Abstract
IMPORTANCE
Biliary atresia is the most common cause of end-stage liver disease in children. Controversy exists as to whether use of steroids after hepatoportoenterostomy improves clinical outcome.
OBJECTIVE
To determine whether the addition of high-dose corticosteroids after hepatoportoenterostomy is superior to surgery alone in improving biliary drainage and survival with the native liver.
DESIGN, SETTING, AND PATIENTS
The multicenter, double-blind Steroids in Biliary Atresia Randomized Trial (START) was conducted in 140 infants (mean age, 2.3 months) between September 2005 and February 2011 in the United States; follow-up ended in January 2013.
INTERVENTIONS
Participants were randomized to receive intravenous methylprednisolone (4 mg/kg/d for 2 weeks) and oral prednisolone (2 mg/kg/d for 2 weeks) followed by a tapering protocol for 9 weeks (n = 70) or placebo (n = 70) initiated within 72 hours of hepatoportoenterostomy.
MAIN OUTCOMES AND MEASURES
The primary end point (powered to detect a 25% absolute treatment difference) was the percentage of participants with a serum total bilirubin level of less than 1.5 mg/dL with his/her native liver at 6 months posthepatoportoenterostomy. Secondary outcomes included survival with native liver at 24 months of age and serious adverse events.
RESULTS
The proportion of participants with improved bile drainage was not statistically significantly improved by steroids at 6 months posthepatoportoenterostomy (58.6% [41/70] of steroids group vs 48.6% [34/70] of placebo group; adjusted relative risk, 1.14 [95% CI, 0.83 to 1.57]; P = .43). The adjusted absolute risk difference was 8.7% (95% CI, −10.4% to 27.7%). Transplant-free survival was 58.7% in the steroids group vs 59.4% in the placebo group (adjusted hazard ratio, 1.0 [95% CI, 0.6 to 1.8]; P = .99) at 24 months of age. The percentage of participants with serious adverse events was 81.4% [57/70] of the steroids group and 80.0% [56/70] of the placebo group (P > .99); however, participants receiving steroids had an earlier time of onset of their first serious adverse event by 30 days posthepatoportoenterostomy (37.2% [95% CI, 26.9% to 50.0%] of steroids group vs 19.0% [95% CI, 11.5% to 30.4%] of placebo group; P= .008).
CONCLUSIONS AND RELEVANCE
Among infants with biliary atresia who have undergone hepatoportoenterostomy, high-dose steroid therapy following surgery did not result in statistically significant treatment differences in bile drainage at 6 months, although a small clinical benefit could not be excluded. Steroid treatment was associated with earlier onset of serious adverse events in children with biliary atresia.
Biliary atresia occurs in 1:5000 to 1:18 000 live births and progresses to end-stage cirrhosis in more than 70% of affected children.1 It is the leading indication for pediatric liver transplantation in the world, accounting for about 50% of transplants in children and about 10% of transplants at any age.1 The disease results from an inflammatory and rapidly fibrosing cholangiopathy that obstructs the lumen of extrahepatic bile ducts and manifests as cholestatic jaundice in the first few weeks after birth. At diagnosis, the primary treatment is a hepatoportoenterostomy (the Kasai procedure), which entails surgical excision of the biliary remnant and creation of bile drainage via a jejunal Roux-en-Y anastomosis to the porta hepatis. Hepatoportoenterostomy results in successful bile drainage in only about half of patients with biliary atresia treated in the United States.2 Even after successful drainage, most infants experience progression of intrahepatic disease, ultimately requiring liver transplantation for survival.3 This poor outcome underscores the need for adjunct therapies to improve survival without liver transplantation.
Following initial reports of corticosteroids (called steroids hereafter) use following hepatoportoenterostomy or as short-term pulses to reverse cessation of previously achieved bile flow after successful hepatoportoenterostomy,4,5 others have reported improved clinical outcomes with postoperative steroid therapy for biliary atresia.6–14 The proposed justification for use of steroids was to reduce biliary inflammation and fibrosis and to promote bile flow. These reports were largely retrospective analyses that used historical controls and varying doses and durations of treatment, yet they became the basis for the widespread use of steroids following hepatoportoenterostomy in the United States and in other countries.11,15 Moreover, due to their designs, these studies could not address potential adverse consequences of this therapy in young infants with biliary atresia. A recent meta-analysis16 was unable to determine if steroids improve patient outcomes because of an insufficient number of well-conducted studies. Based on these conflicting reports and safety concerns regarding the use of steroids in infants, we conducted the Steroids in Biliary Atresia Randomized Trial (START) to determine whether the combination of hepatoportoenterostomy with high-dose steroid therapy was superior to hepatoportoenterostomy alone.
Methods
Study Design
START was a randomizedmulticenter, double-blind, placebo-controlled trial of steroid therapy following hepatoportoenterostomy in infants with biliary atresia conducted at 14 clinical sites in the Childhood Liver Disease Research and Education Network (ChiLDREN) funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Institutional review board approval was obtained at each site and at the data coordinating center; parents or guardians of the children provided written informed consent. Enrollment began in September 2005 and ended in February 2011, with follow-up completed in January 2013.
Patient Population
Infants were recruited if they had biliary atresia and had been enrolled in the ChiLDREN prospective observational database study of cholestasis in infancy (PROBE) and later underwent hepatoportoenterostomy. Inclusion criteria were age of 180 days or younger, serum direct or conjugated bilirubin level of 2 mg/dL or higher and greater than 20% of total bilirubin, postconception age of 36 weeks or older, and weight of 2000 g or greater. Potential participants were excluded from START if they had undergone previous hepatobiliary surgery or had known immunodeficiency, diabetes mellitus, or significant systemic hypertension for age (the complete inclusion and exclusion criteria appear in eTable 1 in Supplement).
Study Intervention and Randomization
Eligible participants were randomized with equal probability to a 13-week course of steroid therapy or matching placebo, which was administered in a double-blind manner starting within 72 hours after hepatoportoenterostomy. Participants in the steroids group received intravenous methylprednisolone (4 mg/kg/d for 2 weeks) and oral prednisolone (2 mg/kg/d for 2 weeks) followed by a tapering protocol for prednisolone for 9 weeks (Table 1). Steroids or placebo were given intravenously for at least 2 postoperative days or until the infant resumed oral or enteral feedings, at which time prednisolone or placebo was given orally for the remainder of the study. Participants in the placebo group received intravenous normal saline or an oral inactive substance that matched the steroid product for appearance and taste. The initial dose was chosen based on 2 reports published before the start of the trial,6,9 one of which showed improved serum bilirubin levels in 76% of patients.6
Table 1.
Dosage and Duration of Steroids or Placebo for START
| Period | Steroidsa | Placebo |
|---|---|---|
| Week 1 | ||
| Days 1–3 | Methylprednisolone, 4 mg/kg/d intravenously, divided twice dailyb | Normal saline intravenously (same volume, twice daily)b |
| Days 4–7 | Prednisolone, 4 mg/kg/d orally, divided twice daily | Placebo with same appearance as steroid pill, orally, twice daily |
| Week 2 | Prednisolone, 4 mg/kg/d, divided twice daily | Placebo, twice daily |
| Week 3–4 | Prednisolone, 2 mg/kg/d, divided twice daily | Placebo, twice daily |
| Week 5–6 | Prednisolone, 1 mg/kg/d | Placebo, once daily |
| Week 7 | Prednisolone, 0.8 mg/kg/d | Placebo, once daily |
| Week 8 | Prednisolone, 0.6 mg/kg/d | Placebo, once daily |
| Week 9 | Prednisolone, 0.4 mg/kg/d | Placebo, once daily |
| Week 10 | Prednisolone, 0.2 mg/kg/d | Placebo, once daily |
| Week 11 | Prednisolone, 0.1 mg/kg/d | Placebo, once daily |
| Week 12–13 | Prednisolone, 0.1 mg/kg, every other day | Placebo, every other day |
| Week 14 | Stop use | Stop use |
Abbreviation: START, Steroids in Biliary Atresia Randomized Trial.
Initial dosage was based on the infant’s weight. Subsequent doses were adjusted based on the infant’s weight measured monthly at each scheduled outpatient visit.
Steroids or placebo were given intravenously for at least 2 postoperative days or until the infant resumed oral or enteral feedings, at which time prednisolone or placebo was given orally for the remainder of the study.
The data coordinating center generated treatment randomization codes with permuted block sizes of 4 (stratified by site) and provided the central pharmacy with a list of assignments for each study site. Study medications were labeled and put into a kit by the central pharmacy and distributed to study site research pharmacists who were instructed to dispense the kits to participants enrolled sequentially. Routine clinical care guidelines for the postoperative care were established for infants enrolled in PROBE and were followed for all participants in this clinical trial (eTable 2 in Supplement).
Measures
Baseline assessments included the collection of demographic, medical, and surgical history; physical examination; presence of biliary atresia splenic malformation (BASM) syndrome, which could influence the response to hepatoportoenterostomy; laboratory parameters; and anthropometric measurements. Race and ethnicity were self-reported according to categories set by the US Office of Management and Budget and are reported to provide descriptive information on these demographic characteristics. Biochemical and serological tests were performed at the clinical laboratories of the participating centers. The assessments were also performed at 2 weeks after hepatoportoenterostomy; at 1, 2, 3, and 6 months after hepatoportoenterostomy; and at 12, 18, and 24 months of age. Antibody titers in response to routine infant immunizations were collected at 18 months of age.
Study Outcomes
The primary end point was defined as successful bile drainage (measured as the percentage of participants with serum total bilirubin level of <1.5 mg/dL; to convert to μmol/L, multiply by 17.104) with his/her native liver at 6 months after hepatoportoenterostomy. Total bilirubin was determined directly using standard laboratory methods, or calculated by the addition of conjugated plus unconjugated bilirubin.17 If these values were missing at the 6-month time point in participants with their native liver, successful bile drainage was imputed if the total bilirubin values were less than 1.5 mg/dL at both time points immediately prior to and after the 6-month time point (ie, 3 months posthepatoportoenterostomy and at 12 months of age), and were considered to have unsuccessful bile drainage otherwise.
Secondary outcomes included duration of successful bile drainage, survival with native liver at 24 months of age, and the proportion of participants with as cites at 12 and 24 months of age. The duration of successful bile drainage was defined as the time (months) between a participant’s first total bilirubin level of less than 1.5 mg/dL and the earlier of next recorded total bilirubin level of 1.5 mg/dL or greater, liver transplantation, or death. Time until loss to follow-up, withdrawn from the study, and completion of the study without experiencing the event were censored. If a participant never achieved successful bile drainage (ie, total bilirubin level ≥1.5 mg/dL for the duration of the study), then duration was set to 0. Unlike other secondary time-to-event end points, the end point for the duration of successful bile drainage began when a participant achieved successful bile drainage instead of the time of hepatoportoenterostomy.
Survival with the native liver was defined as the time from the date of hepatoportoenterostomy to the earlier date when the participant underwent liver transplantation or died (events), was 24 months of age with native liver, withdrew, or was lost to follow-up (censored). The occurrence of ascites was defined as the clinical manifestation of ascites, treatment of ascites, or detection of ascites by sonographic examination.
Safety outcomes included adverse events (total, expected, and unexpected), serious adverse events (defined as death, disability, life-threatening illness, or an event requiring hospitalization), and infectious serious adverse events. The list of a priori expected adverse events appears in eTable 3 in Supplement; each expected adverse event was defined in the START manual of operations. An independent medical monitor reviewed all serious adverse events, providing body system classifications and preparing safety narratives that were reviewed by an independent data and safety monitoring board that was convened quarterly by the National Institute of Dia-betesand Digestive and Kidney Diseases to review study conduct, adverse events, and serious adverse events.
Statistical Analysis
Seventy participants per group were calculated to provide 80% power to detect a 25% absolute treatment difference in the primary end point on the basis of a 2-sample test of proportions, with a 2-sided significance level of .05 and allowing for 20% attrition and 2 interim analyses based on the O’Brien-Fleming spending function. A retrospective study of the level of serum total bilirubin and survival with the native liver in children with biliary atresia treated with hepatoportoenterostomy at the participating centers provided our estimate of 50% for the primary end point in the placebo group.2 The ex-pectation for steroids to improve the primary out come to 75% was based on 2 studies published before the initiation of START reporting that the use of corticosteroids after hepatoportoenterostomy was associated with resolution of jaundice in 76% to 79% of patients.6,9 Although planned, no formal interim analyses of the primary end point were conducted; therefore, the nominal 2-sided α level used for the final analysis was .05 for the primary end point. All other secondary outcomes were also tested at the 2-sided level of .05, with no adjustment for multiplicity; thus, the interpretation of these tests should be considered exploratory.
The primary analysis was based on a modified intention-to-treat approach; all randomized participants who received at least 1 dose of study medication were included. We compared the proportion of participants who had successful bile drainage at 6 months between the steroids and placebo groups, using a generalized linear model based on the binomial distribution with a log link (log-binomial regression),18 with co-variates for treatment group, age at hepatoportoenteros-tomy, and the presence of BASM syndrome as fixed effects and clinical site as a random effect, providing relative risk (RR) ratios of the treatment effect. The adjusted absolute treatment difference and its corresponding 95% confidence interval based on this model were also calculated in a post hoc analysis. Sensitivity analyses were performed to assess the robustness of our findings (1) using 2 mg/dL instead of 1.5 mg/dL as the threshold for total bilirubin level2; (2) defining successful bile drainage based on any time point during the 6-month posthepato-portoenterostomy period; and (3) using a per-protocol analysis set, excluding participants with major protocol deviations, inadequate study medication exposure, or both (inadequate exposure defined as <80% and >120% of the intended study medications, independent of adverse events).
We also conducted a post hoc sensitivity analysis of our imputation method for missing data for the analysis of the primary end point using multiple imputation methods, which assumes that data were missing at random. Missing total biliru-bin at 6 months was multiply imputed using a Markov chain Monte Carlo method that assumes multivariate normality. The imputation model included treatment, age at the time of hepatoportoenterostomy, BASM syndrome, site, total bilirubin values at all time points, and baseline levels of alkaline phosphatase and γ-glutamyltransferase. Then the composite primary end point was calculated for each participant in each of the 10 imputed data sets, and each data set was analyzed separately using the same model as that used for the primary analysis. Results were combined to account for both within- and between-imputation variance.
An additional post hoc analysis was performed to address new information on the use of steroids in children younger than70 days of age at hepatoportoenterostomy.13 We dichotomized age at the time of hepatoportoenterostomy to younger than 70 days and 70 days or older and added an interaction term (treatment × age at hepatoportoenterostomy) to the model used for the primary end point (replacing age at hepatoportoenterostomy as a continuous variable). We also performed subgroup analyses using separate models for the 2 age categories.
Duration of successful bile drainage, survival with the native liver, and other time-to-event outcomes were summarized using Kaplan-Meier methods and tested using Cox proportional hazards models with the same set of covariates as for the primary end point. Prevalence of as cites and other dichotomous efficacy outcomes were analyzed using the same model as used for the primary end point. For dichotomous safety outcomes, the Fisher exact test was used; and for safety outcomes with multiple recurrences per patient, Poisson regression models were used (incorporating an offset for the period of observation from the time of study medication for infectious serious adverse events and no offset for positive blood cultures among infectious serious adverse events). We limited the number of inferential tests because of the large number of potential safety parameters and the expected small incidence of most types of specific adverse events. For continuous safety outcomes, a random-effects model was used to assess the effect of treatment on the safety outcomes over time posthepatoportoenterostomy, with participant as a random effect, treatment and time posthepatoportoenterostomy as fixed effects, and a spatial power correlation structure used to model the correlation among safety outcomes over time for each participant. All analyses were performed using SAS version 9.2 (SAS Institute Inc).
Results
Study Population
There were 257 patients with biliary atresia assessed for eligibility and 141 patients consented to participate in START; 140 participants were randomized, with 70 beginning treatment with steroids and 70 with placebo within 72 hours of hepatoportoenterostomy (1 participant was not randomized because he developed fever and other symptoms postoperatively that raised safety concerns) (Figure 1). Patients who consented to the study were comparable with those who did not consent to participate in START with respect to sex, race, ethnicity, and age at the time of hepatoportoenterostomy (eTable 4 in Supplement). There was greater than expected study retention with 92.9% of participants in the steroids group and 88.6% in the placebo group either completing the final visit at 2 years of age, undergoing liver transplantation, or dying.
Figure 1. Enrollment, Randomization, and Follow-up of Participants in START Through 24 Months of Age.
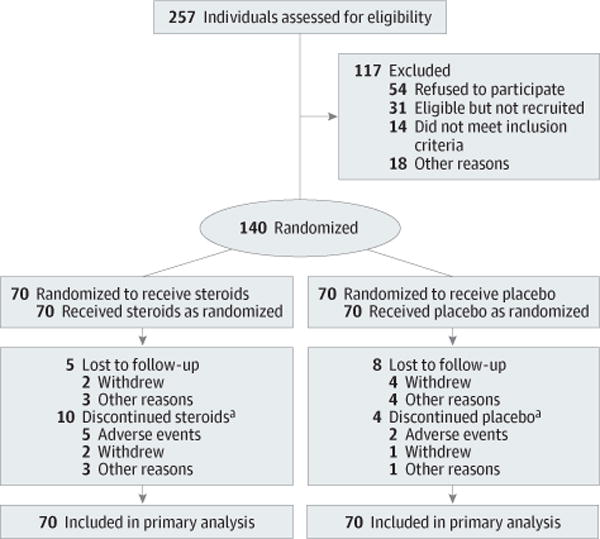
START indicates Steroids in Biliary Atresia Randomized Trial.
aDefined as participants who did not receive at least 80% of their protocol-prescribed study medication.
Demographic and baseline characteristics were comparable between the 2 groups (Table 2). Age at hepatoportoenterostomy (mean [SD] age, 2.3 [0.9] months in the steroids group vs 2.3 [0.8] months in the placebo group) and the percentage of participants with the clinical subtype of BASM syndrome (3% for steroids vs 4% for placebo) were similar in both groups. The degree of hyperbilirubinemia was well balanced between the 2 groups (mean [SD] serum total bilirubin level of 7.5 [2.6] mg/dL in steroids group vs 7.9 [2.8] mg/dL in placebo group), as were biochemical indicators of liver injury and synthetic function.
Table 2.
Participant Characteristics at Enrollment in the Study
| No. (%) of Participants | ||
|---|---|---|
| Steroids (n = 70) |
Placebo (n = 70) |
|
| Male sex | 38 (54) | 30 (43) |
| Racea | ||
| White | 46 (66) | 44 (63) |
| Black | 8 (11) | 11 (16) |
| Other | 16 (23) | 15 (21) |
| Ethnicitya | ||
| Hispanic | 14 (20) | 22 (31) |
| Non-Hispanic | 55 (79) | 48 (69) |
| Refused to respond | 1 (1) | 0 |
| BASM syndrome | 2 (3) | 3 (4) |
| Main types of Ohi classification systemb | ||
| I | 5 (7) | 8 (11) |
| II | 1 (1) | 4 (6) |
| III | 64 (91) | 57 (81) |
| Mean (SD) Values | ||
| Age, mo | 2.3 (0.93) | 2.3 (0.84) |
| z Score | ||
| Weight | −0.8 (1.07) | −0.8 (1.06) |
| Length | −0.7 (1.35) | −0.6 (1.35) |
| Total bilirubin, mg/dL | 7.5 (2.6) | 7.9 (2.8) |
| γ-Glutamyltransferase, U/L | 929 (719) | 731 (569) |
| Alkaline phosphatase, U/L | 619 (341) | 658 (290) |
| Alanine aminotransferase, U/L | 154 (94) | 178 (131) |
| Aspartate aminotransferase, U/L | 236 (215) | 235 (122) |
| White blood cell count,/μL | 13 200 (4300) | 12 900 (4300) |
| Hemoglobin, g/dL | 10.8 (1.9) | 10.4 (1.3) |
| Platelet count, × 103/μL | 473 (179) | 441 (164) |
| International normalized ratio | 1.0 (0.2) | 1.1 (0.4) |
| Albumin, g/dL | 3.6 (0.5) | 3.6 (0.5) |
Abbreviation: BASM, biliary atresia splenic malformation.
SI conversion factors: To convert alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, and γ-glutamyltransferase to μkat/L, multiply by 0.0167; albumin and hemoglobin to g/L, multiply by 10; bilirubin to μmol/L, multiply by 17.104.
Self-reported according to categories set by the US Office of Management and Budget.
Anatomical classification of biliary atresia based on the visual appearance of the extrahepatic biliary tree and the results of intraoperative cholangiography. Type I represents atresia of the common bile duct, type II extends to the hepatic duct, and type III extends to the porta hepatis.19
Primary End Point
In a modified intention-to-treat analysis, treatment with steroids did not increase the proportion of participants that met the primary end point of serum total bilirubin level of less than 1.5 mg/dL with the native liver 6 months after hepatoportoenterostomy compared with placebo (58.6% [41/70] of steroids group vs 48.6% [34/70] of placebo group; adjusted RR, 1.14 [95% CI, 0.83 to 1.57], P = .43; Table 3). The adjusted absolute risk difference was 8.7% (95% CI, −10.4% to 27.7%), with the upper bound exceeding the a priori minimal clinically important difference of 25%.
Table 3.
Primary and Secondary End Points
| No. (%) of Participants | Adjusted RR (95% CI) | Adjusted HR (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Steroids (n = 70) |
Placebo (n = 70) |
||||
| At 6 mo posthepatoportoenterostomya | |||||
| Total bilirubin <1.5 mg/dL and survival with native liver | 41 (58.6) | 34 (48.6) | 1.14 (0.83–1.57)b | .43 | |
| Total bilirubin <1.5 mg/dL | 43 (61.4) | 38 (54.3) | 1.14 (0.82–1.58)c | .44 | |
| Survival with native liver | 55 (78.6) | 52 (74.3) | 1.06 (0.82–1.36)c | .66 | |
| Alive | 68 (97.1) | 68 (97.1) | 1.00 (0.94–1.06)c | .98 | |
| At 24 mo posthepatoportoenterostomyd | |||||
| Survival with native liver and total bilirubin <1.5 mg/dL | 49.4% | 39.8% | 0.8 (0.5–1.2) | .29 | |
| Survival with native liver | 58.7% | 59.4% | 1.0 (0.6–1.8) | .99 | |
| Prevalence of ascitese | |||||
| At age 12 mo | (n = 52) 5 (9.6) |
(n = 47) 3 (6.4) |
1.40 (0.62–3.14) | .41 | |
| At age 24 mo | (n = 42) 1 (2.4) |
(n = 43) 3 (7.0) |
0.30 (0.03–2.92) | .29 | |
Abbreviations: HR, hazard ratio; RR, relative risk.
Good bile drainage defined as serum total bilirubin level of less than 1.5 mg/dL in a participant alive with native liver.
An RR greater than 1 indicates benefit of steroids and a P value for treatment success (good bile drainage) from a log-binomial model with these covariates: treatment group, age of the infant at hepatoportoenterostomy (continuous variable), and biliary atresia splenic malformation (BASM) syndrome as fixed effects and site as a random effect.
An RR greater than 1 indicates benefit of steroids and a P value for components of the primary end point from a log-binomial model with treatment group as a fixed effect and site as a random effect.
Estimate at end of study from the Kaplan-Meier method; the HRs and P values from a Cox proportional hazards model, controlling for treatment group, BASM syndrome, and age of the infant at hepatoportoenterostomy (continuous variable) as fixed effects and site as a random effect.
Among participants with their native liver; the RRs and P values from a log-binomial model with the covariates: treatment group and age of the infant at hepatoportoenterostomy (continuous variable) as fixed effects and site as a random effect.
Sensitivity analyses of the primary end point support the conclusions of the primary analysis. In a per-protocol analysis of 56 participants in the steroids group and 58 in the placebo group, the percentage of the participants meeting the primary end point was similar between the 2 groups (62.5% with steroids and 51.7% with placebo; RR, 1.14 [95% CI, 0.81–1.61], P = .44). Using multiple imputation methods with 10 imputed data sets, there was no statistically significant difference between treatment groups (RR, 1.14 [95% CI, 0.77–1.51], P = .46).
There was no statistically differential effect of steroids by age at hepatoportoenterostomy when younger than 70 days or when aged 70 days or older (P = .67, eTable 5 in Supplement). In the subgroup analysis of 76 participants younger than 70 days at the time of hepatoportoenterostomy, 71.8% (28/39) in the steroids group and 56.8% (21/37) in the placebo group had good bile drainage at 6 months posthepatoportoenteros-tomy; however, this difference was not statistically significant (RR, 1.23 [95% CI, 0.79–1.89], P = .36). There was also no statistically significant treatment difference in the 64 older patients.
Secondary End Points
Survival without liver transplantation for participants treated with steroids was nearly identical to those who received placebo, with 58.7% of participants receiving steroids and 59.4% of those receiving placebo surviving with native liver at 2 years of age (adjusted hazard ratio [HR], 1.0 [95% CI, 0.6–1.8], P = .99; Figure 2A).
Figure 2. Kaplan-Meier Analysis of Key Secondary End Points by Treatment Group.
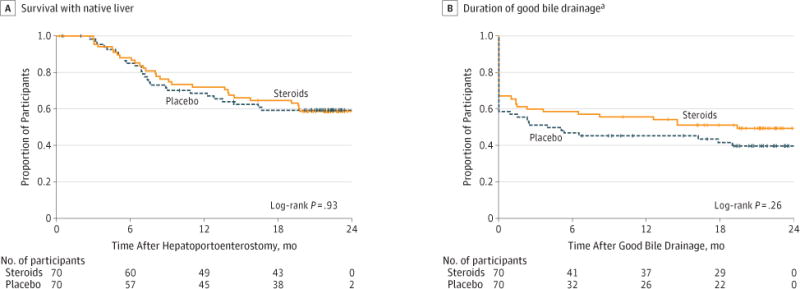
Vertical tick marks indicate censored observations. Participants were censored at time of earlier withdrawal from the study or at the age of 24 months.
aDefined as period when total bilirubin level of less than 1.5 mg/dL achieved for the first time to the first time total bilirubin increased to 1.5 mg/dl or higher, participants underwent liver transplant, or died. Participants that never achieved good bile drainage were considered treatment failures at time 0.
Of those participants who achieved successful bile drainage during the study, treatment with steroids did not significantly influence the duration of serum total bilirubin level of less than 1.5 mg/dL throughout the study (Figure 2B), with 49.4% of participants in the steroids group and 39.8% in the placebo group with their native liver having successful bile drainage at 2 years of age (adjusted HR, 0.8 [95% CI, 0.5–1.2], P = .29). Furthermore, comparison of serum total bilirubin levels at earlier time points and greater than 6 months after hepatoportoenterostomy (time of the primary end point) showed no statistically significant differences between the steroids and placebo groups (eFigure 1 in Supplement).
The prevalence of ascites did not differ statistically between the 2 treatment groups. At 12 months of age, ascites was present in 9.6% (5/52) of the steroids group and 6.4% (3/47) of the placebo group (adjusted RR, 1.40 [95% CI, 0.62–3.14], P = .41), and in 2.4% (1/42) and 7.0% (3/43), respectively, at 24 months of age (adjusted RR, 0.30 [95% CI, 0.03–2.92], P = .29; Table 3).
Safety
Premature discontinuation of steroids due to adverse events was uncommon (5 participants; 7.1%) and similar to placebo (2 did not receive at least 80% and 1 discontinued after receiving >80% for a total of 3 participants [4.3%] discontinuing placebo; P = .72). In contrast, serious adverse events were common in both treatment groups (81.4% [57/70] for steroids vs 80.0% [56/70] for placebo; P > .99), as were unexpected and expected adverse events (Table 4 and eTables 6 and 7 in Supplement). However, infants treated with steroids experienced their first serious adverse events earlier than those receiving placebo; 37.2% (95% CI, 26.9%–50.0%) of the steroids group experienced a first serious adverse event by 30 days posthepa-toportoenterostomy compared with 19.0% (95% CI, 11.5%– 30.4%) of the placebo group (P = .008; Figure 3A). Six participants in the steroids group compared with 1 in the placebo group experienced a surgical serious adverse event (eTable 8 in Supplement). In contrast, during the study period following completion of drug or placebo administration, there were no statistically significant differences in the time to first serious adverse event between the groups (P = .33; Figure 3B).
Table 4.
Adverse Events (AEs) Throughout the Duration of the Study
| Type of AE | No. of AEsa | P Valueb | |
|---|---|---|---|
| Steroids (n = 70) |
Placebo (n = 70) |
||
| Seriousc | 57 (81.4)d | 56 (80.0)d | >.99 |
| Total No. | 204 | 162 | |
| Per participant | 2.91 | 2.31 | |
| Unexpectede | 36 (51.4)d | 36 (51.4)d | >.99 |
| Dermatological | 12 | 11 | |
| Febrile | 21 | 27 | |
| Gastrointestinal | 19 | 19 | |
| Infectious viral | 25 | 9 | |
| Infectious | 38 | 24 | |
| Nutritional | 0 | 6 | |
| Pulmonary | 32 | 16 | |
| Miscellaneousf | 12 | 17 | |
| Expectedg | 44 (62.9)d | 40 (57.1)d | .61 |
| Bacteremia | 31 | 27 | |
| Bone fracture | 2 | 5 | |
| Cataracts | 0 | 0 | |
| Fungemia | 5 | 2 | |
| Gastrointestinal bleeding | 23 | 14 | |
| Hyperglycemia | 1 | 0 | |
| Hypokalemia | 9 | 4 | |
| Impaired wound healing | 0 | 0 | |
| Pancreatitis events | 0 | 0 | |
| Severe irritability events | 3 | 2 | |
| Vaccine-preventable infection | 0 | 1 | |
Expressed as number of events unless otherwise indicated. Any expected or unexpected AE that qualified as serious was counted as such. Details of serious and unexpected AEs are reported in eTables 6 and 7, respectively, in Supplement. A participant may have had more than 1 AE.
Calculated using the Fisher exact test.
Defined as any untoward medical occurrence (whether it was plausibly related to the index surgery) that resulted in death, was life threatening, required inpatient hospitalization, resulted in persistent or serious disability or incapacity, resulted in a congenital anomaly or birth defect, or constituted a medically important condition.
Expressed as No. (%) of participants.
Defined as any other untoward event that did not qualify as an expected AE.
Included hematologic or hepatic injury and immunological, metabolic, orthopedic, or urinary events.
Defined as common events attributable to the initial surgical drainage procedure or the underlying liver disease.
Figure 3. Time to First Serious Adverse Event.
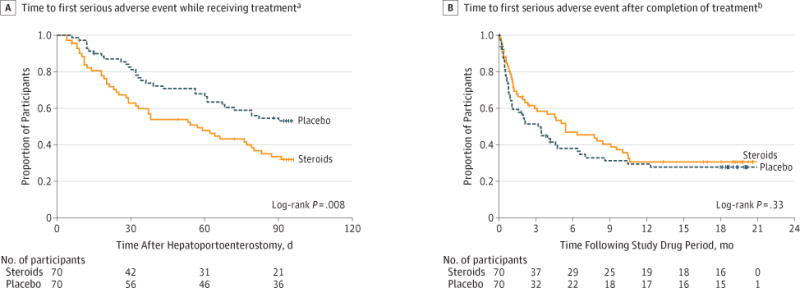
Vertical tick marks indicate censored observations.
aDefined as the time from initiation of study medication to the earliest first serious adverse event or liver transplantation, exit from the study, or last day taking study medication (censored). Serious adverse events occurred significantly earlier in participants receiving steroids compared with placebo.
bDefined as the time from the end of study medication to the earliest first serious adverse event after completion of study drug or placebo (event) or liver transplantation, or exit from the study (censored).
There were no significant treatment differences in weight (posttreatment mean z score range, −0.4 to −1.7 for steroids group vs 0.1 to −1.2 for placebo group, P = .16) and length (post- treatment mean z score range, −0.6 to −1.3 for steroids group vs −0.3 to −1.0 for placebo group, P = .28) or in the number of infectious serious adverse events (P = .40) during the course of the study. An analysis of the occurrence of cholangitis, a known infectious complication following hepatoportoenter-ostomy, showed similar proportions of participants surviving with their native livers with no cholangitis episodes at 24 months of age (eFigure 2 in Supplement).
The proportion of patients with inadequate response to routine childhood immunizations tended to be higher in the steroids group (51.5%) than in the placebo group (38.5%), but was not statistically significant (P = .43; eTable 9 in Supplement).
Discussion
We found that the addition of high-dose steroids following hepatoportoenterostomy did not result in statistically significant differences compared with placebo in the proportion of patients achieving normalization of total serum bilirubin level 6 months posthepatoportoenterostomy, a short-term bio-marker of achieving successful bile drainage,2 although we cannot exclude a small clinical benefit of steroids. We observed no statistically significant differences between the 2 groups in the 2-year survival with the native liver or in the levels of serum total bilirubin after hepatoportoenterostomy at any time point during the duration of the study. Notably, those receiving steroids had a shorter time to the development of serious adverse events while receiving the study drug, raising a potential increase in risks associated with steroid therapy.
The results of this trial differ from previous reports of a benefit of steroid therapy on bile drainage, survival in biliary atresia, or both.6–12 The only other prospective, randomized placebo-controlled trial, which was published after initiation of this trial, showed no steroid effect in the percentage of participants achieving normal bilirubin levels14; however, that study was of smaller size (73 participants vs 140 participants in our trial), involved fewer centers (2 centers vs 14 centers), used lower doses of steroids (starting at 2 mg/kg/d vs 4 mg/kg/d), and for a shorter duration (4 weeks vs 13 weeks).14
Despite theoretical benefits of decreasing tissue inflammation and inducing choleresis,20–22 the use of high doses of steroids starting within 3 days after hepatoportoenterostomy in our study was associated with only an adjusted absolute treatment difference of 8.7% relative to placebo in the proportion of participants with a serum bilirubin level of less than 1.5 mg/dL at 6 months posthepatoportoenterostomy, which fell short of the a priori 25% absolute increase deemed clinically important based on a previous study using a similar steroid dose after hepatoportoenterostomy.6 It is possible that the lack of statistical significance resulted from an overestimation of the effect size. However, if the true benefit of steroids is as large as 25%, we cannot exclude a clinical benefit because the 95% upper confidence bound for the absolute treatment difference was 27.7%. We also cannot exclude that steroids could result in clinical harm because the 95% lower confidence bound for the absolute treatment difference was −10.4%. Secondary outcomes did not support any clinical benefit because total bil-irubin values over time showed similar bilirubin levels at earlier (1 and 3 months posthepatoportoenterostomy) and later (at 12 months of age) time points; and the overall 2-year survival with native liver was nearly identical in both groups (58.7% for steroids vs 59% for placebo).
A previous report14 suggested that steroid therapy was associated with a greater reduction in serum bilirubin 1 month after surgery and a higher percentage of children with normal bilirubin levels at 12 months of age among participants younger than 70 days at hepatoportoenterostomy. In a subsequent open-label trial, these investigators reported that the use of high doses of steroids in the first month postoperatively in participants younger than 70 days was associated with lower serum bilirubin compared with a historical control group and a higher percentage with clearance of jaundice (66% vs 52%), but no improvement in transplant-free survival.13 This study was limited by small cohort size, an open-label design, and the nature of subgroup analyses. We found no evidence of an effect of high doses of steroids in our appropriately sized cohort, whose average age was 69 days atstudy enrollment. Additionally, a subgroup analysis focusing on the 76 participants younger than 70 days at the time of hepatoportoenterostomy showed no statistically significant effect of age at the time of surgery on bile drainage between the steroids or placebo groups.
Safety concerns regarding the use of steroids in infants derive from their known association with a spectrum of severe adverse events, including immunosuppression and associated risk of infection, poor wound healing, hyperglycemia, gastrointestinal bleeding, poor growth, and inadequate response to routine immunizations. With vigilant monitoring and reporting of adverse events and serious adverse events, both the steroids and placebo groups were found to have a high incidence of adverse events, indicating that they were most likely the direct consequences of the severe liver disease typical of biliary atresia. However, during the active treatment period, steroid therapy was associated with a significantly earlier onset of serious adverse events, among which were complications at the sites of surgical anastomoses and intestinal perforation. These findings differ from previous reports of no adverse events associated with steroid use in children with biliary atresia after hepatoportoenterostomy,7–9,12,14 and raise safety concerns for use of these drugs following surgery.
A limitation of this study is the inclusion of participants undergoing surgical and medical treatments in different centers, which introduces a potential influence of the experience of the care team and variation in the surgical procedure on clinical outcome. Previous reports have suggested that the experience of the center influences the outcome of hepatoporto-enterostomy, with better biliary drainage and transplant-free survival in centers performing higher numbers of this procedure.23–25 Other studies have not found a relationship between the annual caseload of a center and improved outcome.26,27 Whether differences relate to experience with hepatoportoenterostomy or a general expertise in complex hepatobiliary surgery and management of severe liver disease in children is not known. To minimize this center effect, all participating sites followed the same postoperative protocol. In addition, we randomized treatments by site and accounted for the influence of site as a random effect when analyzing the primary and secondary end points.
Conclusions
Among infants with biliary atresia who have undergone hepa-toportoenterostomy, high doses of steroids posthepatoportoenterostomy did not result in statistically significant treatment differences in bile drainage at 6 months, although a small clinical benefit could not be excluded. The use of steroids was associated with an earlier onset of serious adverse events. Based on the strength of the evidence, the addition of high-dose steroids as an adjuvant treatment for infants with biliary atresia after hepatoportoenterostomy cannot be recommended.
Supplementary Material
Acknowledgments
Funding/Support: The following National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) grants supported the START study: DK62503 and TR000424 (awarded to Johns Hopkins University School of Medicine), DK62436 and TR000150 (Lurie Children’s Hospital of Chicago), DK62497 and TR000077 (Cincinnati Children’s Hospital Medical Center), DK62453 and TR000154 (University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora), DK62445 (Mount Sinai School of Medicine), DK62481 and TR000003 (Children’s Hospital of Philadelphia), DK62466 and TR000005 (Children’s Hospital of Pittsburgh of UPMC), DK62500 and TR000004 (UCSF Benioff Children’s Hospital), DK62452 and TR000448 (Washington University School of Medicine and St Louis Children’s Hospital), DK62470 (Baylor College of Medicine and Texas Children’s Hospital, Houston), DK84575 and TR000423 (Seattle Children’s Hospital), DK84538 and TR000130 (Children’s Hospital Los Angeles and University of Southern California), DK84585, DK62470, and TR000454 (Emory University School of Medicine and Children’s Healthcare of Atlanta), DK84536 and TR000007 (Indiana University School of Medicine and Riley Hospital for Children), and DK62456 (University of Michigan data coordinating center). The following companies provided support for the START study, each of which provided formula or medications as part of a cooperative agreement with the NIDDK: GlaxoSmithKline supplied ranitidine; Axcan Pharma US Inc, fat-soluble vitamins and tocopherol polyethylene glycol succinate; Axcan Pharma US Inc, ursodiol until 2009; and Mead Johnson Nutrition, Pregestimil.
Role of the Sponsors: The companies listed at the end of the Funding/Support section were provided copies of the START protocol prior to the start of the trial. They did not participate in the design and conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. These industries were given a copy of the manuscript before submission for publication as per policy of the ChiLDREN Network.
Footnotes
Supplemental content at jama.com
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00294684
Author Contributions: Dr Spino had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bezerra, Magee, Shneider, Rosenthal, Haber, Karpen, Schwarz, Shepherd, Suchy, Whitington, Robuck, Sokol.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bezerra, Spino, Magee, Shneider, Rosenthal, Wang, Erlichman, Moore, Sherker, Sokol.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Spino, Magee, Moore.
Obtained funding: Bezerra, Magee, Shneider, Rosenthal, Wang, Karpen, Kerkar, Loomes, Molleston, Murray, Romero, Schwarz, Turmelle, Whitington, Sokol.
Administrative, technical, or material support: Spino, Magee, Sherker, Robuck.
Study supervision: Bezerra, Spino, Magee, Shneider, Sherker, Robuck, Sokol.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bezerra reported receiving grants from Molecular Genetics Laboratory of Cincinnati Children’s Hospital Medical Center outside the submitted work. Dr Shneider reported serving on data monitoring committes for Bristol-Myers Squibb and Vertex on hepatitis B and C, respectively; serving as associate editor for the American Association for the Study of Liver Diseases; and receiving royalites from PMPH-USA for a pediatric gastrointestinal textbook. Dr Rosenthal reported receiving grants from Roche, Bristol-Myers Squibb, Vertex, and Gilead; and receiving consulting fees from General Electric and Hyperion. Dr Haber reported receiving grants from the National Institutes of Health during the conduct of the study while with Children’s Hospital of Philadelphia, University of Pennsylvania. In January 2012, Dr Haber changed employment and now works at Merck in the area of viral hepatitis; however, her current work does not overlap or effect any aspect of the article. Dr Loomes reported receiving book royalties from Lippincott Williams & Wilkins and payment for an article on biliary atresia from Up-to-Date. Dr Molleston reported receiving grants from Schering, Roche, and Vertex outside the submitted work. Dr Schwarz reported serving as a consultant to Roche/Genentech; providing expert testimony for the State of Pennsylvania; and receiving institutional grants from the National Institute of Diabetes, Digestive and Kidney Diseases, Bristol-Myers Squibb, Roche/Genentech, and Vertex. Dr Sokol reported receiving grants from National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health; receiving nonfinancial support from Mead Johnson Nutrition during the conduct of the study; receiving consulting fees from Yasoo Health Inc, Ikaria Pharmaceuticals, Roche Products, and Cardax Pharmaceuticals; and having a patent for use of antioxidants for treatment of cholestasis licensed to Yasoo Health Inc. No other disclosures were reported.
Group Information: The Childhood Liver Disease Research and Education Network (ChiLDREN) additional investigators are NIDDK: Edward Doo, MD, Rebecca Torrance, RN, Rebekah van Raaphorst, MPH, Jay H. Hoofnagle, MD. Johns Hopkins University School of Medicine: Paul Colombani, MD, Henry Lau, MD, Kim Kafka, RN, Anitha Devadason. Lurie Children’s Hospital of Chicago: Riccardo Superina, MD, Sue Kelly, RN. Cincinnati Children’s Hospital Medical Center: Greg Tiao, MD, Frederick Ryckman, MD, Maria Alonso, MD, Jaimie Nathan, MD, John Bucuvalas, MD, Alexander Miethke, MD, James Heubi, MD, William F. Balistreri, MD, Kathleen Campbell, MD, Rohit Kohli, MD, Mike Leonis, MD, Julie Denlinger, RN, Andrea Ferris. University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora: Frederick Karrer, MD, Cara Mack, MD, Michael R. Narkewicz, MD, Shikha S. Sundaram, MD, Mark Lovell, MD, Joan Hines, MPH, Sheryl Faut, RN, Michelle Hite, MA. Mount Sinai School of Medicine, New York: Kishore Iyer, MBBS, Ronen Arnon, MD. Children’s Hospital of Philadelphia: Alan Flake, MD, Elizabeth Rand, MD. Children’s Hospital of Pittsburgh of UPMC: Beverly Bernard, RN, Cartland Burns, MD, Kathryn Bukauskas, RN, Robert Squires, MD, Veena Venkat, MD. UCSF Benioff Children’s Hospital: Camille Langlois, MS, Shannon Fleck. Washington University School of Medicine and St Louis Children’s Hospital: Patrick Dillon, MD, Frances White, MD, Alexander Weymann, MD, David Rudnick, MD, Kathy Harris, Stacy Postma. Baylor College of Medicine and Texas Children’s Hospital, Houston: Kimberly Pieplow, MPH, Mary Brandt, MD, Milton Finegold, MD, Beth Carter, MD, Doug Fishman, MD, Val McLin, MD, David Wesson, MD, Zoe Apted, BA, Alejander DeLaTorre, BBS, Darrell Cass, MD. Seattle Children’s Hospital: Simon Horslen MB, ChB, FRCPCH, Evelyn Hsu, MD, Patrick Healey, MD, Melissa Young, Laura Finn, MD. Children’s Hospital Los Angeles and University of Southern California: Cat Goodhue, NP, Daniel Thomas, MD, Sonia Michael, MD. Emory University School of Medicine and Children’s Healthcare of Atlanta: Carlos Abramowsky, MD, Matthew Clifton, MD, Liezl De La Cruz, BA, Nitika Gupta, MD, DCH, DNM, MRCPCH, Richard Ricketts, MD, Sundari Sekar, MBBS, DGO, Bahig Shehata, MD, Miriam Vos, MD, MSPH. Indiana University School of Medicine and Riley Hospital for Children: Girish Subbarao, MD, Karen West, MD, Beth Byam, RN. University of Michigan Data Coordinating Center: Trivellore Raghunathan, PhD, James Lopez, MD, Emily Fredericks, PhD, Beverley Marchant, BSN, Karen Jones, BS, Kristina Slusser, Yang Casher, MA. Investigators with different institutional affiliations at the beginning of START: Benjamin L. Shneider and Frederick J. Suchy (Mount Sinai School of Medicine, New York, New York), Saul J. Karpen (Texas Children’s Hospital, Houston), Ross Shepherd (Washington University School of Medicine, St Louis, Missouri).
Study Safety Monitor: M. James Lopez, MD, University of Michigan School of Medicine.
Members of the Data and Safety Monitoring Board of START: Keith Oldham, MD (Medical College of Wisconsin, Milwaukee), P. Joan Chesney, MD (St Jude Children’s Research Hospital, Memphis, Tennessee), Richard Ehrenkranz, MD (Yale University School of Medicine, New Haven, Connecticut), Peter Imrey, PhD (Cleveland Clinic Foundation, Cleveland, Ohio), Esther J. Israel, MD (Massachusetts General Hospital for Children, Boston, Massachusetts).
Additional Contributions: We acknowledge the current and former members of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) START and ChiLDREN listed above who played important roles in the development of START and enrollment of participants in the trial. We thank all the ChiLDREN investigators, the research coordinators, the participants, and the families who agreed to participate in this study. We also thank Denise Lagory, RPh, who was the central research pharmacist for the trial. No compensation was received by the individuals for contributions to the trial outside the NIDDK grant funding.
References
- 1.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46(2):566–581. doi: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shneider BL, Brown MB, Haber B, et al. Biliary Atresia Research Consortium A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Chardot C, Buet C, Serinet MO, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. 2013;58(6):1209–1217. doi: 10.1016/j.jhep.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Karrer FM, Lilly JR. Corticosteroid therapy in biliary atresia. J Pediatr Surg. 1985;20(6):693–695. doi: 10.1016/s0022-3468(85)80026-9. [DOI] [PubMed] [Google Scholar]
- 5.Kasai M, Suzuki H, Ohashi E, Ohi R, Chiba T, Okamoto A. Technique and results of operative management of biliary atresia. World J Surg. 1978;2(5):571–579. doi: 10.1007/BF01556048. [DOI] [PubMed] [Google Scholar]
- 6.Dillon PW, Owings E, Cilley R, Field D, Curnow A, Georgeson K. Immunosuppression as adjuvant therapy for biliary atresia. J Pediatr Surg. 2001;36(1):80–85. doi: 10.1053/jpsu.2001.20013. [DOI] [PubMed] [Google Scholar]
- 7.Escobar MA, Jay CL, Brooks RM, et al. Effect of corticosteroid therapy on outcomes in biliary atresia after Kasai portoenterostomy. J Pediatr Surg. 2006;41(1):99–103. doi: 10.1016/j.jpedsurg.2005.10.072. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi H, Yamataka A, Koga H, et al. Optimum prednisolone usage in patients with biliary atresia postportoenterostomy. J Pediatr Surg. 2005;40(2):327–330. doi: 10.1016/j.jpedsurg.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Meyers RL, Book LS, O’Gorman MA, et al. High-dose steroids, ursodeoxycholic acid, and chronic intravenous antibiotics improve bile flow after Kasai procedure in infants with biliary atresia. J Pediatr Surg. 2003;38(3):406–411. doi: 10.1053/jpsu.2003.50069. [DOI] [PubMed] [Google Scholar]
- 10.Muraji T, Higashimoto Y. The improved outlook for biliary atresia with corticosteroid therapy. J Pediatr Surg. 1997;32(7):1103–1107. doi: 10.1016/s0022-3468(97)90408-5. [DOI] [PubMed] [Google Scholar]
- 11.Muraji T, Nio M, Ohhama Y, et al. Japanese Biliary Atresia Society Postoperative corticosteroid therapy for bile drainage in biliary atresia—a nationwide survey. J Pediatr Surg. 2004;39(12):1803–1805. doi: 10.1016/j.jpedsurg.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Tatekawa Y, Muraji T, Tsugawa C. Glucocorticoid receptor alpha expression in the intrahepatic biliary epithelium and adjuvant steroid therapy in infants with biliary atresia. J Pediatr Surg. 2005;40(10):1574–1580. doi: 10.1016/j.jpedsurg.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Davenport M, Parsons C, Tizzard S, Hadzic N. Steroids in biliary atresia: single surgeon, single centre, prospective study. J Hepatol. 2013;59(5):1054–1058. doi: 10.1016/j.jhep.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Davenport M, Stringer MD, Tizzard SA, McClean P, Mieli-Vergani G, Hadzic N. Randomized, double-blind, placebo-controlled trial of corticosteroids after Kasai portoenterostomy for biliary atresia. Hepatology. 2007;46(6):1821–1827. doi: 10.1002/hep.21873. [DOI] [PubMed] [Google Scholar]
- 15.Lao OB, Larison C, Garrison M, Healey PJ, Goldin AB. Steroid use after the Kasai procedure for biliary atresia. Am J Surg. 2010;199(5):680–684. doi: 10.1016/j.amjsurg.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkhy A, Schreiber RA, Milner RA, Barker CC. Does adjuvant steroid therapy post-Kasai portoenterostomy improve outcome of biliary atresia? systematic review and meta-analysis. Can J Gastroenterol. 2011;25(8):440–444. doi: 10.1155/2011/125610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shneider BL, Magee JC, Bezerra JA, et al. Childhood Liver Disease Research Education Network (ChiLDREN) Efficacy of fat-soluble vitamin supplementation in infants with biliary atresia. Pediatrics. 2012;130(3):e607–e614. doi: 10.1542/peds.2011-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agresti A. Categorical Data Analysis. Hoboken, NJ: John Wiley and Sons; 2012. [Google Scholar]
- 19.Ohi R. Biliary atresia: a surgical perspective. Clin Liver Dis. 2000;4(4):779–804. doi: 10.1016/s1089-3261(05)70141-0. [DOI] [PubMed] [Google Scholar]
- 20.Haynes RC, Murad F. Adrenocorticotropic Hormone; Adrenocortical Steroids and Their Synthetic Analogs; Inhibitors of Adrenocortical Steroid Biosynthesis. 6. New York, NY: MacMilan; 1980. [Google Scholar]
- 21.Miner PB, Jr, Gaito JM. Bile flow in response to pharmacologic agents: hepatic DNA as a reference standard. Biochem Pharmacol. 1979;28(7):1063–1066. doi: 10.1016/0006-2952(79)90304-6. [DOI] [PubMed] [Google Scholar]
- 22.Miner PB, Jr, Sutherland E, Simon FR. Regulation of hepatic sodium plus potassium-activated adenossine triphosphatase activity by glucocorticoids in the rat. Gastroenterology. 1980;79(2):212–221. [PubMed] [Google Scholar]
- 23.Davenport M, De Ville de Goyet J, Stringer MD, et al. Seamless management of biliary atresia in England and Wales (1999–2002) Lancet. 2004;363(9418):1354–1357. doi: 10.1016/S0140-6736(04)16045-5. [DOI] [PubMed] [Google Scholar]
- 24.Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. 2009;374(9702):1704–1713. doi: 10.1016/S0140-6736(09)60946-6. [DOI] [PubMed] [Google Scholar]
- 25.Lampela H, Ritvanen A, Kosola S, et al. National centralization of biliary atresia care to an assigned multidisciplinary team provides high-quality outcomes. Scand J Gastroenterol. 2012;47(1):99–107. doi: 10.3109/00365521.2011.627446. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber RA, Barker CC, Roberts EA, Martin SR, Canadian Pediatric Hepatology Research Group Biliary atresia in Canada: the effect of centre caseload experience on outcome. J Pediatr Gastroenterol Nutr. 2010;51(1):61–65. doi: 10.1097/MPG.0b013e3181d67e5e. [DOI] [PubMed] [Google Scholar]
- 27.Serinet MO, Broué P, Jacquemin E, et al. Management of patients with biliary atresia in France: results of a decentralized policy 1986-2002. Hepatology. 2006;44(1):75–84. doi: 10.1002/hep.21219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


