Abstract
Background
To establish the maximum tolerated dose (MTD) and toxicity profile of cyclophosphamide with cisplatin, etoposide, and bleomycin (C-PEB) in children with high-risk malignant germ cell tumors (HR-MGCT).
Procedure
Eligibility criteria included untreated patients ≤ 21 years of age with stage III/IV extragonadal, extra cranial MGCT. Patients received four cycles (repeated every 3 weeks) of cisplatin (20 mg/m2/day × 5 days), etoposide (100 mg/m2/day × 5 days), and bleomycin (15 mg/m2 on Day 1) with escalating doses of cyclophosphamide on Day 1, assigned at the time of enrollment (1.2, 1.8, or 2.4 g/m2). Patients with complete response had therapy discontinued. Patients with residual disease underwent second-look surgery, those with pathologic evidence of residual MGCT or whose markers had not normalized received two more cycles. All other patients had protocol therapy stopped.
Results
Nineteen patients were enrolled between July 2004 and August 2007. Three patients were non-evaluable. Sixteen patients completed four cycles. Eleven had complete response, one had progressive disease and four had partial response. All four with partial response underwent second look surgery followed by two more cycles. Only one patient, on dose 1.8 g/m2, experienced dose-limiting toxicity (DLT) during the first cycle of therapy (grade 3 hyperglycemia). The 4-year EFS and OS (± standard deviation) were 74 ± 7% and 89 ± 10%, respectively.
Conclusion
The addition of cyclophosphamide to the standard PEB regimen (cisplatin, etoposide, and bleomycin) is feasible and well-tolerated at all dose levels used on this study.
Keywords: cyclophosphamide, germ cell tumors, maximum tolerated doses
INTRODUCTION
In a previous randomized study, the Pediatric Oncology Group and the Children’s Cancer Group [1] tested whether the intensification of cisplatin in patients with high-risk MGCT would improve outcomes. The study compared standard-dose cisplatin (20 mg/m2/day × 5 days) versus high-dose (HD) cisplatin (40 mg/m2/day × 5 days), combined with etoposide (100 mg/m2/day × 5 days) and bleomycin (15 mg/m2 on Day 1). This study revealed a 6-year event-free survival (EFS) of 89.6 + 3.6% for patients treated with HD-PEB compared to 80.5 + 4.8% for patients receiving standard PEB (P = 0.0284). Furthermore, although the study was not powered for this comparison, patients with advanced extragonadal tumors also had a consistent, but not statistically significant, improvement in EFS on the HDPEB arm (a 6-year EFS rate of 85 ± 6.2% for HDPEB versus an EFS rate of 73.1 ± 7.4% for PEB). These results suggested that HD-PEB improved the EFS of children with high-risk GCT. However, this combination was associated with significant treatment-related morbidity, particularly severe ototoxicity, in approximately two-thirds of the patients.
An intergroup pediatric study was designed to circumvent the toxicity associated with HD-PEB. Marina et al. [2] treated 25 patients with advanced pediatric extragonadal GCT with HD-PEB and a potential otoprotectant, amifostine, to evaluate the efficacy and toxicity of this regimen. The EFS and OS were similar to those obtained previously in the randomized study. However, amifostine did not protect against HD-PEB associated ototoxicity, since 75% of these patients had significant hearing loss ***(≥Grade 3).
Dose intensification of active agents, including alkylators, results in improved treatment outcome for some pediatric malignancies [3,4]. The dose-intensity of a given agent can be increased either by decreasing the interval between chemotherapy cycles, or by increasing the dose of the active chemotherapeutic agents. If the dose-limiting toxicity (DLT) of the agent to be escalated is neutropenia, then the routine administration of hematopoietic growth factors can minimize the occurrence of the DLT. Alkylating agents, particularly cyclophosphamide, are attractive in dose-escalation studies because their DLT is neutropenia and escalating their dose does not substantially increase other non-hematopoietic toxicities (such as hemorrhagic cystitis or cardiomyopathy) [5,6]. Ifosfamide, an alkylating agent similar to cyclophosphamide, has been used in combination with cisplatin for the treatment of MGCT in adult patients. However, the additive effects of cisplatin and ifosfamide on renal tubular function limit this combination in young children and inform our use of cyclosphosphamide for this trial.
Based on these data, and to continue to evaluate the value of dose-intensification of treatment for children with high-risk GCT, the Children’s Oncology Group (COG) designed a study (AGCT01P1) to establish the maximum tolerated dose (MTD) and toxicity profile of cyclophosphamide combined with cisplatin, etoposide, bleomycin (C-PEB) in previously untreated children with high-risk malignant germ cell tumors.
MATERIAL AND METHODS
Study Patients
Patients were eligible for this study if they: (1) were age 21 years or less; (2) had histological confirmation of previously untreated malignant germ cell tumor with either yolk sac tumor (endodermal sinus tumor), embryonal carcinoma, choriocarcinoma, or teratoma with mixed malignant elements; (3) had stage III or IV as previously described [1]; (4) were enrolled within 21 days of diagnostic surgery; (5) had measurable disease; and (6) had normal renal and hematologic function. The National Cancer Institute and the institutional review boards of the participating institutions approved the protocols. Informed consent was obtained from parents, patients, or both, as deemed appropriate, according to Department of Health and Human Services guidelines.
Pretreatment Evaluation and Staging
Evaluations at study entry included: a medical history and physical examination, and laboratory studies including a complete blood count with differential, a chemistry panel including evaluation of the levels of electrolytes, blood urea nitrogen, creatinine, calcium, magnesium, bilirubin, alanine aminotransferase, total protein, albumin, tumor marker determination (alphafetoprotein [AFP] and beta human chorionic gonadotropin [HCG]). Diagnostic imaging evaluation included a computed tomography (CT) scan or magnetic resonance image (MRI) scan of the primary tumor and a CT scan of the chest. Patients, with elevated AFP or HCG at diagnosis, had those markers assessed before each chemotherapy cycle.
Therapy
Patients received induction therapy consisting of four cycles of chemotherapy with PEB with escalating doses of cyclophosphamide (C-PEB). PEB consisted of cisplatin (20 mg/m2/day × 5 days), etoposide (100 mg/m2/day × 5 days), and bleomycin (15 mg/m2 on Day 1). Cyclophosphamide dose was assigned at the time of enrollment to one of three levels (1.2, 1.8, or 2.4 g/m2), according to the escalation scheme defined below. Cyclophosphamide doses for children less than 12 months of age were dosed according to weight.
Cycles were repeated every 21 days or thereafter, as soon as ANC > 1,000/µl and platelet count > 100,000/µl. At week 12, patients with complete response (CR: disappearance of all known disease and normalization of tumor markers for at least 4 weeks) had therapy discontinued. Patients with radiographic evidence of a residual mass underwent second look surgery. Patients with pathologic evidence of residual malignant GCT or whose markers had not normalized received two more cycles of therapy. All other patients had protocol therapy stopped.
Cyclophosphamide Dose Level Assignment
Patients were assigned a dose level of cyclophosphamide at enrollment (level 1 = 1.2 g/m2; level 2 = 1.8 g/m2; level 3 = 2.4 g/m2). No intra-patient escalation was permitted. The dose, however, could be decreased for individual patients because of toxicity.
Patients were accrued in cohorts of six, starting at level 2 (1.8 g/m2). CTC version 3 was used to code adverse events. Serious adverse events (SAEs) during the first cycle were defined as: (1) any grade 4 non-hematologic toxicity; (2) any non-hematologic grade 3 with the exception of nausea and vomiting controlled with adequate antiemetic prophylaxis, transaminase (AST/ALT) elevations that return to Grade 1 prior to the time for the next treatment cycle, fever or infection, fatigue that returns to ≤ grade 2, before the next dose of a cycle or (3) absolute neutrophil count (ANC) < 1,000/µl or platelet count < 100,000/µl at Day 28. SAEs were reviewed by the COG Data and Safety Monitoring Committee to determine their attribution as a DLT.
While patients enrolled at level 2 were evaluated for DLT, no more than six patients were to be entered at level 1 (1.2 g/m2). If two or more patients at level 2 experienced DLT, the level was considered not feasible to deliver. Otherwise, the next cohort of six patients was to be enrolled at level 3 (2.4 g/m2). Levels 1 and 3 were evaluated in according to the criteria stated above.
Outcomes Definition
Event free survival (EFS) was defined as the period from study enrollment until evidence of an EFS-event (progressive disease, death, and diagnosis of a second malignant neoplasm) or last contact, whichever occurred first. Patients who did not experience an event were censored on the date of last contact.
Survival time (OS) was defined as the period from study enrollment until death or last contact, whichever occurred first. A patient who died was considered to have experienced an OS-event, regardless of the cause of death. Patients who were alive at last contact were considered censored for the analysis of OS.
The survivor functions for EFS and OS were calculated according to the method of Kaplan and Meier [7] Confidence intervals for the Kaplan–Meier estimate were calculated according to the complementary log–log methodology [8].
RESULTS
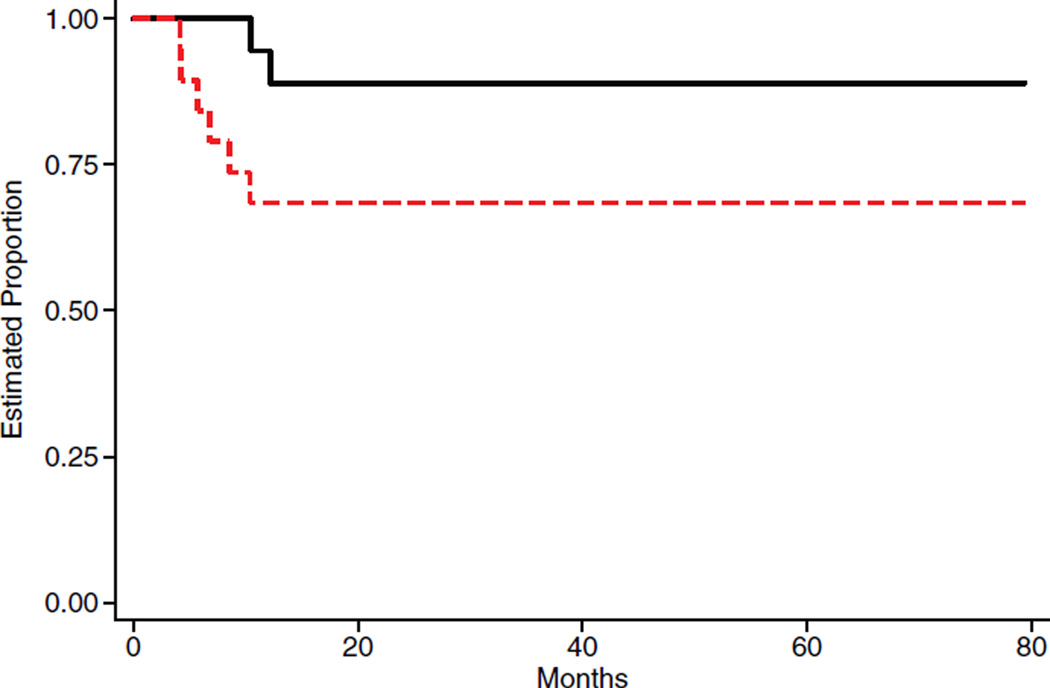
AGCT01P1 opened for enrollment to all COG institutions in July 2004 and closed in August 2007. Nineteen (19) patients were enrolled (Table I). Three patients (5, 12, and 14) were non-evaluable for the assessment of DLT. One of these patients was lost to follow-up prior to Day 28 and two patients were removed from protocol therapy prior to the completion of cycle 1 by physician choice. None of these three experienced DLT prior to termination of protocol therapy. Of the 16 patients who completed four cycles of induction, 11 had complete response, one had progressive disease and four had a partial response. All four patients with partial response had residual mass and underwent second look surgery. Three had histologically confirmed residual malignancy. The other patient had cystic teratoma, but persistently elevated AFP level. All four patients received two additional cycles of their assigned regimen. At the end of consolidation, three were in complete response and one had progressive disease. During follow-up, three patients relapsed, all within 9 months from diagnosis. Two of these patients died as a result of progressive disease. The outcomes of these patients are shown on Figure 1. The 4-year EFS and OS ± standard error were 74% (±7%) and 89% (±10%), respectively.
TABLE I.
Patient and Characteristics, Treatment, Response, Toxicity, and Outcomes
| Patient # | Sex | Age at enrollment (Years) |
Tumor site | Stage | Treatment regimen (g/m2) |
Metastatic site | Initial AFP | AFP normalized | DLT observed | Type of EFS event | Life status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 14 | Retroperitoneal | 3 | 1.2 | 5 | Yes | No | Progressive Disease | Alive | |
| 2 | M | 15 | Mediastinum | 3 | 1.2 | 3,200 | No | No | No event | Alive | |
| 3 | M | 2 | Mediastinum | 3 | 1.2 | 2,129 | No | No | No event | Alive | |
| 4 | F | 2 | Pelvic tumor | 4 | 1.2 | Lung and spinal canal | 52,740 | No | No | Relapse | Alive |
| 5 | F | 2 | Liver | 4 | 1.2 | Lung and abdominal lymph nodes | 4,800 | Yes | Not evaluable | No event | Alive |
| 6 | F | 1 | Vaginal wall | 3 | 1.2 | 7,737 | No | No | No event | Alive | |
| 7 | F | 1 | Orbit | 3 | 1.8 | 18,566 | No | No | No event | Alive | |
| 8 | F | 0 | Retroperitoneal | 4 | 1.8 | Liver | 108,458 | No | No | No event | Alive |
| 9 | M | 17 | Mediastinum | 3 | 1.8 | 4,950 | Yes | No | No event | Alive | |
| 10 | F | 4 | Sacrococcygeal | 4 | 1.8 | Liver and abdominal lymph nodes | 85,910 | No | No | No event | Alive |
| 11 | M | 14 | Mediastinum | 3 | 1.8 | 6 | Yes | Yes | Relapse | Alive | |
| 12 | F | 10 | Ovary | 3 | 1.8 | 7,303 | No | Not evaluable | No event | Alive | |
| 13 | M | 2 | Saccroccoygeal | 4 | 1.8 | Lung and spinal canal | 5,713 | No | No | No event | Alive |
| 14 | F | 6 | Retroperitoneal | 3 | 2.4 | 3 | Yes | Not evaluable | Progressive disease | Dead | |
| 15 | F | 0 | Saccroccoygeal | 3 | 2.4 | 23,050 | No | No | No event | Alive | |
| 16 | M | 1 | Extragonadal | 3 | 2.4 | 47,457 | No | No | No event | Alive | |
| 17 | F | 0 | Uterus | 3 | 2.4 | 7,119 | No | No | No event | Alive | |
| 18 | M | 17 | Mediastinum | 3 | 2.4 | 10,650 | No | No | No event | Alive | |
| 19 | M | 18 | Mediastinum | 3 | 2.4 | 5,034 | No | No | Relapse | Dead |
Fig. 1.
Event free survival and survival. _______ OS _ _ _ _ _EFS
Only one patient, enrolled on dose level 2, experienced DLT during the first cycle of therapy, manifested as grade 3 hyperglycemia. No other patient experienced DLT during cycle 1. Table II shows the incidence of hematological grade 3 and 4 toxicities observed on this study. Neutrophil and platelet recovery for all three cyclophosphamide levels were within the parameters determined as acceptable for this regimen, and did not meet criteria for DLT. Other non-hematological grade 3 and 4 toxicities reported by the investigators were as follows: (1) level 1: hypercalcemia and hypokalemia, 1 patient each; (2) level 2: hyercalcemia, hypoalbuminemia, anorexia, nose bleed and pain, one patient each; (3) level 3: one patient experienced lower extremities weakness. As demonstrated in Table II, neither the incidence of hematologic toxicity or the time to recovery of adequate platelets or neutrophils appeared to vary by dose level of cyclophosphamide.
TABLE II.
Hematological Toxicities (Grade 3 and 4) Experienced on Each Level During the Treatment Course
| Dose level (g/m2) |
Hemoglobin | ANC | Platelets | F&N with/without infection |
Time to recovery ANC (days) |
Time to recovery platelets (days) |
|---|---|---|---|---|---|---|
| 1.2 | 2/5 (40%) | 3/5 (60%) | 1/5 (20%) | 1/5 (20%) | 13–19 | 13–20 |
| 1.8 | 5/6 (84%) | 4/6 (67%) | 5/6 (84%) | 3/6 (50%) | 12–26 | 13–26 |
| 2.4 | 2/5 (40%) | 4/5 (80%) | 3/5 (60%) | 2/5 (40%) | 13–21 | 13–18 |
DISCUSSION
The current study showed that the addition of cyclophosphamide to the standard PEB regimen was feasible and well-tolerated at all dose levels used on this study. However, due to the small number of patients entered on this study we were not able to establish whether the addition of cyclophosphamide to the standard PEB regimen improved the event-free and overall survival of children with high-risk GCT.
It should be noted that cyclophosphamide, like most other alkylating agents, is potentially carcinogenic [9,10]. Hawkins et al. reported on the dose relationship for cyclophosphamide with regard to second tumors in long-term survivors of cancer in childhood. The relative risk of second malignancy, when adjusted for the effect of epipodophyllotoxins and radiotherapy, rose from 1.6 for a dose between 1 and 4.3 g/m2 to 7.4 for doses >13 g/m2 [9]. Therefore, if cyclophosphamide is to be used in combination with the standard PEB regimen as a way to intensify therapy for children with high-risk germ cell tumors, the dose level of 1.8 g/m2 should be used in order to limit the exposure of at least 3/4 of these patients to a cumulative dose of cyclophosphamide of <7.5 g/m2, and therefore decreasing the risks for long-term gonadal toxicity and second cancers.
Although the use of platinum-containing regimens has significantly improved the outcome of children with malignant germ cell tumors, results for those patients identified as having a high risk for relapse continue to be a challenge. First, given that at least 75% of patients with high risk MGCT will survive with standard therapy, it continues to be important to identify the prognostic factors that indicate those patients unlikely to respond in whom intensification of therapy can be justified. For instance, not all extragonadal tumors are equally high-risk. Having a more nuanced approach that includes site as well as age and histology may refine our approach. This work will be enhanced through international cooperation on retrospective data analysis as well as planning for future clinical trials.
Acknowledgments
Grant sponsor: COG Chair’s Grant; Grant number: U10 CA98543-08; Grant sponsor: Statistics and Data Center; Grant number: U10 CA98413-08
REFERENCES
- 1.Cushing B, Giller R, Cullen JW, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children with high-risk malignant germ cell tumors: A Pediatric Intergroup Study—(POG 9049/CCG 8882) J Clin Oncol. 2004;22:2691–2700. doi: 10.1200/JCO.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Marina N, Chang KW, Malogolowkin M, et al. Amifostine does not protect against the ototoxicity of high-dose cisplatin combined with etoposide and bleomycin in pediatric germ cell tumors. A Children’s Oncology Group Study. Cancer. 2005;104:841–847. doi: 10.1002/cncr.21218. [DOI] [PubMed] [Google Scholar]
- 3.Kong N, Heller C, Heller G. Chemotherapy dose intensification correlates strongly with response, median survival and progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JR, Coccia PF. Is more better? Dose intensification in neuroblastoma. J Clin Oncol. 1991;9:902. doi: 10.1200/JCO.1991.9.6.902. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg MA, Antin JH, Guinan EC. Cyclophosphamide cardiotoxicity: An analysis of dosing as a risk factor. Blood. 1986;68:1114–1118. [PubMed] [Google Scholar]
- 6.Stillwell TJ, Benson RCJ. Cyclophosphamide-induced hemorrhagic cystitis. Cancer. 1988;61:451–457. doi: 10.1002/1097-0142(19880201)61:3<451::aid-cncr2820610308>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 9.Hawkins MM, Draper GJ, Kingston JE. Incidence of second primary tumors among childhood cancer survivors. Br J Cancer. 1987;56:339–347. doi: 10.1038/bjc.1987.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushner BH, Heller G, Cheung NK, et al. High risk of leukemia after short-term dose-intensive chemotherapy in young patients with solid tumors. J Clin Oncol. 1998;16:3016–3020. doi: 10.1200/JCO.1998.16.9.3016. [DOI] [PubMed] [Google Scholar]