Abstract
All human immunodeficiency virus type 1 (HIV-1)–infected inocula, such as genital secretions, breast milk, and blood, contain both cell-free virus and infected cells. The relative contributions of cell-free and/or cell-associated virus in establishing an infection in a naive host during the different modes of HIV-1 acquisition remains unclear. Studies aim to elucidate the source of the acquired virus because strategies to prevent acquisition may have differential efficacy against the different modes of transmission. In this review, I will detail some of the challenges in identifying the source of the transmitted virus, genotypic and phenotypic differences among cell-free compared with cell-associated HIV-1, and implications on the efficacy for prevention strategies.
Keywords: HIV-1, cell-associated, transmission, neutralization, replication, envelope, receptor
Although, it has been estimated that around 75 million people have acquired human immunodeficiency virus type 1 (HIV-1) in the world, transmission is fairly inefficient [1]. Sexual contact remains the most common route for HIV-1 acquisition. It has been commonly quoted that 1 in 1000 sexual contacts results in a transmission event, but this estimate can vary dramatically depending on acquisition cofactors, such as the type of sexual contact, circumcision status, and concomitant presence of inflammation or ulceration in the genital mucosa [2]. On the other hand, transmission occurs more frequently with the other less common modes of HIV-1 acquisition, such as mother-to-child transmission and injection drug use [3]. One factor that likely influences the observed differences in transmission frequency among the various routes of acquisition is the amount of infectious virus present in the inoculum. Indeed, numerous studies have shown that levels of cell-free in the blood or genital secretions correlate with infectiousness [4–7]. It should be noted, however, that all infectious sources contain both cell-free virus and infected cells [8]. It is possible that an infected cell from the infectious inoculum enters a naive individual and that de novo virus generation from this cell leads to the new systemic infection. Indeed, a study of mother-to-infant transmission via breast milk suggested that the risk of virus acquisition is more closely correlated with cellular virus loads as opposed to the virus level in cell-free milk [9]. Furthermore, cases of HIV-1 transmission in which the transmitting partner has undetectable cell-free virus suggests that the newly infected subject may have acquired virus from infected cells, which continue to harbor infectious virus even in the presence of antiretroviral therapy [10]. Thus, it is of critical importance that we gain greater understanding about the role of cell-associated virus during mucosal HIV-1 acquisition.
In this review, I will summarize studies that have attempted to elucidate the origin of the transmitted virus in newly infected subjects. I will discuss the challenges associated with determining if the transmitted virus originates from an infected cell or the pool of cell-free virions. Even though cell-associated virus, compared with cell-free virus, has not been examined extensively, I will discuss the theoretical reasons why infected cells may be a major contributor to the infections acquired by newly infected subjects. Finally, I will discuss how transmission of virus from infected cells may negatively impact the efficacy of prevention strategies.
CHALLENGES IN IDENTIFYING THE ORIGIN OF THE TRANSMITTED VARIANTS
Although cell-associated virus potentially plays an important role during all modes of HIV-1 acquisition, it has received a limited amount of research attention, perhaps because cell-free virus is easier to both isolate from infected specimens and manipulate in vitro. Studies have attempted to decipher the origin of transmitted viruses by using sequence and phylogenetic analysis. The most informative studies have examined virus sequences from newly infected individuals early after estimated acquisition and compared them to both blood- and genital secretion–derived cell-free and cell-associated genotypes circulating in the transmitting partner [11–14]. For instance, Butler et al contend that, in 6 homosexual couples, virus sequences in the newly infected subject were consistently more closely related to genotypes of the virus in the seminal plasma as opposed to the infected cells [11]. This conclusion, however, has been challenged by other groups because of potential methodological flaws [15]. In contrast, the same group showed that a newly infected homosexual man was infected by a virus that was more closely related to genotypes present in the transmitting partner's seminal cells as opposed to seminal plasma [14]. Furthermore, other studies of heterosexual couples have failed to demonstrate that the virus circulating in the newly infected subject consistently matches either the cell-free or cell-associated strains in the transmitting partner [12, 13]. These investigations are limited by the ability to sample the appropriate infectious source very soon after transmission was estimated to have occurred; because the HIV-1 genotype diversifies at a relatively fast rate, the sequences of virus from the transmitting partner at the time of transmission may be different from those circulating during sampling. Furthermore, incomplete determination of all sequences present in a chronically infected transmitting partner may also present difficulties in precisely defining the origin of the transmitted strain. Although some but not all chronically infected subjects harbor different mixture of genotypes in cellular as compared to cell-free genital samples, these compartments do not contain completely distinct genotypes [11, 13, 14, 16–18]. These challenges will likely make it exceedingly difficult to use sequencing and phylogenetic studies to definitively determine the source of the transmitted strain.
It is quite possible that the source of the infecting virus may vary among individuals and different routes of transmission. As stated, naive individuals can acquire HIV-1 in multiple ways, such as through sexual contact; from an infected mother, either in utero, during delivery, or through breast-feeding; or by direct exposure to infected blood. The different routes of transmission present diverse challenges for the virus in establishing a new infection. For instance, the virus does not have to traverse an epithelial barrier during parenteral as opposed to mucosal transmission, and this may facilitate virus transfer from infected cells. Previous in vitro studies, however, have documented that infected cells and cell-free virus can transcytose across columnar epithelia, similar to what the virus may encounter in an infant's gastrointestinal tract [19, 20]. While a single column of cells is also present in the some portions of the genital tract, such as the anal and cervical epithelia, the penile and vaginal mucosa have stratified cell layers that likely make it difficult for cell-free and especially cell-associated virus to reach deeper lying target cells. Besides physical barrier differences, the types of infected cells present in the infected inoculum during different routes of transmission may influence whether transmission involves cell-associated or cell-free virus. For example, the quantity of different infected cell types, such as CD4+ T cells, macrophages, and dendritic cells, likely varies in genital secretions, breast milk and blood, which will impact the source of the acquired virus [8]. In addition, previous studies have shown that an exposed individual is more likely to acquire HIV-1 if they share major histocompatibility complex (MHC) class I alleles with the corresponding transmitting partner [21, 22]. HLA discordance presumably allows an exposed individual's cell-mediated immune responses to consider invading infected cells and presumably virions as foreign. This HLA mismatch transmission frequency relationship may be more relevant for cell-associated transmission than for cell-free transmission because of the greater abundance of MHC antigens in infected cells, compared with the number in virions. In aggregate, these caveats suggest that sequence and phylogenetic studies are unlikely to provide a universal answer about the contribution of cell-associated or cell-free virus to HIV-1 transmission because the source may vary both by route of acquisition and among transmission pairs.
THEORETICAL BASIS THAT SUPPORTS THE IMPORTANCE OF CELL-ASSOCIATED VIRUS
Whereas phylogenetic and sequence analysis have failed to demonstrate that either infected cells or cell-free virions are the source of the transmitted virus among newly infected subjects, similar types of studies have definitively shown that, although a source partner may have diverse viruses, a small number of variants productively infect a naive individual [23–25]. Newer investigations have used the sequences isolated from acutely infected subjects to mathematically predict the genotype of the acquired virus, referred to as the transmitted/founder strain (T/F) [26]. Because transmission frequency is low, especially with sexual contact, and only a small number of variants establish an infection in a naive host, this suggests that virus acquisition and systemic dissemination is a relatively low probability event. Modeling of this low probability event by use of a Poisson distribution suggests that newly infected subjects should rarely acquire >1 T/F strain because the likelihood of ≥2 low probability events occurring independently is exceedingly rare [27]. Contrary to this expectation, however, 20%–60% of newly infected subjects harbor >1 virus [23, 28, 29]. The number of strains acquired early after infection correlates with factors present at the time of estimated acquisition, such as genital inflammation and hormonal contraceptive use, which also enhance transmission frequency. Indeed, studies suggest that routes of acquisition that have greater predicted transmission frequency, such as injection drug use, may lead to the acquisition of a greater number of T/F strains [30, 31]. Thus, it could be argued that in certain settings the probability of >1 cell-free virus establishing an infection in a naive host is not necessarily a low probability event. On the other hand, acquisition of >1 virus may be a linked occurrence rather than multiple independents events. One possibility is that acquisition involves cell-associated virus because infected cells can harbor multiple HIV-1 variants [32]. Thus, the likelihood that a single infected cell both enters a naive host and generates de novo viruses is a relatively low probability occurrence. Once this rare event happens, however, multiple variants can establish the new infection because infected cells often harbor >1 virus.
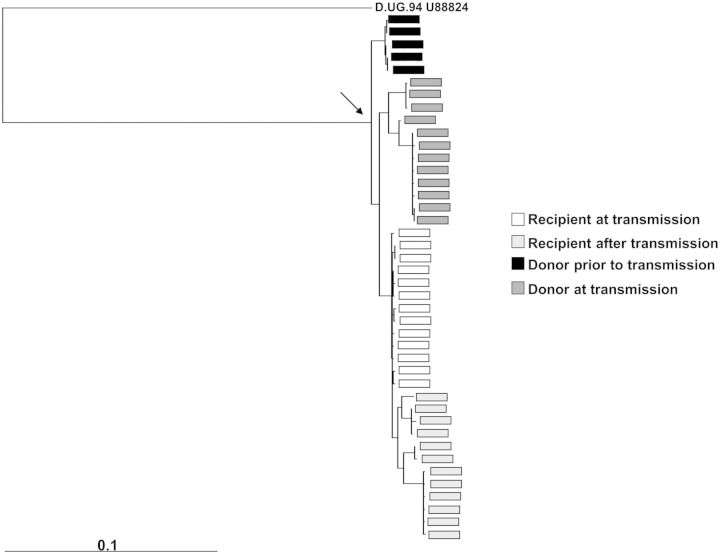
In the absence of definitive data that show that T/F strains originate either from the donor cell-free or cell-associated compartment, further genotypic and phenotypic characteristics may help determine the origin of the acquired virus. Phylogenetic analysis of full-length envelope sequences shows that newly infected subjects preferentially acquire a minority virus that is more closely related to strains circulating earlier during the transmitter's infection (termed ancestral genotypes) than to strains circulating in the transmitter near the time of estimated transmission [33, 34]. We have further shown that the envelope sequences of strains found in newly infected individuals during the acute infection period are more closely related to sequences of strains found in the transmitter prior to as opposed to at the time of transmission (Figure 1) [35]. Furthermore, acquired viruses have significantly shorter and less glycosylated envelopes with less charged V3 loops, compared with the quasispecies circulating in the transmitting partner or during chronic infection [33, 34, 36]. Because viruses increase envelope length, glycosylation, and V3 charge over the course of infection, this suggests that viruses with ancestral genotypes are favored for transmission [37–39].
Figure 1.
Transmitted sequences have ancestral genotypes. In this example, multiple envelope sequences were isolated from plasma specimens collected from a newly infected subject prior to seroconversion (white) and some duration after the acute infection period (light gray). Sequences were also obtained from the linked transmitting partner at the time of estimated transmission (dark gray) and prior to the time of transmission (black). Phylogenetic analysis was conducted as detailed previously [35]. The envelope sequences present in the newly infected subject sampled prior to seroconversion are more closely related to the envelope sequences found in the transmitting partner at a time prior to rather than at the time of estimated transmission. Longitudinal envelopes were collected approximately every 9 months. The arrow points to the most recent common ancestor. The outgroup sequence is labeled at the top of the tree. The bar at the bottom denotes a 10% genetic difference.
Phenotypic data also suggests that the transmitted strains have ancestral properties. Viruses in chronically infected subject often evolve to use the CCR5 and CXCR4 coreceptor, but the majority of newly infected subjects are infected with CCR5-using strains (termed R5 strains) [40]. Over the course of infection, these R5 strains gain the ability to enter cells even if they express the CCR5 receptor at low levels or in an atypical conformation. On the other hand, T/F strains and variants isolated soon after infection cannot enter cells that have low CCR5 levels or a different CCR5 conformation [41–43]. Newer studies further suggest that T/F strains have an enhanced ability to replicate in the presence of type 1 interferons and that viruses lose this relative interferon resistance early over the course of infection [44, 45]. Furthermore, it has been shown that, over the course of infection, evolution in the envelope gene confers greater replication capacity [46]. On the other hand, we have demonstrated in various primary cell culture systems that envelopes of viruses isolated from newly infected subjects soon after the estimated time of transmission confer significantly lower replication capacity than those of viruses circulating in chronically infected transmitting partners (Figure 2) [47]. These findings contrast with those of a recent study that showed that full-length T/F genomes had greater infectivity than unrelated strains from individuals with chronic infection [44]. These 2 studies potentially generated discordant results because examination of the envelope phenotype only, as opposed to the full-length genome, misses the contribution of other portions of the viral genome to infectivity. On the other hand, comparison of the phenotype of the transmitted virus to the phenotype of a small number of unrelated viruses from a person with chronic infection, as opposed to the phenotype of variants circulating in the transmitting partner, potentially introduces selection bias into the comparison. In aggregate, these evaluations suggest that viruses circulating during early infection, compared with the majority of contemporaneous strains circulating in a chronically infected subject, have exclusive R5 tropism, require relatively high CCR5 concentrations, have relative resistance to type 1 interferons, and have potentially decreased replication capacity. It can be argued that viruses with these ancestral properties potentially originate from infected cells because soon after acquisition, HIV can establish a latent state. These infected cells can harbor archived virus that can be activated to generate infectious virus [48]. Collectively, studies from my group and others suggest that envelope features may come full circle during a cycle of transmission and infection within a host because, at the time of transmission, genotypic and phenotypic features of the virus envelope are restored to a more ancestral state.
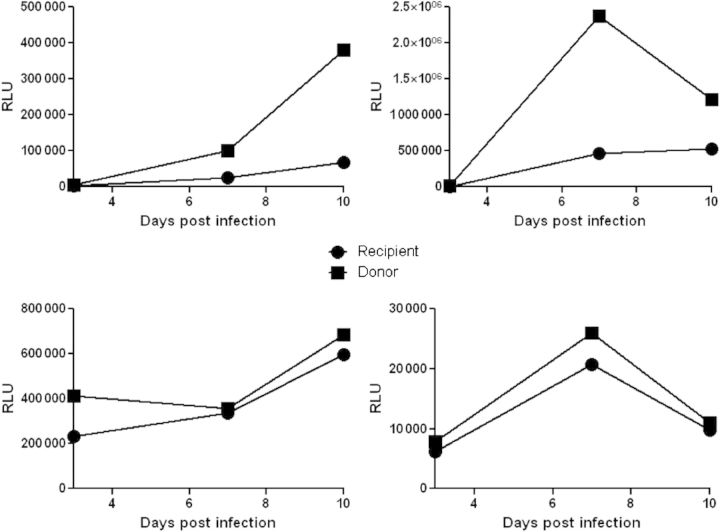
Figure 2.
Envelopes of viruses circulating in newly infected subjects confer lower replication capacity than those of viruses circulating in the transmitting partner. Shown are replication kinetics observed among recombinant viruses incorporating envelopes from a newly infected individual sampled prior to seroconversion (circles) and the linked transmitting partner (squares). Replication was examined in CD4+ T cells from 4 different human immunodeficiency virus type 1–negative volunteer blood donors (A–D). Generation of the replication-competent recombinant virus incorporating the envelopes from each partner and the infection assay was described previously [47]. The CD4+ T cells were exposed to equivalent amounts of infectious virus, estimated using titers from a luciferase reporter cell line, TZM-bl. The amount of infectious virus present in the culture supernatant was assessed as relative light units (RLUs) generated from TZM-bl cells. In CD4+ T cells from all 4 donors, virus incorporating the transmitting partner's envelopes replicates at higher levels than virus from the newly infected individual.
It remains unclear how viruses with ancestral features, such as shorter and less glycosylated envelopes with lower replication capacity, are favored for transmission from the diverse quasispecies present in the transmitting partner. It is well known that shorter envelopes with less glycosylation are generally more neutralization sensitive and that infected subjects develop neutralizing antibodies against previously circulating strains [37, 38, 49, 50]. Cell-free viruses with ancestral envelope genotypes are likely sensitive to neutralization by antibodies present in the chronically infected transmitting partner. Indeed, one study showed that envelopes present in recently infected heterosexual subjects were more sensitive than the transmitter's strains to neutralization by the transmitting partner's plasma [34]. This property, however, was not found in investigations of homosexual couples or in mother and child transmitting pairs [51, 52]. Thus, a transmitting partner's humoral immune response potentially suppresses the cell-free viruses with envelope genotype properties commonly found in variants present in newly infected subjects. On the other hand, we and others have shown that cell-associated virus is generally less susceptible to neutralization than cell-free virus [53–55]. Thus, it is possible that, because cell association confers escape from neutralization, viruses with ancestral envelope features can be acquired by a naive subject even though the transmitting partner harbors neutralizing antibodies against those specific strains. In addition, it is well known that, in vitro, HIV-1 dissemination during cell-to-cell contact occurs more efficiently than cell-free spread [56, 57]. Cell-to-cell transfer enhances HIV-1 transmission by concentrating the relevant receptors and accelerating the rate-limiting step of infection, namely entry within the host cell [58]. Thus, virus envelopes isolated from newly infected subjects, compared with those circulating in the transmitting partner, can have lower replication capacity but still be preferentially transmitted because cell-associated virus has a higher transmission capacity than cell-free HIV-1. In aggregate, cell-associated as opposed to cell-free virus both can bypass circulating donor antibodies that may neutralize the types of viruses favored for transmission and has enhanced ability to infect target cells present at the initial site of invasion.
POTENTIAL IMPORTANCE OF CELL-ASSOCIATED VIRUS FOR PREVENTION STRATEGIES
If transmission involves cell-associated virus rather than cell-free virus, this has significant implications for prevention strategies predicated on generating neutralizing antibodies to prevent HIV transmission. We and others have shown that only certain types of neutralizing antibodies with specific functionalities are equally efficient in blocking cell-associated and cell-free HIV [53–55, 59]. We suggested that steric hindrance retards the ability of some neutralizing antibodies, such as anti-envelope surface unit–directed antibodies, from inhibiting cell-associated infection even though they are highly potent against cell-free virus. On the other hand, other neutralizing antibodies, such as anti-envelope transmembrane–directed antibodies, efficiently block cell-associated virus potentially because they bind target cell membranes, which allows them to be present at the synapse between an HIV-1–laden and naive cell prior to virion transfer [53, 54]. This suggests that vaccines generating broadly neutralizing antibodies prior to HIV-1 exposure may not necessarily prevent virus acquisition from infected cells if they lack the specific functionalities required to block cell-to-cell virion transfer. Furthermore, stopping acquisition of cell-associated virus as opposed to cell-free virus may require significantly higher antibody levels, which add another challenge to developing an effective vaccine. It can be argued that previous primate models of simian immunodeficiency virus infection have demonstrated that transmission can be blocked with passive infusion of broadly neutralizing antibodies [60, 61]. It should be noted, however, that all of these studies have exclusively used cell-free viruses and that challenge has never consisted of infected cells.
SUMMARY AND CONCLUSIONS
Both in vitro studies and in vivo animal models have primarily concentrated on developing strategies that can block transmission of cell-free virus, even though all infectious sources, such as genital secretions, breast milk, and blood, contain both cell-associated and cell-free viruses. Infected cells can harbor viruses with ancestral envelope properties commonly found in the variants isolated from acutely infected subjects. In addition, virus transfer occurs more efficiently with cell-associated virus than with cell-free virus, even in the presence of neutralizing antibodies. Thus, strategies aimed at blocking HIV-1 transmission must be examined for their efficacy against both cell-free and cell-associated viruses.
Notes
Financial support. This work was supported by the National Institutes of Health (grants AI102774 [to M. S.] and AI096398 [to Deborah Anderson]).
Potential conflicts of interest. Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editor considers relevant to the content of the manuscript have been disclosed.
References
- 1.Geneva: UNAIDS: 2013. Global report: UNAIDS report on the global AIDS epidemic 2013. [Google Scholar]
- 2.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:553–63. doi: 10.1016/S1473-3099(08)70156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–8. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler DM, Smith DM, Cachay ER, et al. Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS. 2008;22:1667–71. doi: 10.1097/QAD.0b013e32830bfed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 2010;24:163–87. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–8. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturmer M, Doerr HW, Berger A, Gute P. Is transmission of HIV-1 in non-viraemic serodiscordant couples possible? Antivir Ther. 2008;13:729–32. [PubMed] [Google Scholar]
- 11.Butler DM, Delport W, Kosakovsky Pond SL, et al. The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med. 2010;2:18re1. doi: 10.1126/scitranslmed.3000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeras DI, Hraber PT, Hurlston M, et al. Role of donor genital tract HIV-1 diversity in the transmission bottleneck. Proc Natl Acad Sci U S A. 2011;108:E1156–63. doi: 10.1073/pnas.1103764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frange P, Meyer L, Jung M, et al. Sexually-transmitted/founder HIV-1 cannot be directly predicted from plasma or PBMC-derived viral quasispecies in the transmitting partner. PLoS One. 2013;8:e69144. doi: 10.1371/journal.pone.0069144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianella S, Mehta SR, Young JA, et al. Sexual transmission of predicted CXCR4-tropic HIV-1 likely originating from the source partner's seminal cells. Virology. 2012;434:2–4. doi: 10.1016/j.virol.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath L, Frenkel LM, Foley BT, Mullins JI. Comment on “The origins of sexually transmitted HIV among men who have sex with men”. Sci Transl Med. 2010;2:50le1. doi: 10.1126/scitranslmed.3001416. author reply lr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boeras D, Hawkins P, Mulenga J, Allen S, Hunter E. HIV in genital fluids during heterosexual transmission. Presented at: 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 17.Pillai SK, Good B, Pond SK, et al. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. J Virol. 2005;79:1734–42. doi: 10.1128/JVI.79.3.1734-1742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paranjpe S, Craigo J, Patterson B, et al. Subcompartmentalization of HIV-1 quasispecies between seminal cells and seminal plasma indicates their origin in distinct genital tissues. AIDS Res Hum Retroviruses. 2002;18:1271–80. doi: 10.1089/088922202320886316. [DOI] [PubMed] [Google Scholar]
- 19.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–7. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 20.Hocini H, Becquart P, Bouhlal H, et al. Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J Virol. 2001;75:5370–4. doi: 10.1128/JVI.75.11.5370-5374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald KS, Embree J, Njenga S, et al. Mother-child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 1998;177:551–6. doi: 10.1086/514243. [DOI] [PubMed] [Google Scholar]
- 22.Dorak MT, Tang J, Penman-Aguilar A, et al. Transmission of HIV-1 and HLA-B allele-sharing within serodiscordant heterosexual Zambian couples. Lancet. 2004;363:2137–9. doi: 10.1016/S0140-6736(04)16505-7. [DOI] [PubMed] [Google Scholar]
- 23.Long EM, Martin HL, Jr, Kreiss JK, et al. Gender differences in HIV-1 diversity at time of infection. Nat Med. 2000;6:71–5. doi: 10.1038/71563. [DOI] [PubMed] [Google Scholar]
- 24.Wolinsky SM, Wike CM, Korber BT, et al. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–7. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 25.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 26.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;83:3556–67. doi: 10.1128/JVI.02132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haaland RE, Hawkins PA, Salazar-Gonzalez J, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagar M, Lavreys L, Baeten JM, et al. Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population. AIDS. 2004;18:615–9. doi: 10.1097/00002030-200403050-00005. [DOI] [PubMed] [Google Scholar]
- 30.Bar KJ, Li H, Chamberland A, et al. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J Virol. 2010;84:6241–7. doi: 10.1128/JVI.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagar M, Kirkegaard E, Long EM, et al. Human immunodeficiency virus type 1 (HIV-1) diversity at time of infection is not restricted to certain risk groups or specific HIV-1 subtypes. J Virol. 2004;78:7279–83. doi: 10.1128/JVI.78.13.7279-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung A, Maier R, Vartanian JP, et al. Multiply infected spleen cells in HIV patients. Nature. 2002;418:144. doi: 10.1038/418144a. [DOI] [PubMed] [Google Scholar]
- 33.Sagar M, Laeyendecker O, Lee S, et al. Selection of HIV variants with signature genotypic characteristics during heterosexual transmission. J Infect Dis. 2009;199:580–9. doi: 10.1086/596557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derdeyn CA, Decker JM, Bibollet-Ruche F, et al. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science. 2004;303:2019–22. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 35.Redd AD, Collinson-Streng AN, Chatziandreou N, et al. Previously transmitted HIV-1 strains are preferentially selected during subsequent sexual transmissions. J Infect Dis. 2012;206:1433–42. doi: 10.1093/infdis/jis503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chohan B, Lang D, Sagar M, et al. Selection for human immunodeficiency virus type 1 envelope glycosylation variants with shorter V1-V2 loop sequences occurs during transmission of certain genetic subtypes and may impact viral RNA levels. J Virol. 2005;79:6528–31. doi: 10.1128/JVI.79.10.6528-6531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunnik EM, Euler Z, Welkers MR, et al. Adaptation of HIV-1 envelope gp120 to humoral immunity at a population level. Nat Med. 2010;16:995–7. doi: 10.1038/nm.2203. [DOI] [PubMed] [Google Scholar]
- 38.Sagar M, Wu X, Lee S, Overbaugh J. HIV-1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection and these modifications affect antibody neutralization sensitivity. J Virol. 2006;80:9586–98. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M, Salazar-Gonzalez JF, Derdeyn CA, et al. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006;80:11776–90. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Toma J, Stawiski E, et al. Characterization of human immunodeficiency virus type 1 populations containing CXCR4-using variants from recently infected individuals. AIDS Res Hum Retroviruses. 2009;25:795–802. doi: 10.1089/aid.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redd AD, Laeyendecker O, Kong X, et al. Efficiency of CCR5 coreceptor utilization by the HIV quasispecies increases over time, but is not associated with disease progression. AIDS Res Hum Retroviruses. 2011;28:289–94. doi: 10.1089/aid.2011.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker ZF, Iyer SS, Wilen CB, et al. Transmitted/founder and chronic HIV-1 envelope proteins are distinguished by differential utilization of CCR5. J Virol. 2013;87:2401–11. doi: 10.1128/JVI.02964-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatziandreou N, Arauz AB, Freitas I, et al. Sensitivity changes over the course of infection increases the likelihood of resistance against fusion but not CCR5 receptor blockers. AIDS Res Hum Retroviruses. 2012;28:1584–93. doi: 10.1089/aid.2011.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parrish NF, Gao F, Li H, et al. Phenotypic properties of transmitted founder HIV-1. Proc Natl Acad Sci U S A. 2013;110:6626–33. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fenton-May AE, Dibben O, Emmerich T, et al. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology. 2013;10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troyer RM, Collins KR, Abraha A, et al. Changes in human immunodeficiency virus type 1 fitness and genetic diversity during disease progression. J Virol. 2005;79:9006–18. doi: 10.1128/JVI.79.14.9006-9018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pena-Cruz V, Etemad B, Chatziandreou N, et al. HIV-1 envelope replication and α4β7 utilization among newly infected subjects and their corresponding heterosexual partners. Retrovirology. 2013;10:162. doi: 10.1186/1742-4690-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 49.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–9. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 51.Frost SD, Liu Y, Pond SL, et al. Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J Virol. 2005;79:6523–7. doi: 10.1128/JVI.79.10.6523-6527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Parast AB, Richardson BA, et al. Neutralization escape variants of HIV-1 are transmitted from mother to infant. J Virol. 2006;80:835–44. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sagar M, Akiyama H, Etemad B, Ramirez N, Freitas I, Gummuluru S. Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-Infection. J Infect Dis. 2012;205:1248–57. doi: 10.1093/infdis/jis183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abela IA, Berlinger L, Schanz M, et al. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malbec M, Porrot F, Rua R, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210:2813–21. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–12. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–95. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jolly C, Mitar I, Sattentau QJ. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007;81:13916–21. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan CJ, Williams JP, Schiffner T, et al. High-multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. J Virol. 2014;88:2025–34. doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hessell AJ, Poignard P, Hunter M, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]