Abstract
In 2011 and 2012, the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, National Institutes of Health, held a series of meetings to provide guidance to investigators regarding study design of clinical trials of vaccines and antimicrobial medications that enroll pregnant women. Assessment of congenital anomalies among infants born to women enrolled in these trials was recognized as a challenging issue, and a workgroup with expertise in epidemiology, pediatrics, genetics, dysmorphology, clinical trials, and infectious diseases was formed to address this issue. The workgroup considered 3 approaches for congenital anomalies assessment that have been developed for use in other studies: (1) maternal report combined with medical records review, (2) standardized photographic assessment and physical examination by a health professional who has received specific training in congenital anomalies, and (3) standardized physical examination by a trained dysmorphologist (combined with maternal interview and medical records review). The strengths and limitations of these approaches were discussed with regard to their use in clinical trials. None of the approaches was deemed appropriate for use in all clinical trials. Instead, the workgroup acknowledged that decisions regarding the optimal method of assessment of congenital anomalies will likely vary depending on the clinical trial, its setting, and the agent under study; in some cases, a combination of approaches may be appropriate. The workgroup recognized the need for more research on approaches to the assessment of congenital anomalies to better guide investigators in optimal design of clinical trials that enroll pregnant women.
Keywords: congenital anomalies, birth defects, clinical trials, pregnant women.
Pregnant women have traditionally been excluded from clinical trials because of the potential risk of harm to the fetus that might be associated with prenatal exposure to the product under study [1, 2]. However, in recent years, it has become evident that inclusion of pregnant women in clinical trials of products intended for use during pregnancy might be scientifically and ethically justifiable [3, 4]. To provide guidance to investigators who are enrolling pregnant women in clinical trials of vaccines or antimicrobial medications, the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (DMID/NIAID), National Institutes of Health, organized a series of 3 meetings held in 2011 and 2012 entitled “Enrolling Pregnant Women in Clinical Trials of Vaccines and Therapeutics.” These meetings led to the publication of 2 papers, one on study design and methods of clinical trials that enroll pregnant women [4] and the other on reference values for vital signs and laboratory values for pregnant women [5].
During these meetings, the issue of how to assess congenital anomalies that occur among infants born to women enrolled in clinical trials was discussed, and preliminary recommendations were made [4]. One recommendation was to only report major congenital anomalies as serious adverse events because reporting of minor anomalies could result in inappropriate safety signals. However, meeting attendees recommended that findings on minor anomalies be documented and that information on these anomalies be reviewed periodically by the study team to look for any concerning patterns that could indicate that the agent under study is teratogenic [4]. The importance of training clinical trial personnel on the identification and reporting of major and minor congenital anomalies was also recognized. To provide further guidance to study investigators, an additional DMID/NIAID–sponsored meeting was held on 27 September 2013 and included a workgroup that focused on assessment of congenital anomalies. Workgroup members included experts in epidemiology, pediatrics, genetics, dysmorphology, clinical trials, and infectious diseases. This article summarizes the workgroup's discussions.
MAJOR AND MINOR CONGENITAL ANOMALIES
Major congenital anomalies are defects that are present at birth and that have surgical, medical, or serious cosmetic significance. Major anomalies occur in about 3% of infants, according to data from the Metropolitan Atlanta Congenital Defects Program, a birth defects surveillance system administered by the Centers for Disease Control and Prevention (CDC) [6]. A few examples of major congenital defects are cleft lip, gastroschisis, spina bifida, and congenital heart defects, such as atrial and ventricular septal defects. Minor congenital anomalies are also present at birth but are without medical, surgical, or serious cosmetic significance. Some examples include epicanthal folds, single transverse palmar crease, and fifth finger clinodactyly (Figure 1 [7, 8]). Minor anomalies are useful in the study of birth defects for several reasons [9]. Minor anomalies are often associated with and helpful for making a diagnosis of syndromes of known etiology, such as chromosome abnormalities or single gene disorders [10]. For example, bilateral single transverse palmar creases are seen in >30% of persons with Down syndrome but in only 2% of persons without Down syndrome [11]. In addition, minor anomalies have also been shown to be critical for the identification of the teratogenicity of certain exposures, such as alcohol, hydantoin, and carbamazepine [12–14].
Figure 1.
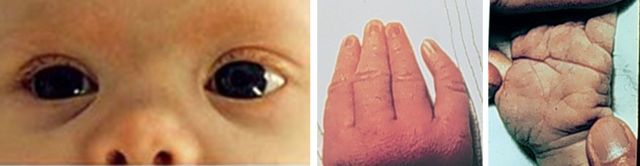
Examples of minor congenital anomalies: Left: Epicanthal folds (defined as presence of folds of skin that cover the medial corners of the eyes) [8]. Middle: fifth finger clinodactyly (defined as incurving of the fifth finger toward the radius) [7]. Right: Single transverse palmar crease (defined as when the distal and proximal transverse palmar creases merge into a single transverse palmar crease) [7]. Left photo is from Elements of Morphology [8]. Middle and right photos are provided courtesy of Dr Jaime L. Frias.
Although minor congenital anomalies are of significance when considering exposures during pregnancy, they are common and not consistently ascertained: in four studies, the rates of minor anomalies identified among newborns ranged from 14.7% to 40.7% [9, 15–17]. In addition, ascertainment of specific minor anomalies among different examiners has been shown to have poor reproducibility, emphasizing the need for efforts to improve the inter-examiner reliability of their ascertainment [18].
NEED FOR STANDARDIZATION
The challenges for collecting data on major and minor congenital anomalies in clinical trials enrolling pregnant women are significant. First, a consistent system for identifying and recording these events has not been developed. However, differences in timing (ie, prenatal vs at birth vs later in life), available technologies, and methods of ascertainment (eg, maternal report, medical records review, or physical examination) all can have a major effect on congenital anomaly data collection. For example, deciding whether to include anomalies that are identified prenatally in pregnancies that are subsequently terminated because of the defect is important and can affect the frequency of certain anomalies, such as anencephaly [19]. Timing of ascertainment of congenital anomalies after birth is also critical. Although congenital anomalies are by definition present at birth, some might not be identified early in life. For example, congenital heart defects are often not diagnosed until later after full transition from fetal circulation to postnatal circulation has occurred [20]. Similarly, some genetic conditions such as Down syndrome might not be immediately recognized at birth but might be diagnosed at a later time [21]. Available technologies also impact the data collected on some anomalies. Defects such as ventricular septal defects are frequently observed among infants in developed countries [20]; however, the frequency of these defects would be much lower among infants in developing countries where echocardiography is less readily available, because definitive diagnosis of these defects requires use of echocardiography or cardiac catheterization or observation at the time of surgical repair or autopsy [21]. The data collected on congenital anomalies also differ based on the method of ascertainment. Ascertainment of major birth defects has been shown to differ widely using different methods such as review of birth certificates, maternal report, and birth defects surveillance systems (which depend on medical records review) [22, 23]. Ascertainment of minor defects would be expected to differ even further: for example, a standardized physical examination would have the ability to identify minor congenital anomalies, but maternal report would almost always miss these anomalies, given that clinicians are unlikely to discuss them with parents. An additional complicating factor is that investigators involved in clinical trials are unlikely to have the expertise to identify and classify major and minor congenital anomalies.
In an effort to work toward standardizing data collection on congenital anomalies for use in clinical trials that include pregnant women, our workgroup considered 3 approaches for assessment of congenital anomalies that have been developed for use in other studies (Table 1): (1) maternal report and review of medical records, used by the North American Antiepileptic Drug (AED) Pregnancy Registry, (2) standardized photographic assessment and physical examination by a health professional who has received specific training in the reporting of congenital anomalies, an approach proposed for use in the National Children's Study (NCS), and (3) a standardized physical examination by a trained dysmorphologist (which follows maternal interview and medical records review), used by the Organization of Teratology Information Specialists [OTIS].
Table 1.
Comparison of Three Approaches Used to Assess Congenital Anomalies: Features, Strengths, and Limitations
| Study | Approach | Ascertains Major or Minor Anomalies | Timing | Strengths | Limitations |
|---|---|---|---|---|---|
| North American Antiepileptic Drug Pregnancy Registry | Maternal report followed by review of medical records of examinations up to 12 wks of life | Major only | Defects identified in first 12 wks |
|
|
| National Children's Study | Photographic protocol (15 images and 3 10-second videos) and Physical Assessment (checklist of 25 features) completed by trained study personnel | Major and minor anomalies | Exam conducted at birth, 6, and 12 mo of age |
|
|
| Organization of Teratology Information Specialists | Physical examination within the first 6 mo of life by trained study dysmorphologists | Major and minor anomalies | Single exam during first 6 mo of life |
|
|
Abbreviations: AED, antiepileptic drug; OTIS, Organization of Teratology Information Specialists.
NORTH AMERICAN ANTIEPILEPTIC DRUG PREGNANCY REGISTRY APPROACH
The North American AED Pregnancy Registry is an ongoing surveillance system of pregnant women who are taking an AED for any reason [24–26]. A pregnancy registry is defined as an observational prospective cohort of women who have had an exposure of interest and have been enrolled voluntarily during gestation, typically before information about pregnancy outcome is known. Information on specific pregnancy outcomes is systematically collected and compared to a scientifically valid reference population [27].
Women enroll in the North American AED Pregnancy Registry by calling a toll-free telephone number. Women are interviewed at enrollment, at 7 months of gestation, and at 8–12 weeks after the expected date of delivery. For the study of major congenital anomalies, women are considered eligible for analysis if they had a live birth, fetal death, or pregnancy termination because of a fetal abnormality. They are considered ineligible for analysis if they had a spontaneous abortion, withdrew from the Registry or were lost to follow-up.
The main outcomes of interest are major congenital anomalies diagnosed before 12 completed weeks after birth [28]. Maternal report is used to ascertain anomalies; information on results of prenatal testing is collected during prenatal interviews, and the postnatal interview is used to collect information on the infant's birth status, including whether the infant has any health problems. Mothers are also asked to sign medical record release forms, and the infant's healthcare providers (including any specialists who have evaluated the infant) are requested to return medical records documenting physical examination findings through the first 12 weeks of life. Written descriptions of physical examination findings are reviewed by the study teratologist, who is blinded to exposure. Physical features that are excluded are minor anomalies, birth marks, deformations, findings observed on prenatal ultrasonography that are not identified by the examining pediatrician, complications of prematurity, genetic disorders, and chromosome abnormalities [28]; these make up about 17%–27% of identified anomalies [29, 30].
Women are considered exposed if they used any AED, as monotherapy or polytherapy, during the first 4 lunar months after the last menstrual period. The primary reference group is composed of women exposed to lamotrigine because it has been the most commonly reported AED in the Registry. The rationale for the primary active reference group is 2-fold. First, this allows comparison among AEDs to answer the most clinically relevant question: which AED is associated with the lowest risk for adverse outcomes? Second, it minimizes confounding by indication, because most subjects in the groups compared (ie, specific AEDs vs lamotrigine) will have a seizure disorder. A secondary internal reference group has been collected since 2003 and consists of pregnant women not taking an AED and without epilepsy who have been recruited among the friends and relatives of AED-exposed participants and followed with the same methodology. In addition, to estimate the expected risk of specific major anomalies, an external reference group is used, which consists of 206 224 infants born at Brigham and Women's Hospital in Boston. This surveillance system was selected because it used the same inclusion/exclusion criteria for outcome definition as the AED Pregnancy Registry; however, it followed infants only up to 5 days after birth [31]. For analyses using this reference group, malformations identified among exposed infants after 5 days of life are excluded.
NATIONAL CHILDREN'S STUDY APPROACH
The NCS is planned as the largest longitudinal study of child health ever conducted in the United States [32]. As a comprehensive epidemiologic survey, one goal of the study is to create a platform from which investigators will be able to conduct future studies. Study protocol development therefore requires significant planning and vision to answer a wide range of questions that might be asked in the future, which has included development and evaluation of methods to identify outcomes of potential interest, such as genetic disorders and congenital anomalies.
A formative research project was designed to develop, evaluate, and implement a tool that would document and categorize physical features by physical examination and digital photography. The baseline physical assessment was planned to include a standardized set of photographs and physical examination at birth, with follow-up assessments at 6 months and 12 months of age. The main objective of the formative research project was to validate a tool that could be used to document physical variations and abnormal features in field settings. A group of clinical geneticists, pediatricians, and an authority on photographic and training modules, comprised the Dysmorphology Assessment Instrument (DAI) Working Group. As a guide to identify variations and anomalies that would need documentation, the DAI Working Group selected the Elements of Morphology (http://elementsofmorphology.nih.gov/index.cgi), a standardization of terms related to human morphology developed by an international group of clinicians with expertise in dysmorphology and published in 2009. The Elements of Morphology includes definitions and illustrations of over 400 phenotypic features [33]. The DAI Working Group met bimonthly by conference call for 6 months, in addition to 2 in-person meetings, one for training in photographic assessment and the other for discussion of features not captured by photography for inclusion in the DAI physical assessment.
The assessment designed by the DAI Working Group has 2 components. The first component is the photographic protocol made up of 15 images and 3 10-second videos. The 15 images included the following views: (1) frontal face; (2) nares; (3) top of head; (4) back of head; (5) ¾ view of face from the left; (6) ¾ view of face from the right; (7) right lateral head; (8) left lateral head; (9) top of right hand; (10) top of left hand; (11) right palm; (12) left palm; (13) top of right foot; (14) top of left foot; and (15) bottom of both feet. The 3 10-second videos included a full body dorsal view, a full body ventral view, and a frontal view of the face. The second component is a proposed physical assessment list. This list was developed by selecting 25 features from the Elements of Morphology that were not captured sufficiently by the photographic protocol (Table 2). An online training module covering both the photographic protocol and physical assessment was developed and placed on a protected website accessible to study collaborators. Field staff, ranging from genetic counselors to research nurses and assistants, then completed the training and implemented the DAI in the field on nearly 250 subjects. An initial evaluation of the photographic protocol by 3 independent dysmorphologists identified over 90% concordance in the detection of various minor anomalies [34]. Further investigations are underway to determine concordance among field staff who had not previously completed previous formal training in dysmorphology.
Table 2.
Proposed National Children's Study Dysmorphology Assessment Instrument Field Data Collection Checklist
| Features of the Head and Face |
|
| Features of the Ear |
|
| Features of the Periorbital Region |
|
| Features of the Lip, Mouth, and Oral Region |
|
| Features of the Hands and Feet |
|
ORGANIZATION OF TERATOLOGY INFORMATION SPECIALISTS APPROACH
The OTIS is a nonprofit organization with member programs throughout the United States and Canada that provide counseling regarding the safety of medications and other exposures during pregnancy and lactation. In addition to these services, OTIS conducts prospective pregnancy outcome research studies (http://www.mothertobaby.org/). For the research portion, OTIS members screen and refer eligible pregnant callers to a coordinating center where all study recruitment, enrollment, data collection, and analyses take place. Women who enroll in OTIS studies are interviewed by telephone one to three times during pregnancy and at least once postpartum regarding exposures, medical and pregnancy histories, demographic information, and pregnancy outcomes. Patients complete a pregnancy diary, and medical records are obtained from the mother's and infant's healthcare providers and delivery hospital.
OTIS studies use a specific method of outcome assessment, which involves a physical examination by a study dysmorphologist of all study infants, including infants born to women exposed and unexposed to the medication or vaccine of interest [14, 35]. Within the first 6 months after birth, after administration of written informed consent, live born infants are examined by one of a group of dysmorphologists. The study dysmorphologists evaluate each infant documenting both major and minor congenital anomalies. Infant examinations are conducted using body measurements and a checklist of minor anomalies that are included in a standard physical evaluation form. Arrangements for infant examinations are handled through the coordinating center, and dysmorphologists perform these examinations blinded to whether the mother was exposed to the agent of interest. Although not routinely included, for infants with prenatal exposure to drugs predicted to affect fetal brain development, a global neurobehavioral assessment conducted at school age could be included.
To ensure consistency, each dysmorphologist participates in a periodic group training exercise in which he or she independently and blindly examines a group of 1–6-month-old infants who had a variety of prenatal exposures. Findings from this exercise are used to further standardize technique and interpretation. In addition, in an interim or final analysis of the actual study data, should a pattern of minor anomalies be identified, the infants exhibiting this pattern are reexamined by one of the other study dysmorphologists to verify the pattern of anomalies. Photographs are taken of infants as a routine part of the examination protocol and help support consensus decisions regarding these infants.
STRENGTHS AND LIMITATIONS OF THESE APPROACHES
Each of these approaches has strengths and limitations for their use as part of clinical trials. Strengths of the AED Pregnancy Registry approach include that it uses data from maternal report and from physical examinations conducted by clinicians providing care to the infant; thus it is less costly than the other methodologies that require a separate examination by a study clinician. This approach also focuses on ascertainment of major congenital anomalies, defects most likely to be of clinical significance. In addition, this approach is the least complicated because physical examinations are not conducted specifically for the study; thus, it is likely to be the easiest to implement as part of a clinical trial. However, this methodology also has several limitations. This approach depends on maternal report and medical record review, neither of which is fully reliable. Mothers may not be able to recall or adequately describe major congenital defects seen in their infants [23], and anomalies might not be adequately documented in medical records, particularly in clinical trials conducted in developing countries where standards of care are significantly different from those in developed countries. In addition, because no physical examination by a study clinician is included in this approach, minor anomalies are not documented; thus, patterns of minor anomalies cannot be assessed. Another limitation is that defects are ascertained through 12 weeks of age; thus, defects diagnosed after 12 weeks of age are not captured. Finally, a neurobehavioral assessment, which is needed to identify neurocognitive and behavioral manifestations that might be associated with prenatal exposure to the agent under study is not included in this approach.
Strengths of the NCS formative research approach include that a standardized photographic assessment is conducted and that health professionals are trained to examine infants for major and minor defects. Therefore, this approach allows training of professionals in many different sites, rather than depending on a small cadre of dysmophologists, as is the case with the OTIS methodology. With this methodology, more examiners are likely to be available, which makes the approach less logistically challenging with regard to travel to different sites. However, use of more examiners means that more persons require training in examination methods and that additional efforts to ensure that examinations by different examiners are consistent are needed. The methodology includes use of a physical examination checklist of 25 minor anomalies, allowing examiners to focus on a limited number of features. However, the inter-examiner reliability among nondysmorphologists has yet to be established; before this methodology can be instituted as part of a clinical trial, testing of agreement among these examiners is needed. Another limitation is that the focus on minor anomalies, which are much more common than major anomalies, could mean that an increased risk for a major malformation could be diluted. Although this approach is less expensive than the OTIS approach, it is more costly than the AED Pregnancy Registry approach. Finally, ascertainment is at birth, 6, and 12 months; thus, defects that might be more apparent at older ages might be missed. Finally, a neurobehavioral assessment is not included.
The OTIS approach has several strengths. A comprehensive physical examination is performed by trained dysmorphologists who assess both major and minor congenital anomalies. This careful examination by experts in dysmorphology is likely to increase the possibility that patterns of anomalies will be identified. A high level of agreement among the examiners used in the OTIS approach has been documented. In addition, the physical examination is complemented by inclusion of photographs. Medical record review is also included, which might allow identification of anomalies that could be missed on physical examination alone (eg, congenital heart defects). The OTIS approach also has several limitations. These include the costly and time-consuming nature of an individual physical examination performed by a dysmorphologist on every study infant. Another issue to be considered is that the number of trained dysmorphologists in the United States is limited. Because these dysmorphologists examine infants that live throughout the United States, scheduling these examinations can be logistically challenging, and studying all infants born to mothers enrolled in a clinical trial, especially one conducted internationally, might be particularly challenging. As with the NCS formative research approach, the focus on minor anomalies could mean that an increase in a major defect could be missed. Additionally, a single assessment is conducted; thus, anomalies that are more apparent at another age might be missed. Finally, the examination does not typically include a neurobehavioral assessment, although one could be added if deemed useful based on the exposure under study.
DISCUSSION
Our workgroup recognized the strengths and limitations of the 3 different approaches and recognized that no single approach would be appropriate for all clinical trials that enroll pregnant women. Workgroup participants noted that none of these approaches have yet been used in the context of a clinical trial, but rather have been developed with the needs of their underlying study in mind. Thus, consideration needs to be given to the clinical trial context, its setting (eg, conducted in the US vs an international setting), and agent under study before any of these approaches can be integrated into the design of a clinical trial. One strategy discussed by the workgroup was to consider using different approaches for different clinical trials, rather than having a “one-size-fits-all” approach for clinical trials of all medications and vaccines. The workgroup discussed some features that might make one methodology preferable over another, depending on the medication or vaccine under study. For example, if studies using animal models suggest that the agent under study might be teratogenic or if a teratogenic effect is biologically plausible, based on what is known about the agent's mechanism of action, a more comprehensive physical examination by a trained dysmorphologist (the OTIS approach) might be preferable. In contrast, a less detailed approach might be sufficient if the agent to be studied belongs to a class of medications for which ample safety data are available, if the medication is unlikely to be used by women of reproductive potential, or if the medication or vaccine is expected to be used for treatment of a condition that occurs later in pregnancy (after the first trimester during which organogenesis is complete). Another issue that might be considered is related to the balance between the potential risks and benefits of the medication or vaccine, with less scrutiny for medications used to treat illness with a high degree of mortality and few therapeutic options. Duration of therapy was also suggested as a possible factor to be considered; however, evidence from other teratogenic exposures suggests that even a short exposure to a teratogenic medication can have negative effects [36, 37].
Another issue discussed among our workgroup was whether these methodologies could be combined. For example, the OTIS approach could be used in the early portion of a clinical trial (eg, the first 100 of a 500 women enrolled); if no signals suggestive of teratogenicity were identified, a less rigorous methodology could be used for the remainder of the study. Another possible combination would be to use the NCS formative research approach but to ensure that an expert dysmorphologist is available for consultation and review of medical records and photographs. With the development of facial analysis software using 2-dimensional photographs [38], future approaches in which dysmorphologists manually review photographs will likely change substantially, potentially improving both cost and efficiency. The workgroup recognized that implementation of any of these three approaches would require training of study personnel.
The workgroup acknowledged that clinical trials will not have the ability to identify all potential safety concerns with regard to congenital anomalies. The number of persons enrolled in clinical trials is typically determined to ensure the trial's ability to assess clinical benefit; thus, clinical trials are underpowered to detect rare adverse events [39], such as congenital defects. A system of post-marketing surveillance to assess safety of vaccines and antimicrobial medications will continue to be needed, even after clinical trials with careful assessment of congenital anomalies are performed [40].
In draft guidance dated February 2014, the Food and Drug Administration (FDA) stated that clinical trial data submitted for FDA review must be submitted electronically in a format that the FDA can process, review, and archive [41]. Issues related to this draft FDA guidance need to be considered as decisions regarding integration of congenital anomalies assessment into clinical trial study designs are made. Standardization of reporting of clinical data allows comparisons of data across different clinical trials. However, standardization requires careful consideration of information that is typically presented in a narrative format, which cannot easily be compared across clinical trials, clinical studies, registries or post-licensure adverse drug reaction databases. It may be worthwhile to consider a clinical summary or clinical narrative database that exists outside of the clinical safety and efficacy databases. Such a database could include photographs that are often critical to the accurate assessment of congenital anomalies, whereas the clinical safety database will contain all relevant data points describing the anomaly for analytic purposes.
In summary, our workgroup recognized that a systematic method of data collection on congenital anomalies needs to be included as part of the design for clinical trials that enroll pregnant women. The 3 methodologies reviewed here all have strengths and limitations. Similar to other decisions made in the design of clinical trials, issues regarding the optimal method of data collection on congenital anomalies will vary depending on the agent under study. To optimize systematic data collection, study teams ideally will include members with expertise in the recognition of congenital anomalies, specifically clinical geneticists or dysmorphologists or staff trained by these specialists. In addition, educational materials on definitions and assessment of congenital anomalies need to be developed so that other study team members are familiar with the standardized assessment of congenital anomalies. Furthermore, development and inclusion of a neurobehavioral assessment into the data collection should be considered for those study agents that might be expected to affect neurocognitive development. The need for research on approaches to the assessment of congenital anomalies was acknowledged; new methodologies, such as the one developed as a formative research study in the NCS, hold promise, but research to understand their reliability is critical before these methodologies can be adopted by investigators as part of clinical trial designs.
Notes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Children's Study or the National Institutes of Health, or the US Department of Health and Human Services.
Acknowledgments. The authors would like to acknowledge the work of the National Children's Study Dysmorphology Assessment Instrument Working Group Members: Lucy Hayes, Brennan Loyola, David A. Stevenson and Elaine Taggart from the University of Utah, Feizal Waffarn from the University of California Irvine, Christina Chambers and Kenneth Lyons Jones from the University of California San Diego, H. Eugene Hoyme from the University of South Dakota, and Susan Astley from the University of Washington. Additionally, the work performed as part of the NCS formative research study was performed by the following NCS Study Centers: (1) University of Utah Study Center, NICHD contract HHSN267200700015C; (2) University of Mississippi Hinds County Study Center, NICHD contract HHSN267200700030C; (3) University of California Irvine, NICHD contract HHSN267200700021C; (4) South Dakota State University Study Center, NICHD contract HHSN275200603416C; and (5) University of Washington, NICHD contract HHSN275200800015C. Westat provided data management support through NICHD contract HHSN275201200005I. Dysmorphology Assessment Instrument formative research was conducted as part of the National Children's Study, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and funded, through its appropriation, by the Office of the Director of the National Institutes of Health, supported in part by NICHD Contracts HSN275201100009C, HHSN267200700030C, HHSN275201100007C, HHSN275201100004C, and HHSN275200800015C.
Financial support. C. R. P.'s work on the article was supported by NIAID contract (HHS contract HHSN272200800013C).
Supplement sponsorship. This article appears as part of the supplement “Including Pregnant Women in Clinical Trials of Antimicrobials and Vaccines,” sponsored by the Bill & Melinda Gates Foundation.
Potential conflicts of interest. J. C. C. received Federal funding as one of PIs on the National Children's study. S. E. F. and S. H. D. received funding from DMID to attend the meeting. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lagoy CT, Joshi N, Cragan JD, Rasmussen SA. Medication use during pregnancy and lactation: an urgent call for public health action. J Womens Health (Larchmt) 2005;14:104–9. doi: 10.1089/jwh.2005.14.104. [DOI] [PubMed] [Google Scholar]
- 2.Shields KE, Lyerly AD. Exclusion of pregnant women from industry-sponsored clinical trials. Obstet Gynecol. 2013;122:1077–81. doi: 10.1097/AOG.0b013e3182a9ca67. [DOI] [PubMed] [Google Scholar]
- 3.Blehar MC, Spong C, Grady C, Goldkind SF, Sahin L, Clayton JA. Enrolling pregnant women: issues in clinical research. Womens Health Issues. 2013;23:e39–45. doi: 10.1016/j.whi.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz FM, Sheffield JS, Beigi RH, et al. Research on vaccines during pregnancy: Protocol design and assessment of safety. Vaccine. 2013;31:4274–9. doi: 10.1016/j.vaccine.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 5.Sheffield JS, Munoz FM, Beigi RH, et al. Research on vaccines during pregnancy: reference values for vital signs and laboratory assessments. Vaccine. 2013;31:4264–73. doi: 10.1016/j.vaccine.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 7.Biesecker LG, Aase JM, Clericuzio C, Gurrieri F, Temple IK, Toriello H. Elements of morphology: standard terminology for the hands and feet. Am J Med Genet A. 2009;149A:93–127. doi: 10.1002/ajmg.a.32596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall BD, Graham JM, Jr, Cassidy SB, Opitz JM. Elements of morphology: standard terminology for the periorbital region. Am J Med Genet A. 2009;149A:29–39. doi: 10.1002/ajmg.a.32597. [DOI] [PubMed] [Google Scholar]
- 9.Leppig KA, Werler MM, Cann CI, Cook CA, Holmes LB. Predictive value of minor anomalies. I. Association with major malformations. J Pediatr. 1987;110:531–7. doi: 10.1016/s0022-3476(87)80543-7. [DOI] [PubMed] [Google Scholar]
- 10.Frias JL, Carey JC. Mild errors of morphogenesis. Adv Pediatr. 1996;43:27–75. [PubMed] [Google Scholar]
- 11.Preus M, Fraser FC. Dermatoglyphics and syndromes. Am J Dis Child. 1972;124:933–43. doi: 10.1001/archpedi.1972.02110180135022. [DOI] [PubMed] [Google Scholar]
- 12.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 13.Hanson JW, Myrianthopoulos NC, Harvey MA, Smith DW. Risks to the offspring of women treated with hydantoin anticonvulsants, with emphasis on the fetal hydantoin syndrome. J Pediatr. 1976;89:662–8. doi: 10.1016/s0022-3476(76)80414-3. [DOI] [PubMed] [Google Scholar]
- 14.Jones KL, Carey JC. The importance of dysmorphology in the identification of new human teratogens. Am J Med Genet C Semin Med Genet. 2011;157C:188–94. doi: 10.1002/ajmg.c.30311. [DOI] [PubMed] [Google Scholar]
- 15.Marden PM, Smith DW, McDonald MJ. Congenital anomalies in the newborn infant, including minor variations: a study of 4,412 babies by surface examination for anomalies and buccal smear for sex chromatin. J Pediatr. 1964;64:357–71. doi: 10.1016/s0022-3476(64)80188-8. [DOI] [PubMed] [Google Scholar]
- 16.Mehes K, Mestyan J, Knoch V, Vinceller M. Minor malformations in the neonate. Helv Paediatr Acta. 1973;28:477–83. [PubMed] [Google Scholar]
- 17.Merlob P, Papier CM, Klingberg MA, Reisner SH. Incidence of congenital malformations in the newborn, particularly minor abnormalities. Prog Clin Biol Res. 1985;163C:51–5. [PubMed] [Google Scholar]
- 18.Holmes LB, Kleiner BC, Leppig KA, Cann CI, Munoz A, Polk BF. Predictive value of minor anomalies: II. Use in cohort studies to identify teratogens. Teratology. 1987;36:291–7. doi: 10.1002/tera.1420360304. [DOI] [PubMed] [Google Scholar]
- 19.Cragan JD, Gilboa SM. Including prenatal diagnoses in birth defects monitoring: experience of the Metropolitan Atlanta Congenital Defects Program. Birth Defects Res A Clin Mol Teratol. 2009;85:20–9. doi: 10.1002/bdra.20508. [DOI] [PubMed] [Google Scholar]
- 20.Bosi G, Garani G, Scorrano M, Calzolari E, Party IW. Temporal variability in birth prevalence of congenital heart defects as recorded by a general birth defects registry. J Pediatr. 2003;142:690–8. doi: 10.1067/mpd.2003.243. [DOI] [PubMed] [Google Scholar]
- 21.Minette MS, Sahn DJ. Ventricular septal defects. Circulation. 2006;114:2190–7. doi: 10.1161/CIRCULATIONAHA.106.618124. [DOI] [PubMed] [Google Scholar]
- 22.Boulet SL, Shin M, Kirby RS, Goodman D, Correa A. Sensitivity of birth certificate reports of birth defects in Atlanta, 1995–2005: effects of maternal, infant, and hospital characteristics. Public Health Rep. 2011;126:186–94. doi: 10.1177/003335491112600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen SA, Mulinare J, Khoury MJ, Maloney EK. Evaluation of birth defect histories obtained through maternal interviews. Am J Hum Genet. 1990;46:478–85. [PMC free article] [PubMed] [Google Scholar]
- 24.Holmes LB, Baldwin EJ, Smith CR, et al. Increased frequency of isolated cleft palate in infants exposed to lamotrigine during pregnancy. Neurology. 2008;70:2152–8. doi: 10.1212/01.wnl.0000304343.45104.d6. [DOI] [PubMed] [Google Scholar]
- 25.Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol. 2004;61:673–8. doi: 10.1001/archneur.61.5.673. [DOI] [PubMed] [Google Scholar]
- 26.Wyszynski DF, Nambisan M, Surve T, et al. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology. 2005;64:961–5. doi: 10.1212/01.WNL.0000154516.43630.C5. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy DL, Uhl K, Kweder SL. Pregnancy exposure registries. Drug Saf. 2004;27:215–28. doi: 10.2165/00002018-200427040-00001. [DOI] [PubMed] [Google Scholar]
- 28.Holmes LB. Need for inclusion and exclusion criteria for the structural abnormalities recorded in children born from exposed pregnancies. Teratology. 1999;59:1–2. doi: 10.1002/(SICI)1096-9926(199901)59:1<1::AID-TERA1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 29.Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. N Engl J Med. 1989;320:19–23. doi: 10.1056/NEJM198901053200104. [DOI] [PubMed] [Google Scholar]
- 30.Holmes LB, Westgate MN. Inclusion and exclusion criteria for malformations in newborn infants exposed to potential teratogens. Birth Defects Res A Clin Mol Teratol. 2011;91:807–12. doi: 10.1002/bdra.20842. [DOI] [PubMed] [Google Scholar]
- 31.Peller AJ, Westgate MN, Holmes LB. Trends in congenital malformations, 1974–1999: effect of prenatal diagnosis and elective termination. Obstet Gynecol. 2004;104:957–64. doi: 10.1097/01.AOG.0000142718.53380.8f. [DOI] [PubMed] [Google Scholar]
- 32.Guttmacher AE, Hirschfeld S, Collins FS. The National Children's Study—a proposed plan. N Engl J Med. 2013;369:1873–5. doi: 10.1056/NEJMp1311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allanson JE, Biesecker LG, Carey JC, Hennekam RC. Elements of morphology: introduction. Am J Med Genet A. 2009;149A:2–5. doi: 10.1002/ajmg.a.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Rahman OA, Hayes L, Loyola B, et al. Development of a Visit Assessment Tool to Address Birth Defects and Dysmorphology. Presented at the Annual Meeting of the David W. Smith Workshop on Malformations and Morphogenesis; August 9, 2012; Lake Lanier Islands, GA. [Google Scholar]
- 35.Chambers CD, Braddock SR, Briggs GG, et al. Postmarketing surveillance for human teratogenicity: a model approach. Teratology. 2001;64:252–61. doi: 10.1002/tera.1071. [DOI] [PubMed] [Google Scholar]
- 36.Miller MT, Stromland K. Teratogen update: thalidomide: a review, with a focus on ocular findings and new potential uses. Teratology. 1999;60:306–21. doi: 10.1002/(SICI)1096-9926(199911)60:5<306::AID-TERA11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 37.Hyoun SC, Obican SG, Scialli AR. Teratogen update: methotrexate. Birth Defects Res A Clin Mol Teratol. 2012;94:187–207. doi: 10.1002/bdra.23003. [DOI] [PubMed] [Google Scholar]
- 38.Douglas TS, Mutsvangwa TE. A review of facial image analysis for delineation of the facial phenotype associated with fetal alcohol syndrome. Am J Med Genet A. 2010;152A:528–36. doi: 10.1002/ajmg.a.33276. [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Loke YK. Drug safety assessment in clinical trials: methodological challenges and opportunities. Trials. 2012;13:138. doi: 10.1186/1745-6215-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman JM. ABCDXXX: The obscenity of postmarketing surveillance for teratogenic effects. Birth Defects Res A Clin Mol Teratol. 2012;94:670–6. doi: 10.1002/bdra.23043. [DOI] [PubMed] [Google Scholar]
- 41.Food and Drug Administration/Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research. Guidance for Industry: Providing Regulatory Submissions in Electronic Format—Submissions Under Section 745A(a) of the Federal Food, Drug, and Cosmetic Act. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM384686.pdf. Accessed 1 June 2014.


