Abstract
Vaginal microbiota and sexually transmitted infections (STIs) are likely to influence the transmission of cell-associated human immunodeficiency virus (HIV). Lactic acid produced by Lactobacillus-dominated microbiota (Nugent score 0–3) will likely inhibit transmission, especially female-to-male transmission. In contrast, polymicrobial microbiota (Nugent score 4–10), community state types IV-A and IV-B, and STIs will likely increase transmission of cell-associated HIV.
Keywords: polymicrobial microbiota, cell-associated HIV, lactic acid, inflammatory cytokines
Both cell-free and cell-associated human immunodeficiency virus (HIV), when delivered vaginally, have been shown to transmit infections [1], and the vaginal environment can greatly alter the likelihood of HIV transmission both from males to females and females to males. In women, polymicrobial vaginal microbiota (bacterial vaginosis [BV] and intermediate by Nugent scores 7–10 and 4–6, respectively [2]) and sexually transmitted infections (STIs) are strongly associated with increased risk for women acquiring HIV infections [3–5]. Conversely, men whose HIV-infected partner has BV are 3 times more likely to become infected than if she has a Lactobacillus-dominated microbiota (Nugent score 0–3) [6]. Several other factors are also likely to increase transmission by cell-associated HIV, including the presence of STIs, epithelial disruptions, and any factor that increases inflammatory cytokines (see [7] and [8] for recent reviews).
During the years between puberty and menopause, the healthy vaginal epithelium is highly protective against HIV infection, as indicated by the low risk of transmission per coital episode, and also by the vagina being a markedly more effective barrier than the rectum [9]. Unlike the columnar epithelium in the rectum, the vagina has a cornified squamous epithelium that is about 28 cell layers thick throughout the menstrual cycle [10]. The stratum corneum on the luminal surface consists of several layers of dead and dying cells incapable of being infected. Moreover, many layers of stratum corneum cells are shed each day, thereby reducing the ability of pathogens to reach target cells deeper in the epithelium. Thus, disruptions in this protective stratum corneum barrier are likely to increase transmission of many types of infections [11]. In humans, and only in humans, the stratum corneum cells contain large amounts of glycogen, and this rich source of glycogen supports what are essentially monocultures of lactobacilli that strongly acidify the vagina with lactic acid. The human vagina is the only one known to supply this large amount of glycogen, and only human vaginas are strongly acidified with lactic acid. As discussed below, there is increasing evidence that acidification with lactic acid provides substantial vaginal protection against infections.
LACTOBACILLI, WHEN DOMINANT, STRONGLY ACIDIFY THE VAGINA WITH LACTIC ACID
Döderlein was the first to suggest that the human vagina is acidified by lactic acid produced by lactobacilli (originally named Döderlein's bacilli) [12]. This was plausible and was generally accepted with little further study until more recently, when it was suggested that because the vagina is hypoxic, the lactic acid might be supplied primarily by the anaerobic metabolism of vaginal epithelial cells [13]. That vaginal lactic acid is actually produced primarily by lactobacilli was confirmed by the demonstration that the ratio of d and l optical isomers of lactic acid in a vaginal sample correlates well with the ratio of d- and l-isomers produced by lactobacilli cultured from the same sample [14]. Humans can only produce the l-isomer. In most of the samples observed in the study, the concentration of the d-isomer was higher than the l-isomer, and the results indicated that <15% of vaginal lactic acid is supplied by the epithelium. Moreover, lactobacilli cultured from vaginal samples acidify the growth medium to an asymptotic pH that correlates with the pH of the vagina from which each sample was obtained, indicating that vaginal pH is determined by the specific strain(s) of resident lactobacilli [15]. That lactic acid is the dominant acid that acidifies the vagina was further confirmed by the demonstration that with Nugent scores 0–3 (Lactobacillus-dominated microbiota), vaginal pH tightly correlates inversely with lactic acid concentration in the vagina [16]. In this carefully controlled study, when lactobacilli dominated, they acidified the vagina to pH 3.5 (SD, 0.3) with 1% (SD, 0.2%) lactic acid [16]. (This strong lactic acid mediated acidity is far greater than reported in earlier studies that suffered from a variety of experimental limitations; vaginal pH is almost always measured with pH indicator dyes that do not reveal pH <4, virtually all prior studies failed to account for alkalization upon exposure to air due to loss of carbon dioxide, and many studies failed to control adequately for the composition of vaginal microbiota and did not exclude women with elevated Nugent scores.)
THE VAGINA IS LIKELY TO BE ESPECIALLY SUSCEPTIBLE TO CELL-ASSOCIATED HIV PRIOR TO PUBERTY AND AFTER MENOPAUSE
Prior to puberty, in the absence of estrogen, the vaginal epithelium is thin and without glycogen, vaginal pH is near neutral, the microbiota is sparse, there is little lubrication, and sexual intercourse is very likely to cause trauma. All these factors are likely to increase transmission by both cell-free and cell-associated HIV.
A somewhat similar condition occurs after menopause. Due in part to the reduction of estrogen, the epithelium thins, the microbiota usually becomes sparser, the pH becomes more neutral, and lubrication becomes less adequate. A recent study of pre-, peri-, and post menopausal women [17] demonstrated a marked shift in community state types (CSTs) from Lactobacillus-dominated microbiota (Lactobacillus crispatus, Lactobacillus iners) to CST IV-A, characterized by Streptococcus and Prevotella, and to CST IV-B, characterized by Atopoium. Both CST IV-A and CST IV-B are polymicrobial with greatly reduced lactobacilli [17]. This study found that vulvovaginal atrophy correlated strongly with the shifts to polymicrobial microbiota, especially the shift from CST I (L. crispatus) to CST IV-A. This is the first study to observe the shifts in microbiota that occur with menopause and makes clear the need to pursue additional longitudinal studies to confirm and extend the findings.
From puberty to menopause, a Lactobacillus-dominated microbiota requires an estrogen-dependent supply of glycogen. Interestingly, maternal estrogen acquired in utero causes the vaginal epithelium of the fetus to deposit glycogen, and for the first few days after birth a Lactobacillus-dominated microbiota is present in the newborn vagina [18].
The amount of glycogen in vaginal secretions decreases during episodes of BV [19], vaginal pH rises, and lactobacilli markedly decrease. This leads to the suggestion that episodes of BV may be caused by a decrease in glycogen produced by the epithelium. However, BV is a polymicrobial overgrowth that most likely increases consumption of glycogen by BV-associated bacteria, especially Gardnerella vaginalis and Megasphaera [20], compared with the rate of glycogen consumption by a much sparser population of bacteria when lactobacilli dominate the microbiota. Indeed, BV-associated bacteria express all the cytolysins and enzymes needed to metabolize glycogen, whereas L. crispatus expresses no cytolysins [20] and also requires the presence of human amylases to metabolize glycogen [21].
During pregnancy, the glycogen stored in epithelial cells increases and the incidence of BV decreases, but there is conflicting evidence as to whether BV early in pregnancy increases risk of preterm birth (ie, [22, 23]). Despite the presence of the thick mucus plug during pregnancy, uterine peristalsis still transports vaginal bacteria [24] and presumably could transport HIV and HIV-infected leukocytes. Uterine peristalsis occurs throughout the menstrual cycle as well as pregnancy, and becomes primarily expulsive only during menstruation and delivery [25, 26]. Thus, uterine peristalsis transports cells, sperm, and bacteria, and most likely viruses, in vaginal fluids, into the uterus and upper tract. The volume of fluid transported is probably rather small, but there is little doubt that uterine peristalsis contributes to upper tract colonization of BV bacteria and to infections, most notably chlamydial infections of the fallopian tubes.
LACTIC ACID, NOT HYDROGEN PEROXIDE, PROTECTS AGAINST HIV AND OTHER STIs
Some strains of lactobacilli, in simplified and aerobic conditions, can produce hydrogen peroxide, and it has been widely believed that hydrogen peroxide together with vaginal peroxidase protects against infections, fends off BV-associated bacteria [27], and thereby enables lactobacilli to dominate the vagina [2]. However, in a direct examination of this appealing and apparently plausible hypothesis, it was shown that lactobacilli can produce little or no hydrogen peroxide in the hypoxic environment of the vagina (<20 µM), that both vaginal secretions and seminal plasma have potent antioxidant activity sufficient to block up to 1 M hydrogen peroxide, and, finally, that in culture media, hydrogen peroxide inhibits growth of lactobacilli more potently than it inhibits growth of BV-associated bacteria [28].
In contrast to this now implausible hydrogen peroxide hypothesis, lactic acid is produced by lactobacilli in the vagina, and in vitro, even at a relatively elevated pH of 4.5, vaginal concentrations of lactic acid potently inactivate a wide range of BV-associated bacteria (viability reduced by >107-fold for all 17 species tested). Finally, unlike hydrogen peroxide, vaginal secretions and seminal plasma do not reduce the potency of lactic acid for inactivating cell-free and cell-associated HIV [29, 30].
LACTIC ACID AS PRODUCED BY LACTOBACILLI INACTIVATES BOTH LEUKOCYTES AND CELL-FREE HIV
Leukocytes cannot survive the acidity produced by healthy vaginal lactobacilli. Indeed, leukocytes, including lymphocytes, monocytes, and macrophages, are rapidly immobilized by pH <5.8 [30], and, at a given pH, 1% lactic acid speeds cell killing (unpublished observations). Leukocytes in vitro do not defend their cytoplasm against acidification when the external pH falls below 5.5 [30]. Moreover, in the human peripheral blood leukocyte reconstituted-severe combined immunodeficient mouse, an animal model that can be infected vaginally only with cell-associated HIV (and not by cell-free HIV), HIV type 1 (HIV-1)–infected human peripheral blood mononuclear cells were blocked from transmitting HIV when the vagina was buffered to pH of approximately 4 [31]. Thus, if an HIV-infected woman sheds cell-associated HIV vaginally, the cells will be rapidly inactivated by vaginal acidity if lactobacilli dominate the vaginal microbiota. This should markedly reduce female-to-male transmission by cell-associated HIV.
Cell-free HIV is also potently inactivated by the vaginal acidity produced by lactobacilli: the Tachedjian laboratory [29] has recently demonstrated that vaginal levels of lactic acid and pH potently inactivate a wide range of HIV isolates, and that the l-isomer of lactic acid is more potent than the d-isomer for many of these isolates. They have also shown that lactic acid is much more potent than hydrogen chloride, or acetic acid, for inactivating HIV. Thus, lactic acid has a much more potent action than acidity alone. The optical isomer dependence raises the possibility that at acidic pH, l-lactic acid can bind to the surfaces of proteins by simultaneously forming 3 hydrogen bonds, with the carboxyl group forming 2 hydrogen bonds to a carboxyl group on the protein surface, and the hydroxyl group forming a third hydrogen bond in a stereo-specific manner. The mechanisms by which lactic acid inactivates HIV and BV-associated bacteria are the subject of ongoing investigations; lactic acid has already been shown to disrupt the outer membrane of gram-negative bacteria [32].
When an ejaculate delivers cell-free and cell-associated HIV, the buffer capacity of the ejaculate is sufficient to transiently neutralize the vagina, and during this transient neutralization both cell-free and cell-associated HIV may transmit infections to the vagina. This will especially be the case for HIV-infected cells delivered in semen if vaginal inflammatory conditions stimulate chemotactic cytokines that cause HIV target cells to migrate into the vaginal epithelium (see [7] for a recent review of the potential role of immune modulation by semen). Unlike the random diffusional motions of free virions, HIV-infected macrophages can chemotax at speeds of approximately 10 µm/second, and lymphocytes can migrate even faster [33]. Thus, HIV-infected leukocytes can potentially migrate within minutes through the relatively thin layer of postejaculate semen and vaginal mucus that coats the vaginal epithelial surface; this layer is likely to be in the range of 100 µm thick, assuming the total volume of vaginal mucus plus semen is approximately 4 mL and the vaginal surface area is approximately 400 cm2. In contrast, the diffusion constant, d, for cell-free HIV diffusing through neutralized undiluted cervical vaginal mucus is approximately 1 µm2/second [34]. Therefore most cell-free HIV delivered in semen would take approximately >104 seconds (>2 hours) to diffuse through 100 µm. Once an HIV virion reaches the epithelium there is a small probability that it can diffuse through occasional channels between cells on the epithelial surface to reach a depth of approximately 10 µm, about 1 cell layer, where it is more likely to be picked up by a Langerhans cell [35]. In summary, cell-associated HIV in semen is likely to migrate actively to the epithelium long before lactobacilli can reacidify the vagina with lactic acid [36]. In contrast, because diffusional transport is so much slower, most cell-free HIV is likely to be inactivated by the return of vaginal acidity before the virions can diffuse all the way to the epithelium.
POLYMICROBIAL MICROBIOTA AND VAGINAL STIs CAN INCREASE TRANSMISSION BY CELL-ASSOCIATED HIV
Polymicrobial microbiota include both BV and intermediate microbiota by Nugent (morphotype) scores [2], and CST IV-A and CST IV-B (by 16S ribosomal RNA [rRNA] analysis) [37]. These polymicrobial communities abolish the strong lactic acid acidification of the vagina by lactobacilli, increasing the vaginal pH to >4.5, typically pH 5–6.5 [37], with a loss of lactic acid and increase in acetic acid, succinic acid, and a variety of diamines (eg, putrescine and spermidine) that can cause a foul, “fishy” odor. Moreover, these polymicrobial microbiotas are associated with increased inflammatory conditions that are only recently being carefully investigated. Most significant for the potential transmission of HIV by cell-associated HIV, leukocytes in the vagina can survive in the presence of most of these conditions if the vaginal pH is above approximately 5.5 [30]. BV is termed “vaginosis” because it does not cause increased discharge of leukocytes, but leukocytes are alive and present at low concentrations (approximately 5 per high-powered field) in BV secretions with elevated pH. Thus, polymicrobial microbiota almost certainly increase the likelihood of transmission by cell-associated HIV both ways, from females to males, and males to females.
In contrast to polymicrobial communities, Lactobacillus-dominated microbiotas do not inflame the epithelium [10], and it is possible that lactic acid in the vagina may have anti-inflammatory effects similar to the action of other short-change fatty acids in the gut [38]. (A recent report to the contrary used conditions that likely killed the test cells with lactic acid [39].) In contrast, polymicrobial communities, especially BV microbiota, significantly increase inflammatory cytokines that may increase susceptibility to HIV [7, 40, 41]. Similarly, many types of STIs also cause inflammatory responses. Moreover, STIs can additionally increase the incidence and prevalence of BV [42–45]. Women with genital herpes simplex virus (HSV) infections often shed virus asymptomatically [46] and likely experience a low-level inflammatory state.
Epidemiologically, several STIs are associated with increased risk of HIV, including especially HSV-2 [47], as well as genital ulcer disease (GUD) [48], syphilis [49] trichomoniasis [50], gonorrhea [51], and human papillomavirus [52]. Most of these STIs cause disruption of the epithelium and increase the presence of target cells for both cell-free HIV and cell-associated HIV. Vaginal products that cause toxic effects, such as the spermicidal detergent used in many vaginal contraceptives, nonoxynol-9, also cause inflammatory responses [53]. Thus, any type of vaginal infection or condition that causes inflammatory responses is likely to increase the probability that cell-associated HIV can transmit infections from males to females and from females to males, especially if the infection and/or polymicrobial community causes a loss of vaginal acidity.
SIGNIFICANCE OF POLYMICROBIAL MICROBIOTA FOR HIV TRANSMISSION
Most women, most of the time, do not have Lactobacillus-dominated vaginal microbiota. Even though lactobacilli are considered to be normal flora, most women most of the time do not have normal flora, but instead have polymicrobial BV or intermediate microbiotas based on Nugent (morphotype) scores, as illustrated by Figure 1 [54, 55]. In women susceptible to BV, polymicrobial microbiota fluctuate frequently between BV, intermediate, and normal [56]. Many episodes of BV last less than a few days, and women susceptible to BV often have ≥1 BV episodes per menstrual cycle [57].
Table 1.
Summary of Expected Risks of Cell-Associated HIV Transmission as a Function of Vaginal Environment
| Vaginal Condition | Estimated Risk of Male-to-Female Transmission | Estimated Risk of Female-to-Male Transmission | Key Factors for Transmission by Cell-Associated HIV |
|---|---|---|---|
| Prepuberty | Highest | High | Cell-associated HIV survives likely trauma, thin epithelium, approximately neutral pH, little lubrication, cervical ectopy |
| Polymicrobial microbiota ± STIs | High | High | Cell-associated HIV survives, elevated pH, little lactic acid, inflammatory responses including cytokines that attract leukocytes, epithelial disruptions by STIs |
| Lactobacillus- dominated No STIs |
Low | Lowest | Vaginally shed cell-associated HIV rapidly inactivated by lactic acid Cell-associated HIV in semen survives but postejaculation leukocytes rapidly immobilized by lactic acid acidity |
| Postmenopause | High | High | Cell-associated HIV survives, polymicrobial microbiota (CST IV-A, CST IV-B), elevated pH, thin epithelium, little lubrication |
Abbreviations: CST, community state type; HIV, human immunodeficiency virus; STI, sexually transmitted infection.
Figure 1.
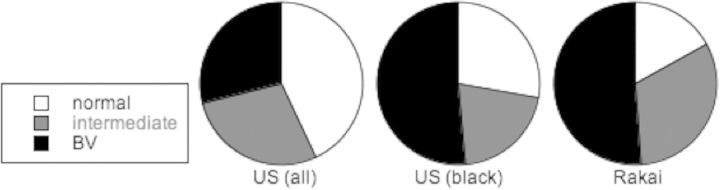
High prevalence of polymicrobial microbiota (intermediate and bacterial vaginosis [BV]; Nugent [morphotype] scores 4–6 and 7–10, respectively), and lower prevalence of normal Lactobacillus-dominated microbiota (Nugent score 0–3) [54, 55]. Abbreviations: US, United States; Rakai, Uganda.
Women with BV are at significantly increased risk of HIV [58, 59]. At present, based on a meta-analysis of numerous trials, this risk is estimated to be about 2-fold. However the magnitude of this risk has been underestimated by the design of many these epidemiologic studies as women were assigned into 2 groups on the day of entry into the trial based on whether they had or did not have BV on that day. Because vaginal microbiota often shifts frequently per menstrual cycle, and many women in both groups will have had many episodes of BV and intermediate microbiota during the trial, the observed associated risk will regress toward the mean (ie, no associated risk). Despite this shortcoming in trial designs, the published risks are nevertheless highly statistically significant. Most important, because polymicrobial vaginal microbiotas are strongly associated with HIV transmission and are highly prevalent (Figure 1), polymicrobial vaginal microbiotas are a high attributable risk factor for HIV.
Not only does BV increase the risk for women acquiring HIV, but as mentioned above, HIV-infected women with BV are 3 times more likely to transmit HIV to their male partner than HIV-infected women with Lactobacillus-dominated microbiota [6]. On the basis of this study, the authors conclude that if this association proves to be causal, “BV could be responsible for a substantial proportion of new HIV-1 infections in Africa.” As discussed above, HIV-infected women with BV are likely to shed cell-free and cell-associated HIV into a vaginal environment that often will not inactivate them, whereas Lactobacillus-dominated microbiota will rapidly inactivate both cell-free and cell-associated HIV shed vaginally prior to intercourse [29, 30].
CONCLUSIONS
Before puberty, and after menopause, risk of male-to-female transmission of cell-associated HIV is likely very high.
The lactic acid produced by a Lactobacillus-dominated microbiota is likely to reduce female-to-male transmission by both cell-free and cell-associated HIV.
In contrast, polymicrobial microbiotas (ie, BV and intermediate by Nugent morphotype scores; CST IV-A and CST IV-B by 16S rRNA analysis) will enable cell-associated HIV to survive in the vaginal lumen and likely increase risk of cell-associated HIV transmission.
Vaginal STIs as well as polymicrobial microbiota have been shown to stimulate the release of inflammatory cytokines that may attract cells infected with HIV to migrate to the epithelium.
Given the high prevalence of polymicrobial microbiota, and the resulting increased risks of STIs, it is plausible to conjecture that the great majority of HIV infections are transmitted during polymicrobial episodes, and that relatively few transmissions occur in the presence of the strong acidity mediated by lactic acid produced by Lactobacillus-dominated microbiota.
Notes
Financial support. This work was supported in part by National Institute of Allergy and Infectious Diseases (grant number 1U19AI096398).
Potential conflicts of interest. Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Barreto-de-Souza V, Arakelyan A, Margolis L, et al. HIV-1 Vaginal transmission: cell-free or cell-associated virus? Am J Reprod Immunol. 2014;71:589–99. doi: 10.1111/aji.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesson HW, Pinkerton SD. Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. J Acquir Immune Defic Syndr. 2000;24:48–56. doi: 10.1097/00126334-200005010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Wawer MJ, Sewankambo N, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:1780. doi: 10.1016/s0140-6736(05)63612-4. [DOI] [PubMed] [Google Scholar]
- 6.Cohen CR, Lingappa JR, Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doncel GF, Anderson S, Zalenskaya I. Role of semen in modulating the female genital tract microenvironment—implications for HIV transmission. Am J Reprod Immunol. 2014;71:564–74. doi: 10.1111/aji.12231. [DOI] [PubMed] [Google Scholar]
- 8.Nardis C, Mosca L, Mastromarino P. Vaginal microbiota and viral sexually transmitted diseases. Ann Ig. 2013;25:443–56. doi: 10.7416/ai.2013.1946. [DOI] [PubMed] [Google Scholar]
- 9.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patton DL, Thwin SS, Meier A, et al. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183:967–73. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 11.Averette HE, Weinstein GD, Frost P. Autoradiographic analysis of cell proliferation kinetics in human genital tissues. I. Normal cervix and vagina. Am J Obstet Gynecol. 1970;108:8–17. doi: 10.1016/0002-9378(70)90195-x. [DOI] [PubMed] [Google Scholar]
- 12.Döderlein A. Uber Scheidensekrete und Scheidenkeime [Vaginal secretions and vaginal microbes] Die Verhandlungen der deutschen Gesellschaft für Gynäkologie. 1892;4:35–50. [Google Scholar]
- 13.Pybus V, Onderdonk AB. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect. 1999;1:285–92. doi: 10.1016/s1286-4579(99)80024-0. [DOI] [PubMed] [Google Scholar]
- 14.Boskey ER, Cone RA, Whaley KJ, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 15.Boskey ER, Telsch KM, Whaley KJ, et al. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect Immun. 1999;67:5170–5. doi: 10.1128/iai.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 2013;8:e80074. doi: 10.1371/journal.pone.0080074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brotman RM, Bradford LL, Conrad M, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21:450–8. doi: 10.1097/GME.0b013e3182a4690b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song YL, Kato N, Matsumiya Y, et al. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol. 1999;37:3062–4. doi: 10.1128/jcm.37.9.3062-3064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirmonsef P, Spear GT. The barrier to HIV transmission provided by genital tract Lactobacillus colonization. Am J Reprod Immunol. 2014;71:531–6. doi: 10.1111/aji.12232. [DOI] [PubMed] [Google Scholar]
- 20.Macklaim JM, Fernandes AD, Di Bella JM, et al. Comparative meta-RNA-seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome. 2013;1:12. doi: 10.1186/2049-2618-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spear GT, French AL, Gilbert D, et al. Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis. 2014 doi: 10.1093/infdis/jiu231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petricevic L, Domig KJ, Nierscher JFJ, et al. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci Rep. 2014;4:5136. doi: 10.1038/srep05136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Hassan SS, Gajer P, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen LK, Becher N, Bastholm S, et al. The cervical mucus plug inhibits, but does not block, the passage of ascending bacteria from the vagina during pregnancy. Acta Obstet Gynecol Scand. 2014;93:102–8. doi: 10.1111/aogs.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunz G, Bell D, Huppert P, et al. Oxytocin—a stimulator of directed sperm transport in humans. Reprod Biomed Online. 2007;14:32–9. doi: 10.1016/s1472-6483(10)60761-4. [DOI] [PubMed] [Google Scholar]
- 26.Kunz G, Leyendecker G. Uterine peristaltic activity during the menstrual cycle: characterization, regulation, function and dysfunction. Reprod Biomed Online. 2002;4(suppl 3):5–9. doi: 10.1016/s1472-6483(12)60108-4. [DOI] [PubMed] [Google Scholar]
- 27.Klebanoff SJ, Hillier SL, Eschenbach DA, et al. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis. 1991;164:94–100. doi: 10.1093/infdis/164.1.94. [DOI] [PubMed] [Google Scholar]
- 28.O'Hanlon DE, Lanier BR, Moench TR, et al. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldunate M, Tyssen D, Johnson A, et al. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013;68:2015–25. doi: 10.1093/jac/dkt156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olmsted SS, Khanna KV, Ng EM, et al. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect Dis. 2005;5:79. doi: 10.1186/1471-2334-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khanna KV, Whaley KJ, Zetilin L, et al. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J Clin Invest. 2002;109:205–11. doi: 10.1172/JCI13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alakomi HL, Skytta E, Saarela M, et al. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol. 2000;66:2001–5. doi: 10.1128/aem.66.5.2001-2005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pixley FJ. Macrophage migration and its regulation by CSF-1. Int J Cell Biol. 2012;2012:501962. doi: 10.1155/2012/501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai SK, Hida K, Shukair S, et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carias AM, McCoombe S, McRaven M, et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol. 2013;87:11388–400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masters WH, Johnson VE. Human sexual response. Boston: Little Brown & Company; 1966. p. 93. [Google Scholar]
- 37.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(suppl 1)):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arpaia N, Rudensky AY. Microbial metabolites control gut inflammatory responses. Proc Natl Acad Sci U S A. 2014;111:2058–9. doi: 10.1073/pnas.1323183111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mossop H, Linhares IM, Bonglovanni AM, et al. Influence of lactic acid on endogenous and viral RNA-induced immune mediator production by vaginal epithelial cells. Obstet Gynecol. 2011;118:840–6. doi: 10.1097/AOG.0b013e31822da9e9. [DOI] [PubMed] [Google Scholar]
- 40.Sturm-Ramirez K, Gaye-Diallo A, Eisen G, et al. High levels of tumor necrosis factor-alpha and interleukin-1beta in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J Infect Dis. 2000;182:467–73. doi: 10.1086/315713. [DOI] [PubMed] [Google Scholar]
- 41.Hedges SR, Barrientes F, Desmond RA, et al. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193:556–62. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 42.Brotman RM, Bradford LL, Conrad M, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis. 2012;39:807–12. doi: 10.1097/OLQ.0b013e3182631c79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masese L, Baeten JM, Richardson BA, et al. Incident herpes simplex virus type 2 infection increases the risk of subsequent episodes of bacterial vaginosis. J Infect Dis. 2014;209:1023–7. doi: 10.1093/infdis/jit634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allsworth JE, Lewis VA, Peipert JF. Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Sex Transm Dis. 2008;35:791–6. doi: 10.1097/OLQ.0b013e3181788301. [DOI] [PubMed] [Google Scholar]
- 45.Cherpes TL, Meyn LA, Krohn MA, et al. Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex Transm Dis. 2003;30:405–10. doi: 10.1097/00007435-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Corey L, Wald A, Davis LG. Subclinical shedding of HSV: its potential for reduction by antiviral therapy. Adv Exp Med Biol. 1996;394:11–6. doi: 10.1007/978-1-4757-9209-6_2. [DOI] [PubMed] [Google Scholar]
- 47.Des Jarlais DC, Arasteh K, McKnight C, et al. HSV-2 infection as a cause of female/male and racial/ethnic disparities in HIV infection. PLoS One. 2013;8:e66874. doi: 10.1371/journal.pone.0066874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickerson MC, Johnston J, Delea TE, et al. The causal role for genital ulcer disease as a risk factor for transmission of human immunodeficiency virus. An application of the Bradford Hill criteria. Sex Transm Dis. 1996;23:429–40. doi: 10.1097/00007435-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Quinn TC, Cannon RO, Glasser D, et al. The association of syphilis with risk of human immunodeficiency virus infection in patients attending sexually transmitted disease clinics. Arch Intern Med. 1990;150:1297–302. [PubMed] [Google Scholar]
- 50.McClelland RS, Sangare L, Hassan WM, et al. Infection with trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 51.Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by Gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202:1907–15. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS. 2012;26:2211–22. doi: 10.1097/QAD.0b013e328358d908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184:418–28. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 54.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–20. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 55.Sewankambo N, Gray RH, Wawer MJ, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–50. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 56.Ravel J, Brotman RM, Gajer P, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013;1:29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van de Wijgert JH, Morrison CS, Cornelisse PG, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr. 2008;48:203–10. doi: 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 59.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]


