirier VC, Lind BK, Manolio TA. Infarctlike lesions in the brain: Prevalence and anatomic characteristics at MR imaging of the elderly -data from the Cardiovascular Health Study. Radiology. 1997;202:47–54. doi: 10.1148/radiology.202.1.8988191. [DOI] [PubMed] [Google Scholar]
- Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Frontiers in aging neuroscience. 2010;2:23. doi: 10.3389/fnagi.2010.00023. doi:10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Cronk BB, Anderson HS, Donnelly JE, Thomas GP, Harsha A, Brooks WM, Swerdlow RH. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–6. doi: 10.1212/01.wnl.0000317094.86209.cb. doi:10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese A, S. J, Schneider DM, Paninski L, Woolley SMN. A generalized linear model for estimating spectrotemporal receptive fields from responses to natural sounds. PLoS ONE. 2011;6:e16104. doi: 10.1371/journal.pone.0016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, Eiriksdottir G, Jonsdottir MK, Lopez OL, Harris TB, Gudnason V, Launer LJ. The effect of midlife physical activity on cognitive function among older adults: AGES--Reykjavik Study. J Gerontol A Biol Sci Med Sci. 2010;65(12):1369–74. doi: 10.1093/gerona/glq152. doi:glq152 [pii] 10.1093/gerona/glq152 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A, C. J, Dinov ID. SOCR Analyses - an Instructional Java Web-based Statistical Analysis Toolkit. J Online Learn Teach. 2009a;5(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Chu A, C.J., Dinov ID. SOCR Analyses: Implementation and Demonstration of a New Graphical Statistics Educational Toolkit. J Stat Softw. 2009b;30(3):1–19. doi: 10.18637/jss.v030.i03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–80. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi: 10.1093/gerona/61.11.1166. doi:61/11/1166 [pii]. [DOI] [PubMed] [Google Scholar]
- Cronk BB, Johnson DK, Burns JM, Alzheimer's Disease Neuroimaging I. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer disease and associated disorders. 2010;24(2):126–30. doi: 10.1097/WAD.0b013e3181a6bf3f. doi:10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elosua R, Marrugat J, Molina L, Pons S, Pujol E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am J Epidemiol. 1994;139(12):1197–209. doi: 10.1093/oxfordjournals.aje.a116966. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Barr LL, Weinstein AM, Banducci SE, Akl SL, Santo NM, Leckie RL, Oakley M, Saxton J, Aizenstein HJ, Becker JT, Lopez OL. Measuring physical activity using accelerometry in a community sample with dementia. Journal of the American Geriatrics Society. 2013;61(1):158–9. doi: 10.1111/jgs.12050. doi:10.1111/jgs.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Raji CA, Lopez OL, Becker JT, Rosano C, Newman AB, Gach HM, Thompson PM, Ho AJ, Kuller LH. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75(16):1415–22. doi: 10.1212/WNL.0b013e3181f88359. doi:WNL.0b013e3181f88359 [pii] 10.1212/WNL.0b013e3181f88359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. doi:1015950108 [pii] 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Lopez OL. Physical activity, brain plasticity, and Alzheimer's disease. Archives of medical research. 2012;43(8):615–21. doi: 10.1016/j.arcmed.2012.09.008. doi:10.1016/j.arcmed.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom AR, Jacobs DR, Jr., Caspersen CJ, Gomez-Marin O, Knudsen J. Test-retest reliability of the Minnesota Leisure Time Physical Activity Questionnaire. J Chronic Dis. 1986;39(7):505–11. doi: 10.1016/0021-9681(86)90195-5. [DOI] [PubMed] [Google Scholar]
- Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database Syst Rev. 2008;(3):CD006489. doi: 10.1002/14651858.CD006489.pub2. doi:10.1002/14651858.CD006489.pub2. [DOI] [PubMed] [Google Scholar]
- Forbes D, Thiessen EJ, Blake CM, Forbes SC, Forbes S. Exercise programs for people with dementia. Cochrane Database Syst Rev. 2013;12:CD006489. doi: 10.1002/14651858.CD006489.pub3. doi:10.1002/14651858.CD006489.pub3. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: Design and Rationale. Annals of epidemiology. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise Engagement as a Moderator of the Effects of APOE Genotype on Amyloid Deposition. Arch Neurol. 2011;69(5):636–43. doi: 10.1001/archneurol.2011.845. doi:archneurol.2011.845 [pii] 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Lee S, Hibar D, Dinov ID, Stein JL, Jack CR, Jr., Weiner MW, Toga AW, Thompson PM, Cardiovascular Health, S., Adni Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010a;31(8):1326–39. doi: 10.1016/j.neurobiolaging.2010.04.006. doi:10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Saharan P, DeGiorgio A, Madsen SK, Hibar DP, Stein JL, Becker JT, Lopez OL, Toga AW, Thompson PM, Alzheimer's Disease Neuroimaging I. Hippocampal volume is related to body mass index in Alzheimer's disease. Neuroreport. 2011;22(1):10–4. doi: 10.1097/wnr.0b013e3283412868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, DeCarli CS, DeChairo BM, Potkin SG, Jack CR, Jr., Weiner MW, Raji CA, Lopez OL, Becker JT, Carmichael OT, Thompson PM, Alzheimer's Disease Neuroimaging I. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A. 2010b;107(18):8404–9. doi: 10.1073/pnas.0910878107. doi:10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45(1):44–51. doi: 10.1016/j.neuroimage.2008.10.064. doi:10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotting K, Reich B, Holzschneider K, Kauschke K, Schmidt T, Reer R, Braumann KM, Roder B. Differential cognitive effects of cycling versus stretching/coordination training in middle-aged adults. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2012;31(2):145–55. doi: 10.1037/a0025371. doi:10.1037/a0025371. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 2012 doi: 10.1016/j.nbd.2012.06.011. doi:S0969-9961(12)00226-4 [pii] 10.1016/j.nbd.2012.06.011 [doi]. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R. Relationship between ApoE, MRI findings, and cognitive function in the cardiovascular health study. Stroke. 1998;29:388–98. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, Bockxmeer FM, Xiao J, Greenop KR, Almeida OP. Effect of physical activity on cognitive function in older adults at risk for Azheimer disease. JAMA. 2008;300(9):1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- Li W, Antuono PG, Xie C, Chen G, Jones JL, Ward BD, Franczak MB, Goveas JS, Li SJ. Changes in regional cerebral blood flow and functional connectivity in the cholinergic pathway associated with cognitive performance in subjects with mild Alzheimer's disease after 12-week donepezil treatment. Neuroimage. 2012;60(2):1083–91. doi: 10.1016/j.neuroimage.2011.12.077. doi:S1053-8119(12)00018-3 [pii] 10.1016/j.neuroimage.2011.12.077 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68(3):311–8. doi: 10.1002/ana.22096. doi:10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O'Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke. 1996;27:1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Chang YF, Sweet RA, DeKosky ST, Gach MH, Carmichael OT, McDade E, Kuller LH. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology. 2012;79(15):1599–606. doi: 10.1212/WNL.0b013e31826e25f0. doi:10.1212/WNL.0b013e31826e25f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson MC, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study Part 1. Arch Neurology. 2003a;60:1385–9. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003b;22(1):1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Archives of internal medicine. 2012;172(8):666–8. doi: 10.1001/archinternmed.2012.379. doi:10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–43. doi: 10.1073/pnas.0611721104. doi:10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Pitkala KH, Raivio MM, Laakkonen ML, Tilvis RS, Kautiainen H, Strandberg TE. Exercise rehabilitation on home-dwelling patients with Alzheimer's disease--a randomized, controlled trial. Study protocol. Trials. 2010;11:92. doi: 10.1186/1745-6215-11-92. doi:10.1186/1745-6215-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier P, Despres JP, Bertrand OF. Identifying which patients with diabetes should be tested for the presence of coronary artery disease--the importance of baseline electrocardiogram and exercise testing. The Canadian journal of cardiology. 2006;22(Suppl A):9A–15A. doi: 10.1016/s0828-282x(06)70973-4. [DOI] [PubMed] [Google Scholar]
- Potter R, Ellard D, Rees K, Thorogood M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. International journal of geriatric psychiatry. 2011;26(10):1000–11. doi: 10.1002/gps.2641. doi:10.1002/gps.2641. [DOI] [PubMed] [Google Scholar]
- Rajagopalan P, Toga AW, Jack CR, Weiner MW, Thompson PM, Alzheimer's Disease Neuroimaging, I Fat-mass-related hormone, plasma leptin, predicts brain volumes in the elderly. Neuroreport. 2013;24(2):58–62. doi: 10.1097/WNR.0b013e32835c5254. doi:10.1097/WNR.0b013e32835c5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010a;31(3):353–64. doi: 10.1002/hbm.20870. doi:10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Lee C, Lopez OL, Tsay J, Boardman JF, Schwartz ED, Bartynski WS, Hefzy HM, Gach HM, Dai W, Becker JT. Initial experience in using continuous arterial spin-labeled MR imaging for early detection of Alzheimer disease. AJNR Am J Neuroradiol. 2010b;31(5):847–55. doi: 10.3174/ajnr.A1955. doi:10.3174/ajnr.A1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 2009;73(22):1899–905. doi: 10.1212/WNL.0b013e3181c3f293. doi:WNL.0b013e3181c3f293 [pii] 10.1212/WNL.0b013e3181c3f293 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Jr., Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging. 2012;33(4):834, e7–16. doi: 10.1016/j.neurobiolaging.2011.08.010. doi:10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer's disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9(6):390–405. doi: 10.1016/j.jamda.2008.02.007. doi:S1525-8610(08)00099-6 [pii] 10.1016/j.jamda.2008.02.007 [doi]. [DOI] [PubMed] [Google Scholar]
- Rolland Y, Pillard F, Klapouszczak A, Reynish E, Thomas D, Andrieu S, Riviere D, Vellas B. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized, controlled trial. Journal of the American Geriatrics Society. 2007;55(2):158–65. doi: 10.1111/j.1532-5415.2007.01035.x. doi:10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A. Physical activity and memory functions: an interventional study. Neurobiol Aging. 2011;32(7):1304–19. doi: 10.1016/j.neurobiolaging.2009.08.001. doi:10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic medicine. 2010;72(3):239–52. doi: 10.1097/PSY.0b013e3181d14633. doi:10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. doi:10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Arch Neurol. 1996;53(2):148–53. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, Mayeux R. Relationship between lifetime occupation and parietal flow: Implications for a reserve against Alzheimer's disease pathology. Neurology. 1995;44:55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- Sturman MT, de Leon CF, Bienias JL, Morris MC, Wilson RS, Evans DA. Body mass index and cognitive decline in a biracial community population. Neurology. 2008;70(5):360–7. doi: 10.1212/01.wnl.0000285081.04409.bb. [DOI] [PubMed] [Google Scholar]
- van Gelder BM, Tijhuis MAR, Kalmijn S, Giampoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men. The FINE study. Neurology. 2004;63:2316–21. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Van Sciver A, Johnson DK, He J, Honea R, Haines B, Goodwin J, Laubinger MP, Anderson HS, Kluding PM, Donnelly JE, Billinger SA, Burns JM. A community-based approach to trials of aerobic exercise in aging and Alzheimer's disease. Contemporary clinical trials. 2012;33(6):1105–16. doi: 10.1016/j.cct.2012.08.002. doi:10.1016/j.cct.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman RM, Saxby BK, Burton EJ, Barber R, Ford GA, O'Brien JT. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology. 2004;63:1892–7. doi: 10.1212/01.wnl.0000144280.59178.78. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. Journal of the American Geriatrics Society. 2007;55(5):758–62. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


