Summary
Light is a crucial input for circadian clocks. In Drosophila, short light exposure can robustly shift the phase of circadian behavior. The model for this resetting posits that circadian photoreception is cell-autonomous: CRYPTOCHROME senses light, binds to TIMELESS (TIM) and promotes its degradation, mediated by JETLAG (JET). However, it was recently proposed that interactions between circadian neurons are also required for phase resetting. We identify two groups of neurons critical for circadian photoreception: the Morning (M)- and the Evening (E)-oscillators. These neurons work synergistically to reset rhythmic behavior. JET promotes acute TIM degradation cell-autonomously in M- and E-oscillators, but also non-autonomously in E-oscillators when expressed in M-oscillators. Thus, upon light exposure, the M-oscillators communicate with the E-oscillators. Since the M-oscillators drive circadian behavior, they must also receive inputs from the E-oscillators. Hence, although photic TIM degradation is largely cell-autonomous, neural cooperation between M- and E-oscillators is critical for circadian behavioral photoresponses.
Introduction
In Drosophila, the self-sustained pacemaker that generates molecular and behavioral circadian rhythms is a negative transcriptional feedback loop: PERIOD (PER) and TIMELESS (TIM) repress CLOCK (CLK) and CYCLE (CYC), which are activators of per and tim transcription (Zhang and Emery, 2012). This mechanism is present in ca. 150 brain neurons (Nitabach and Taghert, 2008). In a standard 12hr light: 12hr dark (LD) cycle, Drosophila exhibits two peaks of activity. The morning (M) peak is driven by the Pigment Dispersing Factor (PDF) positive small ventrolateral neurons (s-LNvs), also referred to as the M-oscillators (Grima et al., 2004; Stoleru et al., 2004). The evening (E) peak is driven by six dorsolateral neurons (LNds), two PDF negative s-LNvs called “5th s-LNvs”, and perhaps a few Dorsal Neurons (DN1s) (Cusumano et al., 2009; Grima et al., 2004; Picot et al., 2007; Stoleru et al., 2004). These cells are known as the E-oscillators. The M-oscillators also function as pacemaker neurons: they maintain behavioral rhythms under constant darkness (DD) and control their pace and phase (Renn et al., 1999; Stoleru et al., 2005).
Circadian rhythms are only beneficial if they are synchronized with the day/night cycle. Light is a crucial cue to entrain the circadian clock. In Drosophila, a brief light pulse in the early night, mimicking a delayed dusk - leads to a phase delay, whereas a late night light pulse resembling an early dawn causes a phase advance (Levine et al., 1994). Light promotes rapid TIM degradation, which is critical to reset the circadian pacemaker and behavioral rhythms (Suri et al., 1998; Yang et al., 1998). Upon light exposure, the intracellular blue-light photoreceptor CRYPTOCHROME (CRY) changes its conformation, binds to TIM and triggers its proteasomal degradation by recruiting a JETLAG (JET)-containing E3 ubiquitin ligase (Busza et al., 2004; Koh et al., 2006; Ozturk et al., 2011; Peschel et al., 2009).
Loss of CRY results in severe photoreception defects: light-induced TIM degradation and behavioral phase shifts are abolished (Dolezelova et al., 2007; Lin et al., 2001; Stanewsky et al., 1998). cry mutant flies also remain rhythmic in constant light (LL), while wild-type flies are arrhythmic under these conditions (Emery et al., 2000). Two jet mutants (jetc and jetr) are also rhythmic in LL (Koh et al., 2006; Peschel et al., 2006). However, this and other circadian photoresponse phenotypes are only observed in flies carrying the long-short tim variant (ls-tim) (Rosato et al., 1997). The long TIM isoform encoded by this variant has reduced affinity for CRY, making flies much less sensitive to light compared to flies carrying the short tim allele (s-tim) (Sandrelli et al., 2007). Thus, although JET promotes TIM degradation, whether it is actually required for TIM degradation and circadian photoresponses remains to be determined.
Although strong evidence supports a cell-autonomous model for circadian photoreception, recent studies indicate that such a mechanism is not sufficient to explain photic resetting of circadian behavior. Indeed, TIM degradation in M-oscillators appears to be neither necessary nor sufficient for phase delays (Tang et al., 2010). Based on the pattern of TIM degradation at Zeitgeber Time (ZT) 15, it was proposed that the DN1s would be important for phase delays (Tang et al., 2010). Moreover, the large (l)-LNvs have been implicated in phase advances (Shang et al., 2008). Ultimately, the DN1s and the l-LNvs would have to communicate with the M-oscillators, since these cells drive circadian behavior in DD, the condition in which phase is measured after exposing flies to a light pulse. Neuronal circuits would thus be important for circadian behavioral photoresponses. Acute TIM degradation in CRY-negative LNds also indicates the existence of non-autonomous photoreceptive mechanisms in the brain (Yoshii et al., 2008).
We used a novel, severe jet mutant and jet RNA interference (RNAi) to map the neuronal circuits controlling circadian photoreception. Our results indicate that both cell-autonomous and non-autonomous photoreception take place within the circadian neural network, and that the M- and E-oscillators are crucial for sensing light and resetting circadian locomotor behavior.
Results
The jetset mutation profoundly disrupts circadian photoresponses
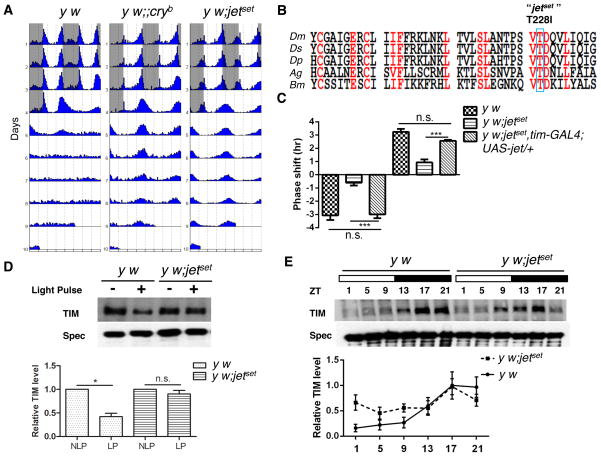
In a screen for mutants affecting Drosophila circadian behavior, we identified a strain that remains robustly rhythmic in LL (Figure 1A, Table S1). This mutant did not complement jetc and jetr (Table S1), and a point mutation causing a Threonine to Isoleucine substitution in JET’s Leucine-Rich Repeats (LRR) was identified (Figure 1B). However, while jetc and jetr show circadian light response defects only with ls-tim (Koh et al., 2006; Peschel et al., 2006), our mutant carries the highly light-sensitive s-tim allele (Sandrelli et al., 2007). It is thus a much more severe loss-of-function mutant, which was named jetset. Furthermore, jetset flies showed almost no behavioral phase shifts when challenged with 5-min light pulses applied early (ZT15) or late (ZT21) at night. Phase shift defects were fully rescued by expression of wild-type JET driven by tim-GAL4, a pan-circadian driver (Figure 1C) (Kaneko et al., 2000). The mutation in the jet gene is thus responsible for jetset’s defective photoresponses. TIM undergoes acute light-dependent degradation after short light pulses at night, and oscillates robustly under LD cycles (reviewed in Zhang and Emery, 2012). TIM did not degrade after a light pulse at ZT21 in jetset mutants (Figure 1D). However, TIM cycling under LD was not abolished, although its amplitude was reduced (Figure 1E). This is probably because JETSET retains residual activity detectable with long exposure to light. Thus, we conclude that both molecular and behavioral circadian photoresponses are affected by jetset. JET is therefore critical for CRY-dependent circadian behavioral photoresponses and for acute TIM degradation.
Figure 1. Identification and characterization of jetset.
(A) y w; jetset flies are rhythmic under LL. Representative double-plotted actograms of y w, cryb and y w; jetset flies. (white indicates the light phase and gray indicates the dark phase).
(B) Sequence alignment of the LRR region of insect JET proteins. The blue box indicates the jetset mutation.
(C) Behavioral phase shifts after short light pulses are profoundly disrupted in jetset mutants. Phase delays and advances are plotted as negative and positive values respectively. Phase shifts were almost completely abolished compared to control (y w) flies. Phase shifting defects were fully rescued by expression of UAS-jet with tim-GAL4. 16 flies were used per genotype for analysis, N=3. Error bars correspond to S.E.M. ***, p < 0.001, n.s., not significant at the 0.05 level as determined by one-way analysis of variance (ANOVA) coupled to post hoc Tukey’s test for multiple comparisons, F (5, 12) = 121.9 with p value < 0.0001.
(D) jetset is defective for acute TIM degradation in response to short light pulses. Upper panel: representative Western blot showing TIM degradation after light pulse in y w and y w; jetset. A light pulse (LP) was given at ZT21 and non-light pulsed (NLP) flies were used as controls. Lower panel: quantification of TIM levels. Upon light pulse, y w flies showed about 50% TIM degradation while jetset did not show any obvious TIM degradation. N=3. For each genotype the LP values are normalized to their NLP control values. Data are plotted as mean ± S.E.M, *, p < 0.05; n.s. – not significant as determined by comparing the LP and NLP groups for each genotype by student’s t test.
(E) TIM oscillations in jetset are dampened under LD conditions. Upper panel: representative Western blots showing TIM oscillation in whole heads at indicated ZT times under a LD cycle. The white bars represent the day and the black bars represent the night. TIM levels were normalized to the SPECTRIN levels. N=5. Lower panel: quantification of TIM levels. TIM expression levels for y w at ZT17 were set to 1 and other values were normalized to it. Data represents mean ± S.E.M.
JET expression in M- and E-oscillators controls light-dependent phase resetting
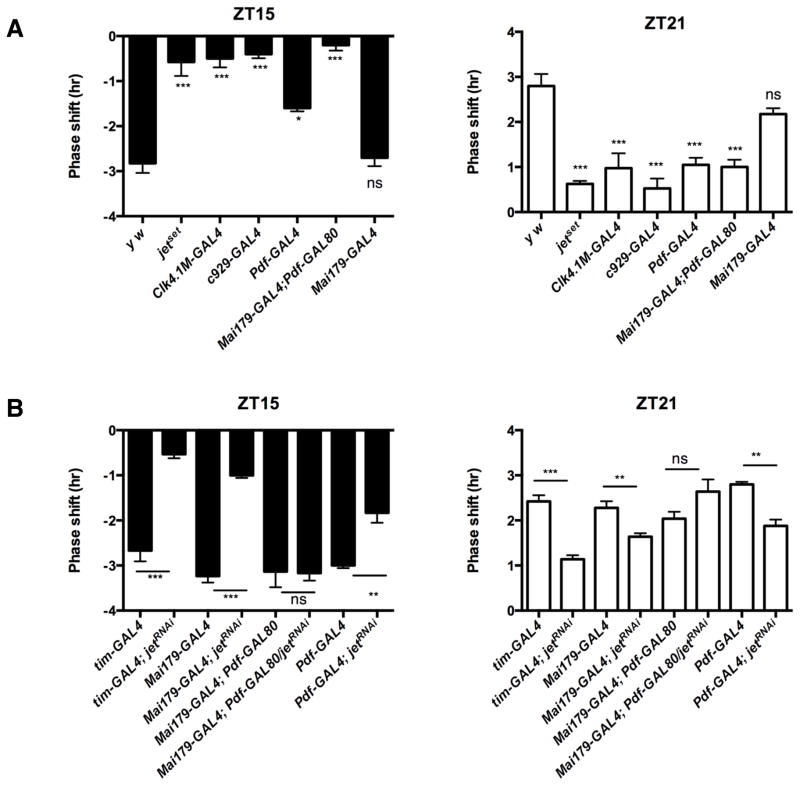
Given its severe phase response defects, we used jetset to map the neural circuit controlling circadian entrainment. GAL4 drivers active in potentially relevant circadian neurons were used to express wild-type JET in jetset flies. When we expressed JET with Clk4.1M-GAL4 (Zhang et al., 2010) only in posterior DN1s – proposed to play a role in phase delays (Tang et al., 2010) - or with c929-GAL4 (Grima et al., 2004) specifically in the l-LNvs – which are important for phase advances (Shang et al., 2008) - phase responses were not rescued, suggesting that these neurons are not sufficient to reset locomotor behavior (Figure 2A). However, JET expression in both M- and E-oscillators with Mai179-GAL4 (Grima et al., 2004) completely restored phase shifts in jetset flies. This indicates that JET expression in these two groups of neurons is critical to phase resetting. To determine the individual contribution of the M- and E-oscillators, we expressed JET only in PDF-positive LNvs (M-oscillators and l-LNvs) using Pdf-GAL4 (Renn et al., 1999). We could only slightly improve the phase delays. Phase advances were not rescued at all. We then combined Mai179-GAL4 with Pdf-GAL80 (Stoleru et al., 2004) to express JET only in the E-oscillators. Unexpectedly, this also could not rescue phase shifts (Figure 2A). Hence, JET must be rescued in both M- and E-oscillators for circadian behavior to be responsive to light pulses.
Figure 2. JET expression in the M- and E-oscillators is critical for circadian photoresponses.
(A) JET expression in the M- and E-oscillators is sufficient to rescue both phase delay and advance defects in jetset. Phase shift in response to light pulse at ZT 15 is shown on the left and the phase shift at ZT21 is shown on the right. All genotypes were compared to y w control. Note that both phase delay (ZT15) and advance (ZT21) were completely rescued only when wild-type JET is expressed in both the M- and E- oscillators using the Mai179-GAL4 driver. With Pdf-GAL4, partial rescue was observed at ZT15 (see also Figure S1B). 16 flies per genotype were used and each experiment was repeated at least four times. Error bars represent S.E.M. ***, p < 0.001; * p < 0.05; n.s., not significant at the 0.05 level as determined by ANOVA coupled to post hoc Tukey’s test, F (6, 33) = 24.77 for phase delay and F (6, 33) = 21.54 for phase advance with p value < 0.0001. See also Figure S1 for additional controls.
(B) Knocking down JET expression in the M- and E-oscillators disrupts phase shifts. Phase delays are plotted on the left and advances on the right. The controls are the different GAL4 driver lines crossed to y w. All the GAL4 drivers were combined with UAS-Dcr2 to enhance RNAi (Dietzl et al., 2007). Each genotype is compared to its GAL4 driver control. ***, p < 0.001; **, p < 0.01; n.s., not significant at the 0.05 level, tested using Student’s t-test. See Figure S2 for additional experiments.
Mai179-GAL4 is weakly expressed in four DN1s (Picot et al., 2007) (Figure S2A). To determine if these neurons are required for phase shifts, we used DvPdf-GAL4, which is expressed in the M-oscillators, l-LNvs, and a subset of Mai179-GAL4 positive E-oscillators, but not in the DN1s (Bahn et al., 2009) (Figure S2B). This driver rescues the E-peak of activity in per0 flies (F. Guo and M. Rosbash, personal communication). We could rescue the phase shifting defects of jetset with this driver (Figure S2C). Thus the DN1s are not required for JET-dependent phase shifts.
To ensure that our identification of the M- and E-oscillators as key neurons for circadian light responses was not the result of a gain-of-function from JET overexpression, we downregulated JET with RNAi (Figure 2B). Consistent with our rescue data, JET knockdown in both M- and E-oscillators severely reduced the amplitude of phase delays and advances. This was observed with Mai179-GAL4 and DvPdf-GAL4 (Figure 2B, S2C). The effects of JET downregulation were more evident at ZT15, probably because CRY levels are lower at this time point (Emery et al., 1998; Yoshii et al., 2008) and flies are thus more sensitive to JET downregulation. Since both Mai179-GAL4 and DvPdf-GAL4 are expressed in l-LNvs (Bahn et al., 2009; Grima et al., 2004) (Figure S2A–B), we also knocked down JET specifically in the l-LNvs with c929-GAL4 (Figure S2C). No effects on phase delays and advances were observed. Thus, JET expression in the l-LNvs is neither necessary nor sufficient for phase shifts. The M- and E-oscillators are therefore essential for behavioral phase shifts.
Also in agreement with our rescue experiments, knocking down JET only in PDF-positive neurons reduced the amplitude of phase shifts, although not to the same degree as knocking down JET in both groups, probably because RNAi does not reduce JET activity as efficiently as the jetset mutation. Surprisingly, when we knocked down JET only in the E-oscillators, no effect on phase responses was observed (see explanation below). Importantly however, the impact of downregulating JET in both M- and E-oscillators on phase shifts is greater than the sum of the effects of knocking down JET in the M- and E-oscillators separately. Thus, both our rescue and RNAi approaches reveal that the M- and E-oscillators collaborate to reset circadian locomotor behavior.
JET controls photic TIM degradation cell-autonomously in M- and E-oscillators, but also non-autonomously in E-oscillators
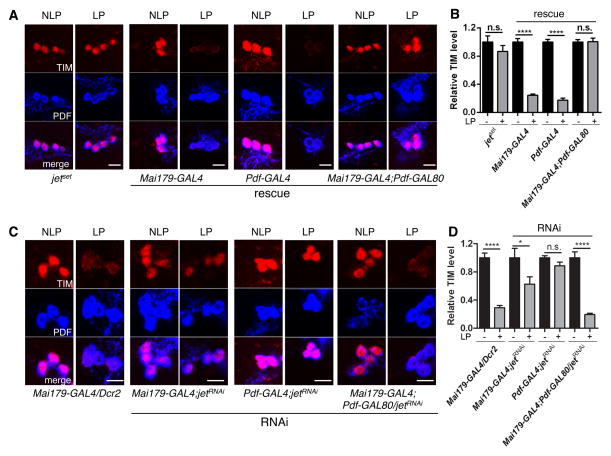
To understand our rescue and RNAi results, we measured TIM degradation after light pulses at ZT15 and 21 in the M- and E-oscillators. In jetset mutants, TIM degradation was abolished in the M-oscillators (Figure 3A–B, S3A). JET rescue in the M-oscillators with both Mai179-GAL4 and Pdf-GAL4 restored photic TIM degradation in these cells. However, expressing JET only in the E-oscillators did not. JET downregulation restricted to the M-oscillators inhibited TIM degradation in M-cells, but E-oscillator downregulation had no effect (Figure 3C–D, S3B). Knocking down JET using Mai179-GAL4 also blocked TIM degradation in the M-oscillators, but less severely than with Pdf-GAL4, probably because Mai179-GAL4 - a weaker driver than Pdf-GAL4 (data not shown) - is less effective in reducing JET activity. Taken together, these results show that JET acts cell-autonomously to trigger TIM degradation in M-oscillators.
Figure 3. Cell-autonomous role of JET in M-oscillators.
(A) Representative confocal images showing TIM degradation in M-oscillators of jetset flies rescued in M- and/or E-oscillators after a light pulse at ZT21. The brains were stained with anti-TIM antibody (red) and anti-PDF antibody (blue). LP represents light pulse, while NLP means no light pulse. From left to right, fly genotypes are 1) jetset 2) Mai179-Gal4, jetset/jetset; UAS-jet/+ 3) Pdf-Gal4, jetset/jetset; UAS-jet/+ 4) Mai179-Gal4, jetset/jetset; UAS-jet/Pdf-GAL80. Scale bars indicate 10 μm.
(B) Quantifications of TIM level y-axis shows the relative TIM level in M-oscillators, normalized to NLP controls for each genotype. Error bars correspond to S.E.M. n.s. - no significance, ****, p < 0.0001 was determined by t-test.
(C) Representative confocal images showing TIM degradation in M-oscillators when JET dsRNAs are expressed in M and/or E-oscillators. From left to right, fly genotypes are 1) Mai179-Gal4/UAS-Dcr2, 2) Mai179-Gal4/UAS-Dcr2; jetRNAi/+, 3) Pdf-Gal4/UAS-Dcr2; jetRNAi/+, 4) Mai179-Gal4/UAS-Dcr2; jetRNAi/Pdf-GAL80.
(D) Quantifications of TIM level. y-axis shows the relative TIM level in M-oscillators, normalized to NLP controls. Error bars correspond to S.E.M. n.s. - no significance, *, p < 0.05, ****, p < 0.0001 was determined by t-test. See also Figure S3 for the similar results obtained at ZT15.
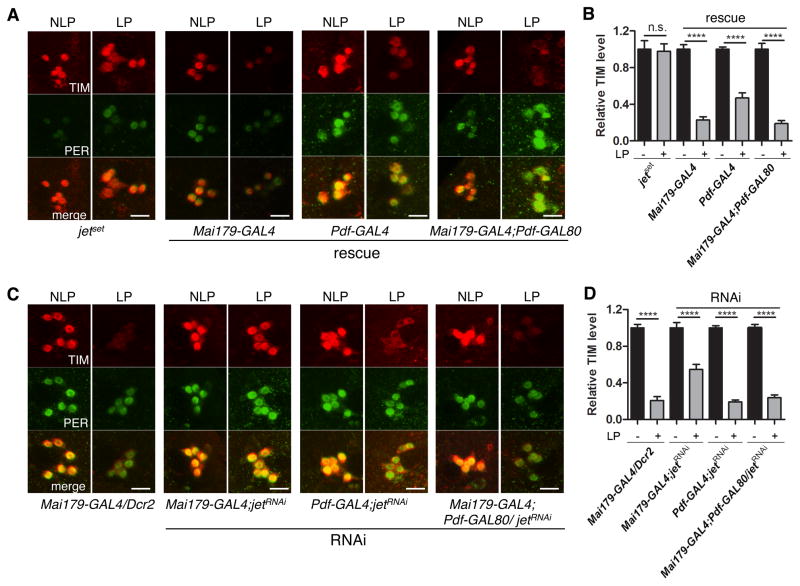
In the E-oscillators of jetset flies, TIM degradation was also eliminated, and rescued by JET expression in these cells, further supporting the cell-autonomous role of JET in TIM degradation (Figure 4A–B, S3A). Unexpectedly however, JET expression restricted to the M-oscillators rescued partially, but significantly TIM degradation in the E-oscillators. These results indicate that JET can function non-autonomously when expressed in the M-oscillators. Moreover, TIM degradation appears to be rescued in most LNds when using Mai179-GAL4, even though this driver is expressed in only three of the six LNds (Grima et al., 2004; Picot et al., 2007) (Figure 4A, S4). Indeed, the intensity of TIM signal in individual light-pulsed LNds overlapped only with that observed in 12% of LNds in non-pulsed control (Figure S4). Similar results were obtained even when Mai179-GAL4 was combined with Pdf-GAL80. This suggests that JET in the E-oscillators can non-autonomously trigger TIM degradation in the three Mai179-GAL4-negative LNds. Downregulating JET in the M- and E-oscillators with Mai179-GAL4 attenuated TIM degradation in the E oscillators (Figure 4C–D, S3B). Interestingly, TIM degradation appeared to be compromised in most LNds (Figure 4C, S4). This suggests again that the Mai179-GAL4-negative LNds, which express low or no CRY (Yoshii et al., 2008), rely predominantly on a JET-dependent non-autonomous mechanism to degrade TIM.
Figure 4. Cell-autonomous and non-autonomous role of JET in E-oscillators.
(A) Representative confocal images showing TIM degradation in LNds of jetset flies rescued in M- and/or E-oscillators, after a light pulse at ZT21. The brains were stained with anti-TIM antibody (red) and anti-PER antibody (green). From left to right, fly genotypes are 1) jetset 2) Mai179-Gal4, jetset/jetset; UAS-jet/+ 3) Pdf-Gal4, jetset/jetset; UAS-jet/+ 4) Mai179-Gal4, jetset/jetset; UAS-jet/Pdf-GAL80. Scale bars indicate 10 μm.
(B) Quantifications of TIM level. y-axis shows the relative TIM level in LNds, normalized to the NLP controls. Error bars correspond to S.E.M. ****, p < 0.0001 was determined by t-test. Note that TIM is degraded in the LNds of Pdf-Gal4, jetset/jetset; UAS-jet/+ flies, even though JET is only expressed in M-oscillators (see also Figure S3C for additional controls).
(C) Representative confocal images showing TIM degradation in LNds when JET dsRNAs are expressed in M and/or E-oscillators, after a light pulse at ZT21. From left to right, fly genotypes are 1) Mai179-Gal4/UAS-Dcr2, 2) Mai179-Gal4/UAS-Dcr2; jetRNAi/+, 3) Pdf-Gal4/UAS-Dcr2; jetRNAi/+, 4) Mai179-Gal4/UAS-Dcr2; jetRNAi/Pdf-GAL80.
(D) Quantifications of TIM level. y-axis shows the relative TIM level in LNds compared with the average level in three neighboring non-circadian neurons. TIM levels are normalized to NLP controls. Error bars correspond to S.E.M. ****, p < 0.0001 was determined by t test. Note that down-regulating JET only in E-oscillators does not affect TIM degradation, but blocking JET expression in both M and E-oscillators does. See also Figure S3 and S4.
Importantly, downregulating JET with Mai179-GAL4 did not completely block TIM degradation in the E-oscillators (Figure 4C–D, S3B), while the jetset mutation did. Thus, the E-oscillators retained residual JET activity in jet RNAi flies. This explains an apparent paradox in our behavioral results. On one hand, rescuing JET expression in M-oscillators only weakly rescues phase shifts in jetset flies. On the other hand, downregulating JET specifically in E-oscillators has no effect on phase shifts. In the latter case, residual JET activity in E-oscillators and non-autonomous JET activity from M-oscillators result in full TIM degradation in E-oscillators. Hence normal phase shifts are observed. In the former situation, non-autonomous JET activity from the M-oscillators is not sufficient to trigger full TIM degradation, because there is not enough autonomous JET activity in E-oscillators. Thus, phase shifts are poorly rescued. This illustrates the importance of both autonomous and non-autonomous JET activity, and the role played by interactions between M- and E-oscillators in circadian photoreception.
Discussion
Circadian photoreception is based on a cell-autonomous mechanism. However, recent studies indicate that resetting circadian behavior in response to light input requires neural interactions (Shang et al., 2008; Tang et al., 2010). Our results show that the M- and E-oscillators are critical for circadian photoresponses and act synergistically to shift the timing of the locomotor rhythms in response to light. Indeed JET is required in both the M- and E-oscillators, whereas individually, these neuronal groups cannot, or only weakly, phase-shift locomotor rhythms. Moreover, JET promotes both cell-autonomous and non-autonomous acute TIM degradation in circadian neurons. Thus, circadian behavior relies heavily on network interactions during its photic resetting.
The identification of the E-oscillators as critical cells for both phase delays and advances was unexpected. Indeed, the DN1s were proposed to be important for phase delays (Tang et al., 2010), and the l-LNvs were found to be needed for phase advances (Shang et al., 2008). However, our experiments indicate that JET is neither required, nor sufficient in DN1s and l-LNvs for phase shifts. The l-LNvs might thus secrete a neurotransmitter in a JET-independent manner, and this only happens when the light pulse is administered late at night.
Our finding that JET in the M-oscillators can non-autonomously trigger TIM degradation in the E-oscillators was also unanticipated. How JET does so is unclear, but it must involve rapid communication between the M- and E-oscillators, because we measured TIM degradation only one hour after the light pulse. JET might regulate acutely neuronal activity, possibly with CRY’s help. Indeed, this photoreceptor influences neuronal activity in a light-dependent manner, and is required for phase-shifts in M-oscillators (Fogle et al., 2011; Tang et al., 2010). Interestingly, the reverse is not true: JET in the E-oscillators has no effect on TIM degradation in the M-oscillators. Since the E-oscillators are essential for phase shifts and the M-oscillators drive circadian behavior (Stoleru et al., 2005), the formers have to communicate with the latters through a JET-independent mechanism. Although JET in the E-oscillators cannot promote TIM degradation in M-oscillators, our rescue experiments suggest that it can do so in the Mai179-GAL4-negative LNds. Indeed, JET expression restricted to the E-oscillators restored TIM degradation in most LNds (Figure S4). In addition, JET expression in M-oscillators promoted TIM degradation in most LNds as well. The non-E-oscillator LNds are CRY negative, which suggests that they rely on a non-autonomous mechanism for TIM degradation (Yoshii et al., 2008). Our results indicate that JET’s non-autonomous function in TIM degradation might be critical to spread light information broadly in the circadian neural network.
Strong evidence supports the idea that acute TIM degradation is required for circadian behavioral photoresponses (Suri et al., 1998; Yang et al., 1998). However, a recent study has challenged the notion that TIM degradation in M-oscillators is critical for phase shifts, or at least for phase delays (Tang et al., 2010). Our results suggest that TIM degradation is critical in E-oscillators, whether it is achieved cell-autonomously or not, since partial block of TIM degradation in E-oscillators is associated with compromised phase advances and delays (Figure 2, 4, Table S2). In the M-oscillators, the requirement for TIM degradation remains uncertain. On one hand, JET is required in these neurons and promotes TIM degradation cell-autonomously. On the other hand, this JET-dependent TIM degradation could be unnecessary for behavioral phase-shifts: JET in M-oscillators could contribute to phase shifts entirely non-autonomously. We note that TIM degradation is severely blocked in M-oscillators when JET is downregulated, but phase delays are only partially disrupted (Table S2). This would fit with the idea that TIM degradation in M-oscillators is not required for phase shifts, although we cannot rule out that TIM degradation occurred with a slower kinetics. In any case, we propose that after light pulses, TIM degradation in E-oscillators resets their molecular pacemaker, which allows them to help the M-oscillators to resynchronize their own circadian pacemaker. The M-oscillators then readjust the whole circadian neural network. This bears similarities with light synchronization in mammals. The Suprachiasmatic Nucleus (SCN) - the mammalian neural circadian pacemaker - receives light input through dedicated retinal ganglion cells in the retina (Hattar et al., 2006). Cells in the core of the SCN appear to be particularly sensitive to this light input. They communicate with robust pacemaker neurons of the shell, which then reset the whole circadian neural network (Yan et al., 2007).
Materials and methods
Protein extraction and Western blots
Flies were entrained to a standard LD cycle and frozen on the 4th day at the indicated time points. For acute photic TIM degradation, flies were exposed to a 10-min light pulse (1500 lux) at ZT21 and returned to darkness for 1 hr. Protein extraction and Western blots were performed as described in Busza et al. (2004).
Behavioral monitoring and analysis
Behavior under LL was monitored and analyzed as previously described (Emery et al., 2000). To measure photic phase shifts, flies were entrained to a LD cycle for 5 days and exposed to a 5-minute light pulse (1500 lux) at ZT15 and 21. They were then monitored in DD for six days. The phase of their behavior was compared to non-pulsed controls. We used the off-set of subjective evening activity, as it is the most reliable phase marker across genotypes. It is defined as the time at which the activity of a group of flies (averaged from day 2–6 post light pulse) drops to 50% of peak value.
Whole Mount Immunocytochemistry
Whole-mount immunohistochemistry for fly brains was done as previously described (Zhang et al., 2010). All samples were viewed on a Zeiss LSM5 Pascal confocal microscope.
Supplementary Material
Table S1: Circadian locomotor behavior under constant light and constant darkness, Related to Figure 1.
Table S2: TIM degradation in M- and E-oscillators and behavioral phase shifts after light pulses, Related to Figure 2, 3 and 4.
Figure S1: Behavioral phase shifts in control flies, Related to Figure 2.
A. Left panel shows behavioral phase shifts in response to a light pulse at ZT15 and the right panel shows the response to a light pulse at ZT21. y-axis indicates the amplitude of the phase shift in hours and the x-axis indicates the genotypes. Neither the GAL4 driver lines without UAS-jet nor the UAS-jet transgene without a GAL4 driver could correct the phase shifting defects of jetset flies. As a positive control jetset/+; UAS-jet flies were also included. As expected, these flies can phase delay and advance their behavior since they are heterozygous for the recessive jetset mutation.
B. Behavioral phase-shifts of cryb flies with CRY expression limited to the M-oscillators. In a previous study, CRY expression limited to the M-oscillators was found to fully rescue phase shifts in cryb mutants at ZT21, and partially at ZT15 (Emery et al., 2000). While we obtained similar results at ZT15 when rescuing jetset mutants, we observed no rescue at ZT21 (figure 2A). Thus, we measured phase shifts in cryb flies in which CRY expression is rescued with Pdf-GAL4 exactly the same way as we did for jetset rescues. Left and the right panels correspond to phase shifts observed after light pulses at ZT15 and ZT21, respectively. Phase delays and phase advances are partially rescued when CRY expression was driven by Pdf-GAL4. This is in line with our JET rescue data (figure 2A): the M-oscillators alone are not sufficient. We note that CRY rescue is stronger than JET rescue, probably because CRY overexpression increases sensitivity to light (Emery et al., 1998; Emery et al., 2000; Klarsfeld et al., 2004; Tang et al., 2010). Also, although the rescue at ZT15 appears clearly partial, the difference with wild-type closely missed statistical significance, because of higher than usual variability with the y w control. We are confident that our interpretation that this is a partial rescue is correct, since very similar results were obtained in a previous report (Emery et al., 2000). Moreover, we also observe partial rescue with JET (figure 2A). **, P < 0.01, *, P < 0.05, n.s., not significant at the 0.05 level as determined by one-way analysis of variance (ANOVA) coupled to post hoc Tukey’s test for multiple comparisons, F (2, 6) = 15.06, with P value = 0.0046 for phase delays. The phase advance was also analyzed similarly by ANOVA, F (2, 6) = 21.30 with P value = 0.0019.
Figure S2: DN1s and l-LNvs are not required for phase shifts, R elated to Figure 2.
A. Expression pattern of the Mai179-GAL4 enhancer trap line. The brains of flies expressing GFP under the control ofMai179 -GAL4were dissected and stained for anti-GFP (green), anti-PDF (blue) and anti-PER (red). Upper panel shows the whole brain, and bottom panel shows a very weak expression of GFP when driven by Mai179-GAL4 in DN1as (left) and two DN1ps (right). This pattern of expression is very similar to that described previously (Cusumano et al., 2009).
B. Expression pattern of the DvPdf-GAL4 enhancer trap line. Upper panel shows the dorsal region, where there is no GFP expression in the DN1s. Middle panel shows the expression in the LNds. DvPdf-GAL4is expressed in four LNds. Bottom panel shows that one of these DvPdf-GAL4 positive LNds (green, pointed by an arrow) expresses CRY (blue). The CRY-positive and Mai179-GAL4positive LNds are the same neurons (Yoshii et al., 2008). Thus, Mai179-GAL4and DvPdf-GAL4expression overlap in one LNd in addition to the 5th sLNv (Bahn et al., 2009). All images are Z-stacks. Scale bars indicate 10 μm.
C. The DN1s and l-LNvs are not required for behavioral phase shifts. Upper panel shows that rescue of JET expression using DvPdf-GAL4 restores the phase shifting defects of jetset mutants at both ZT15 and 21, indicating that JET expression is not required in the DN1s for circadian behavioral photoresponses. 16 flies per genotype were used for all the behavioral analysis and each experiment was repeated three times. Error bars represent S.E.M. **, P < 0.01, n.s., not significant at the 0.05 level as determined by one-way analysis of variance (ANOVA) coupled to post hoc Tukey’s test for multiple comparisons, F (2, 6) = 15.31 and P = 0.0044 for phase delay, and F (2, 6) = 10.59 and P = 0.0108 for phase advance. Lower panel shows jet downregulation using DvPdf-GAL4 and c929-GAL4. The jetRNAi flies were compared to their GAL4control. Downregulating JET expression in the l-LNvs using c929-GAL4has no effect on phase shifts, indicating that these cells are not required for JET dependent photoresponses. Error bars represent S.E.M. **, P < 0.01; *, P < 0.05 tested using student’s t-test. n.s., not significant at the 0.05 level tested using student’s t-test.
Figure S3: TIM degradation in the M- and E- oscillators after a ZT15 light pulse, Related to Figure 3 and 4.
A. Quantification of TIM levels in the M-oscillators (left) and E-oscillators (right) in neuron-specific rescued jetset. y-axis shows relative TIM levels normalized to no light pulse controls for each genotype. Error bars correspond to S.E.M. n.s. - no significance, ****, P < 0.0001; ***, P < 0.001 as determined by t-test. Abbreviations of the genotypes are the same as in Fig 3A.
B. TIM levels in M-oscillators (left) and E-oscillators (on right) when jet is knocked down using RNAi. Relative TIM levels normalized to no light pulsed control are plotted on the y-axis. Statistics are the same as in Fig S3A. Abbreviations of the genotypes are the same as in Fig 3C.
C. TIM levels in the LNds of jetset flies carrying UAS-jet but no GAL4 driver. UAS-jet alone does not rescue the TIM degradation jetset phenotype. Thus there is no leaky expression of JET in LNds. LP was given at ZT21.
Figure S4: Distribution of TIM signals in individual LNds with or without light pulses at ZT21, Related to Figure 4.
Left: Each spot represents the relative TIM signal in an individual LNd. Note that most LNds appear to behave similarly within a genotype (and within a brain), which shows that TIM degradation in Mai179-GAL4 negative LNds is triggered by non-autonomous signals. Error bars correspond to S.E.M. The fly genotypes are 1) Mai179-Gal4, jetset/jetset; UAS-jet/+, 2) Mai179-Gal4, jetset/jetset; UAS-jet/Pdf-GAL80, 3) Mai179-Gal4/UAS-Dcr2; jetRNAi/+, 4) Mai179-Gal4/UAS-Dcr2; jetRNAi/Pdf-GAL80. LP is abbreviated for light pulse. Number of neurons quantified are indicated.
Right: Percentage overlap of TIM staining intensity between light-pulsed and non-pulsed LNds. If Mai179-positive and –negative LNds behaved as separate groups, overlap should be 50%, since 3 of the 6 LNds are Mai179-positive. Indeed, only the rescued cells should show TIM degradation, or only the LNds that do not express jet dsRNAs. This is clearly not the case. In each case the percentage was clearly less or more than 50% suggesting that the most LNds behaved as a single population. Chi-square test with Yate’s correction confirms our interpretation that the LNds do not behave as two equally divided populations: p< 0.0001 for all four genotypes.
Highlights.
JET is essential for circadian photoresponses.
JET can function both cell-autonomously and non-autonomously in circadian neurons.
The M- and E-oscillators are critical for circadian photoresponses.
The M- and E-oscillators collaborate to reset circadian behavior with light inputs.
Acknowledgments
We thank M. Freeman for providing EMS-mutagenized fly lines, D. Szydlik, J. Ling, and C. Yuan for technical support, F. Guo and M. Rosbash for communicating results before publication, the TRiP stock center for jet RNAi flies, R. Stanewsky, C. Helfrich-Foerster and the Hybridoma Bank for PER, CRY and PDF antibodies. This work was supported by NIH grant GM066777, to P.E.
Footnotes
Author contributions
P.E and Y.Z. supervised the project and designed the experiments. P.L, Y.Z., and D.W. performed the experiments and analysis. Y.Z., P.L., and P.E, wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahn JH, Lee G, Park JH. Comparative analysis of Pdf-mediated circadian behaviors between Drosophila melanogaster and D. virilis. Genetics. 2009;181:965–975. doi: 10.1534/genetics.108.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- Cusumano P, Klarsfeld A, Chelot E, Picot M, Richier B, Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci. 2009;12:1431–1437. doi: 10.1038/nn.2429. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dolezelova E, Dolezel D, Hall JC. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Hall JC, Rosbash M. A unique circadian-rhythm photoreceptor. Nature. 2000;404:456–457. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- Fogle KJ, Parson KG, Dahm NA, Holmes TC. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331:1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Park JH, Cheng Y, Hardin PE, Hall JC. Disruption of synaptic transmission or clock-gene-product oscillations in circadian pacemaker cells of Drosophila cause abnormal behavioral rhythms. J Neurobiol. 2000;43:207–233. doi: 10.1002/(sici)1097-4695(20000605)43:3<207::aid-neu1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Koh K, Zheng X, Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Casey CI, Kalderon DD, Jackson FR. Altered circadian pacemaker functions and cyclic AMP rhythms in the drosophila learning mutant dunce. Neuron. 1994;13:967–974. doi: 10.1016/0896-6273(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Lin FJ, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Photic signaling by cryptochrome in the drosophila circadian system. Mol Cell Biol. 2001;21:7287–7294. doi: 10.1128/MCB.21.21.7287-7294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Ozturk N, Selby CP, Annayev Y, Zhong D, Sancar A. Reaction mechanism of Drosophila cryptochrome. Proc Natl Acad Sci U S A. 2011;108:516–521. doi: 10.1073/pnas.1017093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N, Chen KF, Szabo G, Stanewsky R. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr Biol. 2009;19:241–247. doi: 10.1016/j.cub.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Peschel N, Veleri S, Stanewsky R. Veela defines a molecular link between Cryptochrome and Timeless in the light-input pathway to Drosophila’s circadian clock. Proc Natl Acad Sci U S A. 2006;103:17313–17318. doi: 10.1073/pnas.0606675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf Neuropeptide Gene Mutation and Ablation of PDF Neurons Each Cause Severe Abnormalities of Behavioral Circadian Rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Rosato E, Trevisan A, Sandrelli F, Zordan M, Kyriacou CP, Costa R. Conceptual translation of timeless reveals alternative initiating methionines in Drosophila. Nucleic Acids Res. 1997;25:455–458. doi: 10.1093/nar/25.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrelli F, Tauber E, Pegoraro M, Mazzotta G, Cisotto P, Landskron J, Stanewsky R, Piccin A, Rosato E, Zordan M, et al. A molecular basis for natural selection at the timeless locus in Drosophila melanogaster. Science. 2007;316:1898–1900. doi: 10.1126/science.1138426. [DOI] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M. Feature Article: Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0809577105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta M, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agusto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Suri V, Qian Z, Hall JC, Rosbash M. Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron. 1998;21:225–234. doi: 10.1016/s0896-6273(00)80529-2. [DOI] [PubMed] [Google Scholar]
- Tang CH, Hinteregger E, Shang Y, Rosbash M. Light-mediated TIM degradation within Drosophila pacemaker neurons (s-LNvs) is neither necessary nor sufficient for delay zone phase shifts. Neuron. 2010;66:378–385. doi: 10.1016/j.neuron.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Karatsoreos I, Lesauter J, Welsh DK, Kay S, Foley D, Silver R. Exploring spatiotemporal organization of SCN circuits. Cold Spring Harb Symp Quant Biol. 2007;72:527–541. doi: 10.1101/sqb.2007.72.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Emerson M, Su HS, Sehgal A. Response of the timeless protein to light correlates with behavioral entrainment and suggests a nonvisual pathway for circadian photoreception. Neuron. 1998;21:215–223. doi: 10.1016/s0896-6273(00)80528-0. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Forster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Emery P. Molecular and Neural Control of Insects Circadian Rhythms. In: Gilbert LI, editor. Insect Molecular Biology and Biochemistry. Academic Press; 2012. pp. 513–551. [Google Scholar]
- Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Circadian locomotor behavior under constant light and constant darkness, Related to Figure 1.
Table S2: TIM degradation in M- and E-oscillators and behavioral phase shifts after light pulses, Related to Figure 2, 3 and 4.
Figure S1: Behavioral phase shifts in control flies, Related to Figure 2.
A. Left panel shows behavioral phase shifts in response to a light pulse at ZT15 and the right panel shows the response to a light pulse at ZT21. y-axis indicates the amplitude of the phase shift in hours and the x-axis indicates the genotypes. Neither the GAL4 driver lines without UAS-jet nor the UAS-jet transgene without a GAL4 driver could correct the phase shifting defects of jetset flies. As a positive control jetset/+; UAS-jet flies were also included. As expected, these flies can phase delay and advance their behavior since they are heterozygous for the recessive jetset mutation.
B. Behavioral phase-shifts of cryb flies with CRY expression limited to the M-oscillators. In a previous study, CRY expression limited to the M-oscillators was found to fully rescue phase shifts in cryb mutants at ZT21, and partially at ZT15 (Emery et al., 2000). While we obtained similar results at ZT15 when rescuing jetset mutants, we observed no rescue at ZT21 (figure 2A). Thus, we measured phase shifts in cryb flies in which CRY expression is rescued with Pdf-GAL4 exactly the same way as we did for jetset rescues. Left and the right panels correspond to phase shifts observed after light pulses at ZT15 and ZT21, respectively. Phase delays and phase advances are partially rescued when CRY expression was driven by Pdf-GAL4. This is in line with our JET rescue data (figure 2A): the M-oscillators alone are not sufficient. We note that CRY rescue is stronger than JET rescue, probably because CRY overexpression increases sensitivity to light (Emery et al., 1998; Emery et al., 2000; Klarsfeld et al., 2004; Tang et al., 2010). Also, although the rescue at ZT15 appears clearly partial, the difference with wild-type closely missed statistical significance, because of higher than usual variability with the y w control. We are confident that our interpretation that this is a partial rescue is correct, since very similar results were obtained in a previous report (Emery et al., 2000). Moreover, we also observe partial rescue with JET (figure 2A). **, P < 0.01, *, P < 0.05, n.s., not significant at the 0.05 level as determined by one-way analysis of variance (ANOVA) coupled to post hoc Tukey’s test for multiple comparisons, F (2, 6) = 15.06, with P value = 0.0046 for phase delays. The phase advance was also analyzed similarly by ANOVA, F (2, 6) = 21.30 with P value = 0.0019.
Figure S2: DN1s and l-LNvs are not required for phase shifts, R elated to Figure 2.
A. Expression pattern of the Mai179-GAL4 enhancer trap line. The brains of flies expressing GFP under the control ofMai179 -GAL4were dissected and stained for anti-GFP (green), anti-PDF (blue) and anti-PER (red). Upper panel shows the whole brain, and bottom panel shows a very weak expression of GFP when driven by Mai179-GAL4 in DN1as (left) and two DN1ps (right). This pattern of expression is very similar to that described previously (Cusumano et al., 2009).
B. Expression pattern of the DvPdf-GAL4 enhancer trap line. Upper panel shows the dorsal region, where there is no GFP expression in the DN1s. Middle panel shows the expression in the LNds. DvPdf-GAL4is expressed in four LNds. Bottom panel shows that one of these DvPdf-GAL4 positive LNds (green, pointed by an arrow) expresses CRY (blue). The CRY-positive and Mai179-GAL4positive LNds are the same neurons (Yoshii et al., 2008). Thus, Mai179-GAL4and DvPdf-GAL4expression overlap in one LNd in addition to the 5th sLNv (Bahn et al., 2009). All images are Z-stacks. Scale bars indicate 10 μm.
C. The DN1s and l-LNvs are not required for behavioral phase shifts. Upper panel shows that rescue of JET expression using DvPdf-GAL4 restores the phase shifting defects of jetset mutants at both ZT15 and 21, indicating that JET expression is not required in the DN1s for circadian behavioral photoresponses. 16 flies per genotype were used for all the behavioral analysis and each experiment was repeated three times. Error bars represent S.E.M. **, P < 0.01, n.s., not significant at the 0.05 level as determined by one-way analysis of variance (ANOVA) coupled to post hoc Tukey’s test for multiple comparisons, F (2, 6) = 15.31 and P = 0.0044 for phase delay, and F (2, 6) = 10.59 and P = 0.0108 for phase advance. Lower panel shows jet downregulation using DvPdf-GAL4 and c929-GAL4. The jetRNAi flies were compared to their GAL4control. Downregulating JET expression in the l-LNvs using c929-GAL4has no effect on phase shifts, indicating that these cells are not required for JET dependent photoresponses. Error bars represent S.E.M. **, P < 0.01; *, P < 0.05 tested using student’s t-test. n.s., not significant at the 0.05 level tested using student’s t-test.
Figure S3: TIM degradation in the M- and E- oscillators after a ZT15 light pulse, Related to Figure 3 and 4.
A. Quantification of TIM levels in the M-oscillators (left) and E-oscillators (right) in neuron-specific rescued jetset. y-axis shows relative TIM levels normalized to no light pulse controls for each genotype. Error bars correspond to S.E.M. n.s. - no significance, ****, P < 0.0001; ***, P < 0.001 as determined by t-test. Abbreviations of the genotypes are the same as in Fig 3A.
B. TIM levels in M-oscillators (left) and E-oscillators (on right) when jet is knocked down using RNAi. Relative TIM levels normalized to no light pulsed control are plotted on the y-axis. Statistics are the same as in Fig S3A. Abbreviations of the genotypes are the same as in Fig 3C.
C. TIM levels in the LNds of jetset flies carrying UAS-jet but no GAL4 driver. UAS-jet alone does not rescue the TIM degradation jetset phenotype. Thus there is no leaky expression of JET in LNds. LP was given at ZT21.
Figure S4: Distribution of TIM signals in individual LNds with or without light pulses at ZT21, Related to Figure 4.
Left: Each spot represents the relative TIM signal in an individual LNd. Note that most LNds appear to behave similarly within a genotype (and within a brain), which shows that TIM degradation in Mai179-GAL4 negative LNds is triggered by non-autonomous signals. Error bars correspond to S.E.M. The fly genotypes are 1) Mai179-Gal4, jetset/jetset; UAS-jet/+, 2) Mai179-Gal4, jetset/jetset; UAS-jet/Pdf-GAL80, 3) Mai179-Gal4/UAS-Dcr2; jetRNAi/+, 4) Mai179-Gal4/UAS-Dcr2; jetRNAi/Pdf-GAL80. LP is abbreviated for light pulse. Number of neurons quantified are indicated.
Right: Percentage overlap of TIM staining intensity between light-pulsed and non-pulsed LNds. If Mai179-positive and –negative LNds behaved as separate groups, overlap should be 50%, since 3 of the 6 LNds are Mai179-positive. Indeed, only the rescued cells should show TIM degradation, or only the LNds that do not express jet dsRNAs. This is clearly not the case. In each case the percentage was clearly less or more than 50% suggesting that the most LNds behaved as a single population. Chi-square test with Yate’s correction confirms our interpretation that the LNds do not behave as two equally divided populations: p< 0.0001 for all four genotypes.