Abstract
Human immunodeficiency virus (HIV)–infected leukocytes have been detected in genital secretions from HIV-infected men and women and may play an important role in the sexual transmission of HIV. However, they have been largely overlooked in studies on mechanisms of HIV transmission and in the design and testing of HIV vaccine and microbicide candidates. This article describes the characteristics and quantities of leukocytes in male and female genital secretions under various conditions and also reviews evidence for the involvement of HIV-infected cells in both horizontal and vertical cell-associated HIV transmission. Additional research is needed in this area to better target HIV prevention strategies.
Keywords: HIV, transmission, cell-associated, mucosal, semen, vagina, rectum, infected leukocytes, AIDS
Unprotected intercourse is the most common route through which human immunodeficiency virus type 1 (HIV) is transmitted [1, 2]. Genital secretions from men and women contain leukocytes, which can be present in high numbers during episodes of genital tract inflammation or infection. HIV-infected cells have been detected in semen and cervicovaginal secretions from HIV-infected men and women [3]. Since intracellular HIV is protected from environmental factors that can attenuate the infectiousness of free HIV virions and can be efficiently transmitted to target cells via virologic synapses, HIV-infected cells in genital secretions could play an important role in sexual and maternal-to-fetal transmission of HIV. This review focuses on the quantities, characteristics, and function of uninfected and HIV-infected leukocytes in genital tract secretions.
LEUKOCYTES IN MALE GENITAL TRACT SECRETIONS
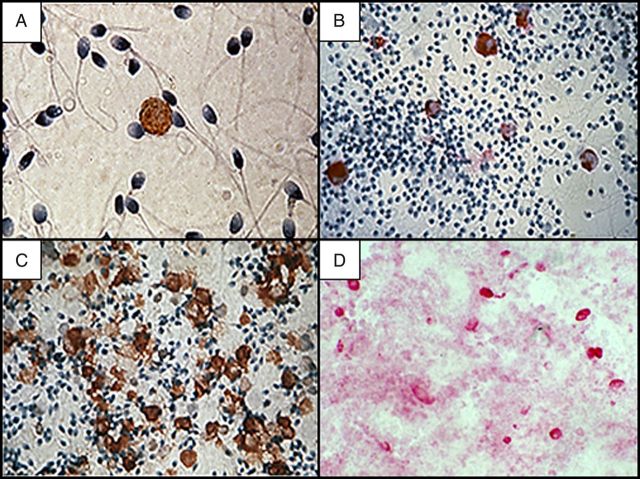
The principal cell types in human semen are spermatozoa, immature germ cells, and white blood cells (WBCs; Figure 1). WBCs have been detected in semen by various methods, including peroxidase stain (eg, the Endtz test), immunohistologic, and enzymatic (eg, granulocyte elastase) assays [4]. Recently, flow cytometry has also been used, with results comparing favorably to the more traditional methods of semen WBC assessment [5].
Figure 1.
Leukocytes in human genital secretions, detected by immunohistochemistry. A, CD4+ T cell in semen. B, CD68+ macrophages in semen. C, CD45+ leukocytes in semen from a man with leukocytospermia. D, CD68+ macrophages in cervicovaginal secretions. Original magnification ×400.
WBCs potentially enter semen from various sites along the reproductive tract, including the rete testis, epididymis, prostate, and urethra, where they are thought to play an immunosurveillance role [6]. Most of the studies of WBCs in semen that use immunohistologic analysis or flow cytometry indicate that semen from healthy non–HIV-infected men contains 105 WBCs/mL; the majority are polymorphonuclear leukocytes (PMNs), but substantial numbers of macrophages and CD4+ T cells are also present [7–9]. In addition, CD8+ T lymphocytes, B lymphocytes, and, most recently, dendritic cells have been detected in human semen [7–10].
Seminal plasma contains a rich variety of bioactive cytokines, chemokines, growth factors, prostaglandins, and other immunomodulatory mediators that can potentially affect the viability and function of seminal leukocytes and cells in the female genital tract after intercourse [11]. Seminal plasma has been reported to be cytotoxic to peripheral blood–derived mononuclear cells [12, 13] and to adversely affect macrophage function [14, 15]. However, many of the early studies used long periods of exposure to seminal plasma and tissue culture medium supplemented with fetal calf serum, which contains high concentrations of amine oxidase; this enzyme oxidizes spermine in seminal plasma creating toxic intermediaries [16]. Recent studies have found that following short so-called physiologic exposures to seminal plasma, lymphocytes retain viability and function [12, 13]. In one flow cytometry study of the viability of various leukocyte populations in semen, CD3+ cells were found to be >60% viable [7]. Markers of T-cell activation, such as interleukin 2 (IL-2) receptors and CD69, are often detected on lymphocytes in human semen from both HIV-infected and uninfected men [17, 18], indicating that seminal T cells are in an activated state. Furthermore, lymphocytes isolated from fresh semen are viable and retain their function; several studies have demonstrated cell-mediated cytotoxic and other functions of semen-derived T cells in vitro [19–21].
Genital Inflammation and Infections
Concentrations of WBCs in semen are highly variable, and genital inflammation is a common occurrence. The prevalence of leukocytospermia, an asymptomatic genital inflammatory condition characterized by >106 WBCs/mL semen [22], is 5%–10% in healthy non–HIV-infected men [23–25] and up to 24% in HIV-infected men [26]. Concentrations of PMNs correlate with other WBC types in semen [27]; thus, leukocytospermic semen contains substantially elevated concentrations of PMNs, macrophages, and CD4+ T cells [28]. The principal etiology of leukocytospermia is thought to be subclinical genital infections [29]. Leukocytospermia has been associated with asymptomatic detection of Chlamydia trachomatis [30] and Epstein-Barr virus (EBV) [31] DNA in semen, whereas other studies have failed to show a strong relationship between bacterial or viral infection in the male genital tract and leukocytospermia [32, 33]. Seminal WBC concentrations correlate positively with various cytokines [25]. Thus, it is not surprising that levels of a number of proinflammatory and other cytokines, including interleukin 1β (IL-1β) [32], IL-2 [34], interleukin 6 (IL-6) [35], interleukin 8 (IL-8) [34], and tumor necrosis factor α (TNF-α) [36], are elevated in semen of men with leukocytospermia.
The World Health Organization reports an estimated 499 million new cases of genital tract infections each year caused by the leading sexually transmitted pathogens, Neisseria gonorrhoeae, Chlamydia trachomatis, Treponema pallidum, and Trichomonas vaginalis [37]. The primary symptoms of urethritis caused by sexually transmitted infections (STIs) in men include dysuria and urethral discharge [38], which contains elevated levels of PMNs [39]. Interestingly, there is not much information available on seminal WBC subpopulations in men with symptomatic STIs, although it is likely that, as with leukocytospermia, leukocytic infiltrates associated with symptomatic bacterial infections contain elevated numbers of macrophages and lymphocytes in addition to PMNs. Viral STIs, including several members of the human herpesvirus (HHV) family (HSV types 1 and 2, EBV, and human cytomegalovirus) and human papillomaviruses, are also highly prevalent (>1 billion infections worldwide [37]) and have been associated with leukocytospermia in some studies [40].
HIV-Infected Cells in Semen
Since the initial discovery in 1983 that HIV could be cultured from seminal cells [41], a number of laboratories have demonstrated that HIV can be cultured from both seminal cells and cell-free seminal plasma. Overall, the recovery rate of infectious HIV from seminal cells has been much higher (median, 20%; range, 4%–55%) than that from seminal plasma (median, 5.9%; range, 3%–11%; P < .0001) [3]. Since most of these studies were performed before widespread use of antiviral therapy and viral load assessment, most of the subjects were chronically infected (ie, HIV seropositive) and naive to highly active antiretroviral therapy. The relatively low recovery rate of infectious HIV from seminal plasma contrasts with the high rate of HIV RNA detection by quantitative polymerase chain reaction (PCR) [42], suggesting that much of the cell-free HIV in semen is replication incompetent or inactivated. A number of factors have been identified in seminal plasma that may inactivate cell-free HIV, including anti-HIV antibodies [43], α and β chemokines [25], and antimicrobial peptides (SLPI, lactoferrin, and defensins [44]). The low culture rate could also reflect the toxicity of seminal plasma to target peripheral blood mononuclear cells (PBMCs) used for culturing HIV [13, 14].
Only a few studies have used quantitative HIV DNA PCR assays to assess the prevalence or number of HIV-infected cells in semen. In these studies, the prevalence of HIV proviral DNA in semen samples has ranged from 21% to 65%, and the HIV DNA level has ranged from not detectable to 80 000 copies/mL [3]. Interestingly, in 2 of the larger studies that assessed both HIV RNA and DNA copy numbers in semen, these 2 parameters were not correlated [42, 45]. Elevated proviral HIV DNA levels in semen have been associated with (1) reduced peripheral CD4+ T-cell counts [46], (2) acute HIV infection [47], (3) leukocytospermia and STIs [46, 48], and (4) vasectomy [45]. After initiation of HAART, levels of both HIV RNA and DNA are reduced in semen, although HIV proviral DNA–bearing cells can persist in semen for several months [42, 49] and have been shown to be infectious in vitro [50].
The question of whether spermatozoa transmit HIV infection has been controversial for several years. HIV reportedly infects or binds to testicular germ cells and spermatozoa under certain conditions, but isolated viable motile sperm from HIV-infected men are rarely HIV positive by PCR and are therefore not likely a major factor in the sexual transmission of HIV [51, 52]. Both macrophages and T cells, but not spermatozoa, isolated from semen of HIV-infected men by magnetic beads were capable of transmitting HIV to target PBMCs in vitro [53]. Macrophages usually outnumber CD4+ T cells in semen, especially in HIV-infected men, in whom seminal CD4+ lymphocytes are commonly depleted. In a study of 98 ART-naive HIV-positive men, the median ratio of macrophages to CD4+ lymphocytes in semen was 22:1 [17]. In some HIV-positive men with leukocytospermia, the seminal macrophage cell count exceeds 107 cells/mL [17]. These data indicate that macrophages are the most abundant HIV host cells in semen and a likely principal mediator of cell-associated HIV transmission. Dendritic cells, which can capture and transfer HIV, have also been detected in semen, and their numbers are elevated in semen of men with genital tract inflammation [10]. Other important HIV host cells, such as Langerhans cells, have not been detected in semen, although it is possible that some viable HIV-infected Langerhans cells from penile skin, especially the inner foreskin [54], are shed into the vagina or rectum during intercourse.
HIV-infected leukocytes have been detected in pre-ejaculatory fluid, a urethral secretion secreted from the glands of Littre and Cowper glands during sexual stimulation [55]. Evidence that cells in pre-ejaculatory fluid are infectious was provided by an epidemiologic study that found that delayed application of condoms is a risk factor for HIV transmission [56].
Elevated seminal PMN counts and leukocytospermia have been associated with increased levels of both cell-free and cell-associated HIV in semen [3], as well as with increased levels of IL-1β, TNF-α, IL-6, and other proinflammatory cytokines that could activate HIV replication in infected cells [25, 32, 57]. A recent study showed that seminal IL-6, TNF-α, and IL-8 concentrations were elevated in semen samples positive for cell-associated but not cell-free HIV (Politch et al, unpublished data). Epidemiologic studies indicate that STIs substantially enhance HIV transmission [58]. Urethritis caused by N. gonorrhoeae was associated with a 10-fold increase in HIV RNA copy numbers in semen, which declined following successful antibiotic treatment [59]. Other studies have demonstrated increased HIV RNA shedding from genital ulcers caused by various STI pathogens [60]. Most of these studies have only measured cell-free HIV RNA, but since symptomatic infections and inflammation are associated with elevated WBC levels in semen, it is probable that the number of HIV-infected cells in semen is also increased. One study showed that both HIV RNA and proviral DNA levels were elevated in semen from men with a recent STI [48].
LEUKOCYTES IN FEMALE GENITAL TRACT SECRETIONS
HIV host cells (ie, monocytes, macrophages, CD4+ T cells, and dendritic cells) have been described in vaginal and cervical tissue [61], but few studies have quantified or characterized these cell populations in human vaginal and cervical secretions. HIV host cells are detectable but usually not numerous in cervicovaginal secretions from healthy uninfected [62] and HIV-infected women [63]. Two recent studies have characterized lymphocyte subtypes in cervicovaginal lavage or cytobrush samples. A large fraction of T lymphocytes were positive for the integrin α4β7 and expressed the HIV coreceptor CCR5 and the early activation marker CD69 but not CXCR4. As with semen, cervical CD4+ T cells were severely depleted in HIV-positive subjects [64, 65].
There are limited data regarding the viability of leukocytes isolated from cervicovaginal fluid. The viability of lymphocytes in vaginal secretions from healthy reproductive aged women is often poor, most likely because of toxic effects of low pH conditions commonly found in the human vagina [66]. In prepubertal and postmenopausal women, the vaginal pH is closer to neutral, and leukocytes in cervical vaginal secretions may be more viable. Likewise, vaginal secretions at menses are neutralized by the presence of blood, and viable lymphocytes have been recovered from menstrual blood and used in functional assays [67]. Studies that have used cytobrush sampling to obtain lymphocytes for functional assays have demonstrated >85% viability [68, 69].
Cervicovaginal secretions from women with certain STIs have elevated leukocyte counts. N. gonorrhoeae and C. trachomatis infections can induce massive inflammatory infiltrates [70]. Bacterial vaginosis (BV), on the other hand, appears to have little or no effect on vaginal leukocyte counts [70, 71], but HIV-infected cells in vaginal secretions from women with BV could have preserved viability and higher infectiousness because of near-neutral pH conditions. This hypothesis is supported by the observation that BV is associated with an increased incidence of HIV transmission and acquisition [72]. Seminal fluid neutralizes the pH of vaginal secretions following intercourse [73, 74] and could thus prolong the viability of infected leukocytes in the vagina; seminal fluid also contains high concentrations of chemokines that recruit leukocytes, especially macrophages and dendritic cells, to the human cervix after coitus [75]. These HIV host cells may play an important role in sexual transmission or acquisition of HIV.
HIV-Infected Leukocytes in Female Genital Secretions
Several studies have used qualitative cell-associated HIV DNA assessment as a marker for infected leukocytes. An increased prevalence of HIV DNA in vaginal secretions has been associated with cervicitis, candidiasis and STIs [76–89], hormonal contraception [83] and vitamin A or selenium deficiency [83, 90], although this latter relationship may be more complex [91]. As mentioned above, menstrual blood introduces viable CD4+ lymphocytes into vaginal secretions; HIV proviral DNA [92, 93] has been detected in vaginal samples collected at menses, and one of the first studies to culture infectious HIV from vaginal secretions provided evidence that samples collected during menses were more infectious than those collected at other times during the menstrual cycle [94]. These data, combined with epidemiological reports of increased female-to-male HIV transmission as a result of sexual contact during menses [95, 96], suggest that menses may be a time of increased risk for female-to-male cell-associated HIV transmission. The prevalence of HIV-infected cells in vaginal secretions is reduced in women receiving antiretroviral therapy [97, 98], although as with semen, the reduction in HIV-infected cells following initiation of HAART appears to lag behind the reduction in cell-free HIV load [99]. Only a few studies have quantified HIV DNA in cells from cervicovaginal secretions [3]; the maximum number of HIV proviral copies was on the order of 104 copies per lavage sample.
It is difficult to culture infectious HIV from cervicovaginal secretions because of heavy contamination with endogenous bacteria and fungi. Most successful studies have cultured HIV from the filtered cell-free fraction and have produced HIV culture rates ranging from 11% to 22% [3]. Only one study to date has compared the HIV culture rate from cell-free versus cell-associated fractions of cervicovaginal lavage samples: HIV was cultured from 12 of 55 cell-free supernatants (22%) and 5 of 22 cell lysates (23%) [100]. Although correlates of HIV culture from cervicovaginal cells have not been studied, we hypothesize that HIV-infected leukocytes from reproductive-aged women with normal vaginal flora are inactivated by lactic acid produced by lactobacilli and are therefore less infectious [66]. We predict that HIV-infected genital leukocytes from women with neutral vaginal pH due to conditions such as BV and low estrogen states [101] are more infectious than those from reproductive-aged women with vaginal pH of 3.5–5.0 and more capable of cell-associated HIV transmission. The effect of factors present in female genital secretions on the infectiousness of either cell-associated or cell-free HIV is reviewed in this issue of the Journal.
Role of HIV-infected Genital Leukocytes in Mother-to-Child Transmission (MTCT)
Cell-associated HIV transmission has been implicated in MTCT as a result of breast-feeding. A 10-fold increase in the number of infected cells per milliliter of breast milk was associated with 3-fold increased risk of HIV transmission [102]. Similarly, cell-associated HIV transmission has been implicated in MTCT during parturition, as several studies have demonstrated a correlation between increased MTCT and the isolation of cellular HIV DNA from the mother's cervicovaginal samples [103, 104] and the presence of HIV-infected cells in the baby's oropharyngeal cavity [105]. The hypothesis of MTCT of HIV via fetal ingestion of infected maternal cells during parturition is further supported by a recent study demonstrating that HIV-infected cells can migrate across human fetal oral epithelial tissues and retain their infectiousness. In contrast, HIV-infected cells lost their infectiousness while crossing adult oral epithelium, because of the expression of anti-HIV beta-defensins 2 and 3 [106]. This latter finding is consistent with reports that oral transmission of HIV in adults is rare [107, 108]. Our laboratory quantified HIV RNA and DNA in cervicovaginal secretions from women in the WITS cohort during the third trimester of pregnancy; levels of HIV DNA, but not RNA, and proviral heterogeneity were positively associated with perinatal HIV transmission [109, 110]. A recent study, designed to determine the timing of HIV transmission, reported that 5 of 9 infants were infected at the time of delivery, whereas 4 of 9 were infected during pregnancy [111]. These results stress the need to further evaluate methods to block MTCT of cell-associated HIV via breast milk and genital secretions.
CONCLUSION
There is increasing evidence that HIV-infected WBCs in male and female genital secretions may be important vectors of both horizontal and vertical HIV mucosal transmission. These cells are attractive targets for microbicide and vaccine interventions to prevent HIV transmission, but relatively little information is available about these cells or factors that affect their abundance and infectiousness. Future research should be conducted to further characterize the phenotypes of HIV-infected cells in genital secretions and their abundance, viability, survival time, and infectiousness under various conditions. Studies should also be conducted on mechanisms of cell-associated HIV transmission. Such information could provide clues leading to the control or eradication of these infectious vectors to achieve an ultimate goal of HIV prevention.
Notes
Financial support. This work was supported by the National Institutes of Health (grant U19 AI096398 to D. J. A.).
Potential conflicts of interest. Author certifies no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–57. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. Targeting Trojan Horse leukocytes for HIV prevention. AIDS. 2010;24:163–87. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Politch JA, Anderson DJ. Semen white blood cell assay. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. 4th ed. Cambridge: Cambridge University Press; 2009. pp. 613–7. [Google Scholar]
- 5.Ricci G, Perticarari S, Boscolo R, et al. Leukocytospermia and sperm preparation--a flow cytometric study. Reprod Biol Endocrinol. 2009;7:128. doi: 10.1186/1477-7827-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson DJ, Pudney J. Mucosal immunology of the human male genital tract and experimental models. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal immunology. 3rd ed. Vol 2. New York: Elsevier Academic Press; 2005. pp. 1647–59. [Google Scholar]
- 7.Olivier AJ, Liebenberg LJ, Coetzee D, Williamson AL, Passmore JA, Burgers WA. Isolation and characterization of T cells from semen. J Immunol Methods. 2012;375:223–31. doi: 10.1016/j.jim.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson MJ, Barratt CL, Cooke ID. Prospective study of leukocytes and leukocyte subpopulations in semen suggests they are not a cause of male infertility. Fertil Steril. 1993;60:1069–75. doi: 10.1016/s0015-0282(16)56412-7. [DOI] [PubMed] [Google Scholar]
- 9.Wolff H, Anderson DJ. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil Steril. 1988;49:497–504. [PubMed] [Google Scholar]
- 10.Duan YG, Zhang Q, Liu Y, et al. Dendritic cells in semen of infertile men: association with sperm quality and inflammatory status of the epididymis. Fertil Steril. 2014;101:70–77 e3. doi: 10.1016/j.fertnstert.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Doncel GF, Anderson S, Zalenskaya I. Role of semen in modulating the female genital tract microenvironment—implications for HIV transmission. Am J Reprod Immunol. 2014;71:564–74. doi: 10.1111/aji.12231. [DOI] [PubMed] [Google Scholar]
- 12.Fiore JR, La Grasta L, Di Stefano M, Buccoliero G, Pastore G, Angarano G. The use of serum-free medium delays, but does not prevent, the cytotoxic effects of seminal plasma in lymphocyte cultures: implications for studies on HIV infection. New Microbiol. 1997;20:339–44. [PubMed] [Google Scholar]
- 13.Okamoto M, Byrn R, Eyre RC, Mullen T, Church P, Kiessling AA. Seminal plasma induces programmed cell death in cultured peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 2002;18:797–803. doi: 10.1089/08892220260139549. [DOI] [PubMed] [Google Scholar]
- 14.James K, Harvey J, Bradbury AW, Hargreave TB, Cullen RT. The effect of seminal plasma on macrophage function—a possible contributory factor in sexually transmitted disease. AIDS Res. 1983;1:45–57. doi: 10.1089/aid.1.1983.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence P, Portran D, Terrasse R, et al. Selective transmigration of monocyte-associated HIV-1 across a human cervical monolayer and its modulation by seminal plasma. AIDS. 2012;26:785–96. doi: 10.1097/QAD.0b013e328351426e. [DOI] [PubMed] [Google Scholar]
- 16.Allen RD, Roberts TK. Role of spermine in the cytotoxic effects of seminal plasma. Am J Reprod Immunol Microbiol. 1987;13:4–8. doi: 10.1111/j.1600-0897.1987.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 17.Politch JA, Mayer KH, Anderson DJ. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;50:283–9. doi: 10.1097/QAI.0b013e3181989870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshadri S, Flanagan B, Vince G, Lewis Jones DI. Leucocyte subpopulations in the seminal plasma and their effects on fertilisation rates in an IVF cycle. Andrologia. 2012;44:396–400. doi: 10.1111/j.1439-0272.2012.01293.x. [DOI] [PubMed] [Google Scholar]
- 19.Musey L, Ding Y, Cao J, et al. Ontogeny and specificities of mucosal and blood human immunodeficiency virus type 1-specific CD8(+) cytotoxic T lymphocytes. J Virol. 2003;77:291–300. doi: 10.1128/JVI.77.1.291-300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quayle AJ, Coston WM, Trocha AK, Kalams SA, Mayer KH, Anderson DJ. Detection of HIV-1-specific CTLs in the semen of HIV-infected individuals. J Immunol. 1998;161:4406–10. [PubMed] [Google Scholar]
- 21.Sheth PM, Danesh A, Shahabi K, et al. HIV-specific CD8+ lymphocytes in semen are not associated with reduced HIV shedding. J Immunol. 2005;175:4789–96. doi: 10.4049/jimmunol.175.7.4789. [DOI] [PubMed] [Google Scholar]
- 22.WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press; 2010. [Google Scholar]
- 23.Tomlinson MJ, White A, Barratt CL, Bolton AE, Cooke ID. The removal of morphologically abnormal sperm forms by phagocytes: a positive role for seminal leukocytes? Hum Reprod. 1992;7:517–22. doi: 10.1093/oxfordjournals.humrep.a137682. [DOI] [PubMed] [Google Scholar]
- 24.Yanushpolsky EH, Politch JA, Hill JA, Anderson DJ. Antibiotic therapy and leukocytospermia: a prospective, randomized, controlled study. Fertil Steril. 1995;63:142–7. [PubMed] [Google Scholar]
- 25.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–35. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- 26.Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012;26:1535–43. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Politch JA, Wolff H, Hill JA, Anderson DJ. Comparison of methods to enumerate white blood cells in semen. Fertil Steril. 1993;60:372–5. doi: 10.1016/s0015-0282(16)56116-0. [DOI] [PubMed] [Google Scholar]
- 28.Wolff H, Anderson DJ. Male genital tract inflammation associated with increased numbers of potential human immunodeficiency virus host cells in semen. Andrologia. 1988;20:404–10. [PubMed] [Google Scholar]
- 29.Anderson DJ, Politch JA. White blood cells in semen and their impact on fertility. In: Centola GM, Ginsburg KA, editors. Evaluation and treatment of the infertile male. Cambridge: Cambridge University Press; 1996. pp. 263–76. [Google Scholar]
- 30.Hosseinzadeh S, Eley A, Pacey AA. Semen quality of men with asymptomatic chlamydial infection. J Androl. 2004;25:104–9. doi: 10.1002/j.1939-4640.2004.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 31.Neofytou E, Sourvinos G, Asmarianaki M, Spandidos DA, Makrigiannakis A. Prevalence of human herpes virus types 1–7 in the semen of men attending an infertility clinic and correlation with semen parameters. Fertil Steril. 2009;91:2487–94. doi: 10.1016/j.fertnstert.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 32.Bezold G, Politch JA, Kiviat NB, Kuypers JM, Wolff H, Anderson DJ. Prevalence of sexually transmissible pathogens in semen from asymptomatic male infertility patients with and without leukocytospermia. Fertil Steril. 2007;87:1087–97. doi: 10.1016/j.fertnstert.2006.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cottell E, Harrison RF, McCaffrey M, Walsh T, Mallon E, Barry-Kinsella C. Are seminal fluid microorganisms of significance or merely contaminants? Fertil Steril. 2000;74:465–70. doi: 10.1016/s0015-0282(00)00709-3. [DOI] [PubMed] [Google Scholar]
- 34.Rajasekaran M, Hellstrom WJ, Naz RK, Sikka SC. Oxidative stress and interleukins in seminal plasma during leukocytospermia. Fertil Steril. 1995;64:166–71. [PubMed] [Google Scholar]
- 35.Shimoya K, Matsuzaki N, Ida N, et al. Detection of monocyte chemotactic and activating factor (MCAF) and interleukin (IL)-6 in human seminal plasma and effect of leukospermia on these cytokine levels. Am J Reprod Immunol. 1995;34:311–6. doi: 10.1111/j.1600-0897.1995.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 36.Omu AE, Al-Qattan F, Al-Abdul-Hadi FM, Fatinikun MT, Fernandes S. Seminal immune response in infertile men with leukocytospermia: effect on antioxidant activity. Eur J Obstet Gynecol Reprod Biol. 1999;86:195–202. doi: 10.1016/s0301-2115(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Sexually transmitted infections (STIs) Geneva: WHO; 2013. [Google Scholar]
- 38.Kasturi SS, Osterberg EC, Tannir J, Brannigan RE. The effect of genital tract infection and inflammation on male infertility. In: Lipshultz LI, Howards SS, Niederberger CS, editors. Infertility in the male. Cambridge: Cambridge University Press; 2009. pp. 295–330. [Google Scholar]
- 39.Lomas DA, Natin D, Stockley RA, Shahmanesh M. Chemotactic activity of urethral secretions in men with urethritis and the effect of treatment. J Infect Dis. 1993;167:233–6. doi: 10.1093/infdis/167.1.233. [DOI] [PubMed] [Google Scholar]
- 40.Kaspersen MD, Hollsberg P. Seminal shedding of human herpesviruses. Virol J. 2013;10:226. doi: 10.1186/1743-422X-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho DD, Schooley RT, Rota TR, et al. HTLV-III in the semen and blood of a healthy homosexual man. Science. 1984;226:451–3. doi: 10.1126/science.6208608. [DOI] [PubMed] [Google Scholar]
- 42.Mayer KH, Boswell S, Goldstein R, et al. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin Infect Dis. 1999;28:1252–9. doi: 10.1086/514775. [DOI] [PubMed] [Google Scholar]
- 43.Belec L, Georges AJ, Steenman G, Martin PM. Antibodies to human immunodeficiency virus in the semen of heterosexual men. J Infect Dis. 1989;159:324–7. doi: 10.1093/infdis/159.2.324. [DOI] [PubMed] [Google Scholar]
- 44.Anderson DJ. Genitourinary Immune Defense. In: Holmes KK, Sparling PF, Piot P, Wasserheit JN, Corey L, Cohen M, editors. Sexually transmitted diseases. 4th ed. New York: McGraw-Hill; 2007. [Google Scholar]
- 45.Krieger JN, Nirapathpongporn A, Chaiyaporn M, et al. Vasectomy and human immunodeficiency virus type 1 in semen. J Urol. 1998;159:820–5. discussion 825–6. [PubMed] [Google Scholar]
- 46.Xu C, Politch JA, Tucker L, Mayer KH, Seage GR, III, Anderson DJ. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J Infect Dis. 1997;176:941–7. doi: 10.1086/516539. [DOI] [PubMed] [Google Scholar]
- 47.Tindall B, Evans L, Cunningham P, et al. Identification of HIV-1 in semen following primary HIV-1 infection. AIDS. 1992;6:949–52. doi: 10.1097/00002030-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Atkins MC, Carlin EM, Emery VC, Griffiths PD, Boag F. Fluctuations of HIV load in semen of HIV positive patients with newly acquired sexually transmitted diseases. BMJ. 1996;313:341–2. doi: 10.1136/bmj.313.7053.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;14:117–21. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–9. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 51.Marina S, Marina F, Alcolea R, et al. Human immunodeficiency virus type 1–serodiscordant couples can bear healthy children after undergoing intrauterine insemination. Fertil Steril. 1998;70:35–9. doi: 10.1016/s0015-0282(98)00102-2. [DOI] [PubMed] [Google Scholar]
- 52.Quayle AJ, Xu C, Tucker L, Anderson DJ. The case against an association between HIV-1 and sperm: molecular evidence. J Reprod Immunol. 1998;41:127–36. doi: 10.1016/s0165-0378(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 53.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–8. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 54.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. AIDS. 2006;20:1491–5. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 55.Pudney J, Oneta M, Mayer K, Seage G, III, Anderson D. Pre-ejaculatory fluid as potential vector for sexual transmission of HIV-1. Lancet. 1992;340:1470. doi: 10.1016/0140-6736(92)92659-4. [DOI] [PubMed] [Google Scholar]
- 56.Calzavara L, Burchell AN, Remis RS, et al. Delayed application of condoms is a risk factor for human immunodeficiency virus infection among homosexual and bisexual men. Am J Epidemiol. 2003;157:210–7. doi: 10.1093/aje/kwf195. [DOI] [PubMed] [Google Scholar]
- 57.Comhaire F, Bosmans E, Ombelet W, Punjabi U, Schoonjans F. Cytokines in semen of normal men and of patients with andrological diseases. Am J Reprod Immunol. 1994;31:99–103. doi: 10.1111/j.1600-0897.1994.tb00853.x. [DOI] [PubMed] [Google Scholar]
- 58.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 59.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–73. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 60.Dyer JR, Eron JJ, Hoffman IF, et al. Association of CD4 cell depletion and elevated blood and seminal plasma human immunodeficiency virus type 1 (HIV-1) RNA concentrations with genital ulcer disease in HIV-1-infected men in Malawi. J Infect Dis. 1998;177:224–7. doi: 10.1086/517359. [DOI] [PubMed] [Google Scholar]
- 61.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 62.Anderson DJ, Politch JA, Tucker LD, et al. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res Hum Retroviruses. 1998;14(suppl 1)):S43–9. [PubMed] [Google Scholar]
- 63.Bardeguez AD, Skurnick JH, Perez G, Colon JM, Kloser P, Denny TN. Lymphocyte shedding from genital tract of human immunodeficiency virus-infected women: immunophenotypic and clinical correlates. Am J Obstet Gynecol. 1997;176:158–65. doi: 10.1016/s0002-9378(97)80029-4. [DOI] [PubMed] [Google Scholar]
- 64.McKinnon LR, Nyanga B, Chege D, et al. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–42. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- 65.Quayle AJ, Kourtis AP, Cu-Uvin S, et al. T-lymphocyte profile and total and virus-specific immunoglobulin concentrations in the cervix of HIV-1-infected women. J Acquir Immune Defic Syndr. 2007;44:292–8. doi: 10.1097/QAI.0b013e31802c5b3a. [DOI] [PubMed] [Google Scholar]
- 66.Olmsted SS, Khanna KV, Ng EM, et al. Low pH immobilizes and kills human leukocytes and prevents transmission of cell-associated HIV in a mouse model. BMC Infect Dis. 2005;5:79. doi: 10.1186/1471-2334-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabbaj S, Hel Z, Richter HE, Mestecky J, Goepfert PA. Menstrual blood as a potential source of endometrial derived CD3+ T cells. PLoS One. 2011;6:e28894. doi: 10.1371/journal.pone.0028894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bere A, Denny L, Naicker P, Burgers WA, Passmore JA. HIV-specific T-cell responses detected in the genital tract of chronically HIV-infected women are largely monofunctional. Immunology. 2013;139:342–51. doi: 10.1111/imm.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liebenberg LJ, Gamieldien H, Mkhize NN, et al. Stability and transport of cervical cytobrushes for isolation of mononuclear cells from the female genital tract. J Immunol Methods. 2011;367:47–55. doi: 10.1016/j.jim.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levine WC, Pope V, Bhoomkar A, et al. Increase in endocervical CD4 lymphocytes among women with nonulcerative sexually transmitted diseases. J Infect Dis. 1998;177:167–74. doi: 10.1086/513820. [DOI] [PubMed] [Google Scholar]
- 71.Cook RL, Redondo-Lopez V, Schmitt C, Meriwether C, Sobel JD. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30:870–7. doi: 10.1128/jcm.30.4.870-877.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121:4610–7. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouvet JP, Gresenguet G, Belec L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin Microbiol Infect. 1997;3:19–23. doi: 10.1111/j.1469-0691.1997.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 74.Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 75.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–54. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 76.Clemetson DB, Moss GB, Willerford DM, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–4. [PubMed] [Google Scholar]
- 77.Cowan FF, Pascoe SJ, Barlow KL, et al. Association of genital shedding of herpes simplex virus type 2 and HIV-1 among sex workers in rural Zimbabwe. AIDS. 2006;20:261–7. doi: 10.1097/01.aids.0000198086.39831.4a. [DOI] [PubMed] [Google Scholar]
- 78.John GC, Nduati RW, Mbori-Ngacha D, et al. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J Infect Dis. 1997;175:57–62. doi: 10.1093/infdis/175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kreiss J, Willerford DM, Hensel M, et al. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170:1597–601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 80.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 81.McClelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–10. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 82.Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis. 2000;181:58–63. doi: 10.1086/315188. [DOI] [PubMed] [Google Scholar]
- 83.Mostad SB, Overbaugh J, DeVange DM, et al. Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet. 1997;350:922–7. doi: 10.1016/S0140-6736(97)04240-2. [DOI] [PubMed] [Google Scholar]
- 84.Spinillo A, Zara F, Gardella B, Preti E, Mainini R, Maserati R. The effect of vaginal candidiasis on the shedding of human immunodeficiency virus in cervicovaginal secretions. Am J Obstet Gynecol. 2005;192:774–9. doi: 10.1016/j.ajog.2004.10.609. [DOI] [PubMed] [Google Scholar]
- 85.Spinillo A, Debiaggi M, Zara F, Maserati R, Polatti F, De Santolo A. Factors associated with nucleic acids related to human immunodeficiency virus type 1 in cervico-vaginal secretions. BJOG. 2001;108:634–41. doi: 10.1111/j.1471-0528.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 86.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183:1017–22. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 87.Manhart LE, Mostad SB, Baeten JM, Astete SG, Mandaliya K, Totten PA. High Mycoplasma genitalium organism burden is associated with shedding of HIV-1 DNA from the cervix. J Infect Dis. 2008;197:733–6. doi: 10.1086/526501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson BL, Wang CC, Delong AK, et al. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin Infect Dis. 2008;47:1216–21. doi: 10.1086/592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitchell C, Balkus JE, McKernan-Mullin J, et al. Associations between genital tract infections, genital tract inflammation, and cervical cytobrush HIV-1 DNA in US versus Kenyan women. J Acquir Immune Defic Syndr. 2013;62:143–8. doi: 10.1097/QAI.0b013e318274577d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baeten JM, Mostad SB, Hughes MP, et al. Selenium deficiency is associated with shedding of HIV-1--infected cells in the female genital tract. J Acquir Immune Defic Syndr. 2001;26:360–4. doi: 10.1097/00126334-200104010-00013. [DOI] [PubMed] [Google Scholar]
- 91.McClelland RS, Baeten JM, Overbaugh J, et al. Micronutrient supplementation increases genital tract shedding of HIV-1 in women: results of a randomized trial. J Acquir Immune Defic Syndr. 2004;37:1657–63. doi: 10.1097/00126334-200412150-00021. [DOI] [PubMed] [Google Scholar]
- 92.Benki S, Mostad SB, Richardson BA, Mandaliya K, Kreiss JK, Overbaugh J. Increased levels of HIV-1-infected cells in endocervical secretions after the luteinizing hormone surge. J Acquir Immune Defic Syndr. 2008;47:529–34. doi: 10.1097/QAI.0b013e318165b952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mostad SB, Jackson S, Overbaugh J, et al. Cervical and vaginal shedding of human immunodeficiency virus type 1-infected cells throughout the menstrual cycle. J Infect Dis. 1998;178:983–91. doi: 10.1086/515665. [DOI] [PubMed] [Google Scholar]
- 94.Vogt MW, Witt DJ, Craven DE, et al. Isolation patterns of the human immunodeficiency virus from cervical secretions during the menstrual cycle of women at risk for the acquired immunodeficiency syndrome. Ann Intern Med. 1987;106:380–2. doi: 10.7326/0003-4819-106-3-380. [DOI] [PubMed] [Google Scholar]
- 95.Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on Heterosexual Transmission of HIV. BMJ. 1992;304:809–13. doi: 10.1136/bmj.304.6830.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mattson CL, Bailey RC, Agot K, Ndinya-Achola JO, Moses S. A nested case-control study of sexual practices and risk factors for prevalent HIV-1 infection among young men in Kisumu, Kenya. Sex Transm Dis. 2007;34:731–6. doi: 10.1097/01.olq.0000261335.42480.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Graham SM, Holte SE, Peshu NM, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21:501–7. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 98.Nunnari G, Sullivan J, Xu Y, et al. HIV type 1 cervicovaginal reservoirs in the era of HAART. AIDS Res Hum Retroviruses. 2005;21:714–8. doi: 10.1089/aid.2005.21.714. [DOI] [PubMed] [Google Scholar]
- 99.Henning TR, Kissinger P, Lacour N, Meyaski-Schluter M, Clark R, Amedee AM. Elevated cervical white blood cell infiltrate is associated with genital HIV detection in a longitudinal cohort of antiretroviral therapy-adherent women. J Infect Dis. 2010;202:1543–52. doi: 10.1086/656720. [DOI] [PubMed] [Google Scholar]
- 100.Henin Y, Mandelbrot L, Henrion R, Pradinaud R, Coulaud JP, Montagnier L. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J Acquir Immune Defic Syndr. 1993;6:72–5. [PubMed] [Google Scholar]
- 101.Hillier SL. Normal Genital Flora. In: Holmes KK, Sparling PF, Stamm W, et al., editors. Sexually transmitted diseases. 4th ed. New York: McGraw Hill Medical Publications; 2008. pp. 298–308. [Google Scholar]
- 102.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–8. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.John GC, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–12. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 104.Montano M, Russell M, Gilbert P, et al. Comparative prediction of perinatal human immunodeficiency virus type 1 transmission, using multiple virus load markers. J Infect Dis. 2003;188:406–13. doi: 10.1086/376838. [DOI] [PubMed] [Google Scholar]
- 105.Gaillard P, Verhofstede C, Mwanyumba F, et al. Exposure to HIV-1 during delivery and mother-to-child transmission. AIDS. 2000;14:2341–8. doi: 10.1097/00002030-200010200-00015. [DOI] [PubMed] [Google Scholar]
- 106.Tugizov SM, Herrera R, Veluppillai P, et al. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011;409:211–22. doi: 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Page-Shafer K, Sweet S, Kassaye S, Ssali C. (C2) Saliva, breast milk, and mucosal fluids in HIV transmission. Adv Dent Res. 2006;19:152–7. doi: 10.1177/154407370601900127. [DOI] [PubMed] [Google Scholar]
- 108.Tudor-Williams G, Lyall EG. Mother to infant transmission of HIV. Curr Opin Infect Dis. 1999;12:21–6. doi: 10.1097/00001432-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 109.Tuomala RE, O'Driscoll PT, Bremer JW, et al. Cell-associated genital tract virus and vertical transmission of human immunodeficiency virus type 1 in antiretroviral-experienced women. J Infect Dis. 2003;187:375–84. doi: 10.1086/367706. [DOI] [PubMed] [Google Scholar]
- 110.Panther LA, Tucker L, Xu C, Tuomala RE, Mullins JI, Anderson DJ. Genital tract human immunodeficiency virus type 1 (HIV-1) shedding and inflammation and HIV-1 env diversity in perinatal HIV-1 transmission. J Infect Dis. 2000;181:555–63. doi: 10.1086/315230. [DOI] [PubMed] [Google Scholar]
- 111.Chaillon A, Samleerat T, Zoveda F, et al. Estimating the timing of mother-to-child transmission of the human immunodeficiency virus type 1 using a viral molecular evolution model. PLoS One. 2014;9:e90421. doi: 10.1371/journal.pone.0090421. [DOI] [PMC free article] [PubMed] [Google Scholar]