Abstract
mTOR is a serine/threonine kinase and plays a critical role in mammalian cell growth, survival, and metabolism. mTOR is present in two cellular complexes: mTORC1 and mTORC2. Dysregulation of the mTOR pathway has been related to tumorigenesis, poor prognosis and/or chemotherapy resistance in a variety of malignancies. Inhibition of mTORC1 by Rapamycin and its analogs has been explored to treat a number of tumors. However, the effectiveness of patient response is limited and not all patients respond. Second generation of mTOR inhibitors have recently been developed to target mTOR kinase activity and to suppress both mTORC1 and mTORC2. Dual mTORC1/mTORC2 inhibitors generally are more efficacious in preclinical studies and clinical trials. We and others have recently found that dual mTORC1/mTORC2 inhibitors sensitize T-cell acute lymphocytic leukemia and rhabdomyosarcoma cells to DNA damaging agents by suppression of expression of FANCD2 of the Fanconi anemia pathway, an important DNA repair mechanism that is associated with drug resistance of multiple types of cancer. This review will highlight mTOR and the Fanconi anemia pathway in cancer, with a particular attention to our newly discovered connection between mTOR and the Fanconi anemia pathway.
Keywords: mTOR, Fanconi anemia pathway, FANCD2, DNA repair, Tumorigenesis, Drug resistance, mTOR targeting
Introduction
The mammalian target of Rapamycin (mTOR) is a serine/threonine kinase that signals through two parallel molecular complexes: mTOR complex 1 (mTORC1) and mTORC2 [1]. mTORC1 contains Raptor, mLST8, PRAS40 and Deptor, while mTORC2 contains Rictor, mLST8, mSIN1 and Protor [1]. In response to nutrients, growth factors, and intracellular energy status, mTORC1 is activated by signaling through phosphatidylinositol-3-OH kinase (PI3K), PDK1 and Akt [2]. mTORC1 activation leads to phosphorylation of the translational regulators S6K1 and 4E-BP to regulate protein synthesis, cell growth and metabolism. mTORC2, on the other hand, can regulate cell survival via phosphorylating Akt on Ser473 [2,3]. The critical role of mTOR is attested by the lethality of mice harboring full-body knockout of mTOR and by tissue-specific knockout of mTOR complex components [4-24].
mTOR has been suggested to regulate DNA damage response (DDR) that is important for maintenance of genomic stability [25-27]. Mammalian cells have evolved a complex network of DDR to sense and respond to genotoxic agents [28]. Activation of DDR leads to the repair of DNA damage and genomic restoration, with cell death being an alternative outcome [29]. A major DDR network consists of Fanconi anemia (FA) DNA repair pathway; mutation of each of the 15 known FANC genes causes FA syndrome in human, which is often manifested by bone marrow failure [30-32].
This brief review will outline mTOR and the FA pathway in cancer, with a particular attention to our newly discovered connection between mTOR and the FA pathway.
mTOR in cancer
mTOR plays a crucial role in regulating cancer cell growth, proliferation, survival, migration and tumor angiogenesis [33]. Hyperactivation of mTOR signaling has been reported in many cancers (e.g. renal cell carcinoma, chronic myeloid leukemia) [34,35]. Deregulation of mTOR signaling in cancer occurs through complex mechanisms, including overexpression or activation of oncogenic Ras, PI3K, Akt, epidermal growth factor receptor (EGFR), BCR-ABL or loss of function of tumor suppressor genes phosphatase and tensin homolog (PTEN), tuberous sclerosis complex (TSC), and LKB1 [35-37]. mTOR downstream targets are also deregulated in a number of tumors. For example, S6K1 overexpression or activation has been detected in several cancer cell lines including breast cancer cells [33,34]. It has been shown that 4E-BP1 is overexpressed in gastrointestinal cancer and is hyperactivated in breast cancer and ovarian tumors [33].
Deregulation of mTOR pathway has also been implicated in chemotherapy resistance mechanisms. Overexpression of mTOR and S6K1 has been associated with TRAIL resistance of glioblastoma [38]. The resistance of NB4 promyelocytic cells to retinoid acid is related to defects in the regulation of 4E-BP1 and 4E-BP2 [39]. mTOR activation is linked to vincristine resistance of pro-B lymphoma cells [40]. Activation of mTOR and S6K1 is indicated in cisplatin or Adriamycin resistance of hepatocellular carcinoma, lung carcinoma, T-cell acute lymphocytic leukemia (T-ALL), and fibrosarcoma cells [41]. In addition, the aberrant activation of mTOR signaling has been detected in chemo-, radio- and/or hormone-resistant chordoma, breast cancer, pancreatic cancer, and prostate cancer cells [42-45].
Because of its pivotal role in tumorigenesis and drug resistance, mTOR has presented itself as a valid target for the treatment of various cancers. Targeting mTORC1 by rapamycin and its analogs has been explored in preclinical studies and clinical trials to treat tumors of diverse cellular origin. A rapamycin analog Temsirolimus was approved for treating renal cell carcinoma by the US Food and Drug Administration (FDA) in 2007. However, in general the length of patient response to rapamycin or rapamycin analogs is limited and not all patients respond. This could be attributable to several factors. First, rapamycin and its analogs release the negative feedback loop between S6K and Akt, resulting in Akt hyperactivation. Second, rapamycin and its analogs can only transiently inhibit the activity of 4E-BP1, leading to suboptimal inhibition of protein synthesis. Finally, rapamycin and its analogs have minimum inhibitory effect on mTORC2 activity [36,37,46,47]. It is noted that mTORC1 and mTORC2 may be complementary to each other in regulating cellular functions with mTORC1 governing cell growth, proliferation and metabolism, while mTORC2 regulating cell survival [2,3]. That said, it is unlikely that mTORC1 inhibitors suppress cell survival. Even worse, mTORC1 inhibitors may enhance cell survival through Akt activation that is known to be regulated by mTORC2 [2,3], and therefore, reminiscent of compensatory increase of mTORC2 activity.
To overcome the limitations of mTORC1 inhibitors, a new generation of mTOR inhibitors has recently been developed to target mTOR kinase activity and thus both mTORC1 and mTORC2. Compared to rapamycin and its analogs, these dual mTORC1/mTORC2 inhibitors, overall, have significantly higher anticancer efficacy. Among them, AZD8055, OSI-027 and INK128 have entered into clinical trials [35]. Readers are referred to a number of excellent reviews on this topic published recently [34,47-49]. It is notable that dual mTORC1/mTORC2 inhibitors may also be more effective than rapamycin and its analogs in combinatory therapy. For example, a dual mTORC1/mTORC2 inhibitor pp242 synergized with bortezomib in inhibition of multiple myeloma (MM) cell growth and survival, whereas this synergistic effect was not seen when the cells were treated with rapamycin in combination with bortezomib [50,51]. An important issue concerning dual mTORC1/mTORC2 inhibitors is that they may activate ERK/MAPK pathway [50,52], leading to cancer cell hyperproliferation. However, this is likely a cell type-specific event. While MM cells treated with pp242 showed an increased ERK phosphorylation [50,52], we observed an attenuated ERK activation in pp242-treated T-ALL cells [53]. Another caveat is that dual mTORC1/mTORC2 inhibitors may induce autophagy, which could promote cancer cell survival [49]. These drawbacks may be circumvented by combining dual mTORC1/mTORC2 inhibitor with ERK inhibitor or autophagy inhibitor. Indeed, even mTORC1 inhibitor can synergize with autophagy inhibitor in inhibition of endothelial cell growth [54].
The FA DNA repair pathway in cancer
The FA signaling pathway is essential for DNA damage repair and thus for maintaining genomic stability. Upon DNA damage, the FA core complex containing FANCA and FANCF, among others, induces monoubiquitylation of FANCD2, which in turn leads to FANCD2 translocation to nuclear foci and subsequent DNA repair [31,55]. Mutation or silencing of genes controlling the FA pathway causes a genomic instability syndrome, namely FA, that is defective for DDR resulting in cellular hypersensitivity to DNA damaging agents [30].
Gain-of-function in the FA pathway (e.g. overexpression of FA proteins) appears to play a key role in underlying drug resistance of multiple types of cancer. The FA pathway has been identified as a key pathway in both chemo- and radio-resistance of mesothelioma [56]. FANCF and FANCD2 contribute to melphalan resistance of MM cells [57-59]. DNA alkylating agent resistance of glioma and cisplatin resistance of ovarian cell cells are attributable to increased FANCD2 monoubiquitination and FANCD2 nuclear foci formation [60,61]. In head and neck cancer cells, proficient FANCD2 foci formation is associated with cisplatin resistance [62]. In addition, FANCD2 is related to etoposide resistance of lung cancer cells and doxorubicin resistance of breast cancer cells [63]. Thus, it has been a long sought-after goal in the FA field that rational targeting the FA pathway may provide a chemo-sensitizing avenue for more effective DNA damaging chemotherapy [63-67].
Linkage of mTOR to the FA pathway
mTOR is known to regulate DDR [25,26]. By gene targeting approach, we have recently shown that deletion of mTOR in mouse hematopoietic stem and progenitor cells causes a defective DDR to a variety of DNA damage agents (e.g. melphalan, mitomycin C, γ-ionizing radiation) [68]. Mechanistically, we demonstrate that mTOR deficiency inhibits expression of FANCD2 [68]. This regulation of FANCD2 expression by mTOR is conserved in mouse myeloid cells and human B lymphoblasts, because ablation of mTOR in mouse myeloid cells or inhibition of mTOR in human B lymphoblast by dual mTORC1/mTORC2 inhibitors (e.g. pp242, Torin 1) suppresses FANCD2 expression [53,68]. We further show that mTOR deficiency or inhibition increases phosphorylation and nuclear translocation of nuclear factor (NF)-κB, which results in an enhanced NF-κB binding to FANCD2 promoter to suppress FANCD2 expression [68]. It appears that mTOR negatively regulates NF-κB activity by direct binding to IKK and that mTOR knockout or inhibition disrupts the mTOR-IKK complex to release IKK for binding to and activation by TAK1 leading to increased NF-κB nuclear activity [68] (Figure 1A). Interestingly, mTOR-NF-κB-FANCD2 signaling circuitry seems not to be dependent on mTORC1 or mTORC2, because deletion of either the mTORC1 component Raptor or the mTORC2 component Rictor has no effect on FANCD2 expression [68].
Figure 1. Working model of mTOR regulation of FANCD2 expression through NF-κB pathway in normal and cancer cells.
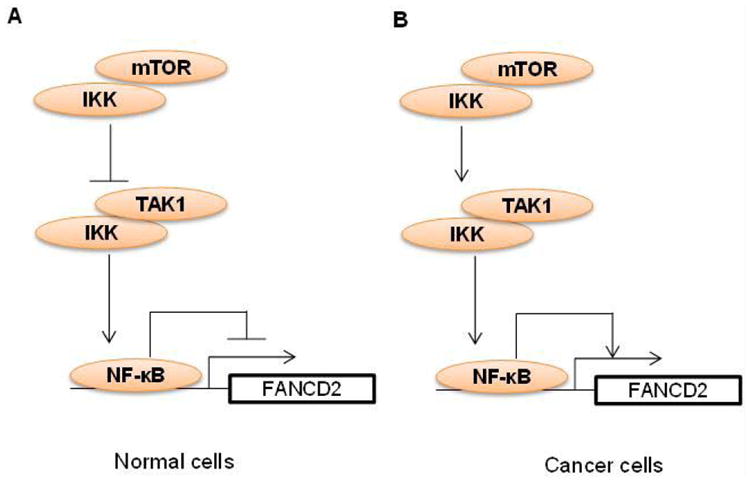
(A) In normal cells, mTOR binds to IKK, leading to sequestration of IKK from binding to and activation by TAK1. As a result, NF-κB activation and binding to FANCD2 promoter is suppressed, which causes de-repression of FANCD2 gene expression. (B) In cancer cells, mTOR binding to IKK induces IKK binding to and activation by TAK1. IKK activation leads to NF-κB activation and binding to FANCD2 promoter, which in turn promotes FANCD2 gene expression.
We have also found that mTOR-NF-κB-FANCD2 signaling hub exists in human T-ALL cells [53]. However, in T-ALL cells, the downregulation of FANCD2 by mTOR inhibition correlates with a deactivation of NF-κB (our unpublished data), suggesting that NF-κB plays a positive role in mediating FANCD2 expression in these cells, reminiscent of that in melphalan-resistant MM cells [59] (Figure 1B). In line with our discovery, mTOR has been found to regulate FANCD2 expression in rhabdomyosarcoma cells [69], particularly in alveolar rhabdomyosarcoma harboring the PAX3/FOXO1 fusion gene [70]. It is worth noting that in T-ALL cells, we did not detect an inhibitory effect of rapamycin on FANCD2 expression, consistent with the mTORC1-independent role of mTOR in the regulation of FANCD2 expression in mouse hematopoietic stem and progenitor cells. However, in rhabdomyosarcoma cells, it appears that mTORC1 accounts for mTOR effect on FANCD2 expression [69]. Thus, whether mTOR regulates FANCD2 expression through mTORC1 is contingent on cell types. Interestingly, suppression of FANCD2 expression by mTOR inhibition deactivates DNA damage sensors ATM and ATR kinases [69]. Thus, mTOR may mediate DDR through governing FANCD2 expression and subsequent activation of ATM-Chk2 and ATR-Chk1 pathways that otherwise have been suggested to activate the FA core complex and to facilitate FANCD2 translocation to nuclear foci [55,71] (Figure 2). These recent findings raise the prospect of using mTOR inhibitors, particularly dual mTORC1 and mTORC2 inhibitors, in combination with DNA damaging chemotherapy as a potential future approach for the treatment of cancers. Indeed, we show that dual mTORC1 and mTORC2 inhibitor pp242, AZD8055, or INK128, but not rapamycin, sensitizes T-ALL cells for Arac-, etoposide-or cisplatin-induced DNA damage and apoptosis. Accordingly, the combinatory treatment significantly prolongs lifespan of T-ALL cell line- or patient cell-xenograted mice [53].
Figure 2. Working model of mTOR-FANCD2 pathway-mediated DDR.
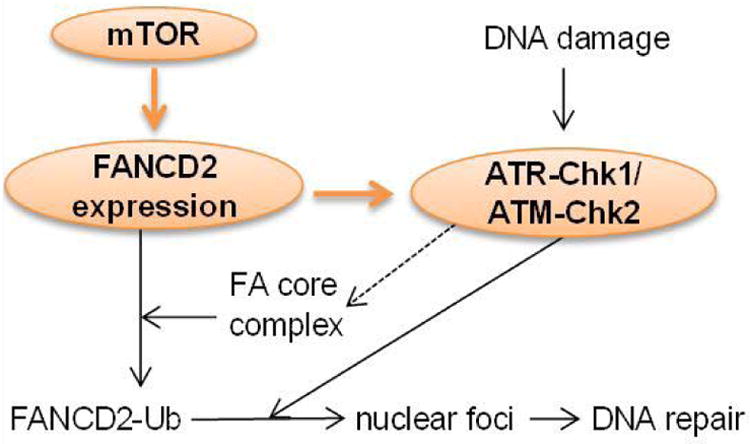
mTOR activation leads to FANCD2 expression, which in turn activates ATM-Chk2 and ATR-Chk1 pathway, resulting in the activation of the FA core complex, FANCD2 mono-ubiquitination and FANCD2 translocation to nuclear foci to mediate DNA repair. FANCD2-Ub: ubiquitylated FANCD2.
Conclusion and Prospective
While targeting mTOR is promising for cancer treatment, inhibition of mTORC1 by rapamycin or its analogues has limited clinical efficacy. The development of more potent dual mTORC1/mTORC2 inhibitors represents a major advance in mTOR targeting. However, because dual mTORC1/mTORC2 inhibitors work by competing with ATP to bind to mTOR kinase domain, they may elicit off-target effects on related kinases and thus not be as specific as rapamycin and its analogues [47]. Nonetheless, dual mTORC1/mTORC2 inhibitors work well and are well tolerated in a xenograft study of prostate cancer [72]. We have also shown that there is a therapeutic window that combined dual mTORC1/mTORC2 inhibitor pp242 with AraCcan significantly extend life span of mouse xenografts of T-ALL [53]. Nevertheless, any clinical benefit of dual mTORC1/mTORC2 inhibitors needs to be weighed against their greater toxicity in comparison with rapamycin and its analogues.
mTOR plays a critical role in anti-cancer drug resistance. The molecular mechanism of mTOR underlying drug resistance remains poorly studied. With that FANCD2 monoubiquitination and FANCD2 nuclear foci formation have been shown to be prognostic in drug resistance [60,61], we and others suggest that greater FANCD2 transcript and protein levels may underlie mTOR-regulated drug resistance [53,69,70]. A correlation of mTOR-associated genes with FANCD2 gene expression has been demonstrated in alveolar rhabdomyosarcoma patients [70]. Thus, FANCD2 transcript and protein contents could potentially serve as a predictive biomarker for responsiveness of drug-resistant cancer patients to mTOR inhibition. That is to say, targeting mTOR-FANCD2 pathway by using dual mTORC1/mTORC2 inhibitors may represent a novel and selective sensitizing strategy to combat drug resistance of cancer patients to other anticancer agents, particularly DNA damaging agents. The identification of FANCD2 as a biomarker of mTOR targeting responsiveness is apparently beneficial for patient stratification and eventual clinical success of mTOR inhibition. However, it should be realized that mTOR-FANCD2 signing node also exists in normal cells [68]. Thus, targeting this pathway may yield severe side effects, for instance, bone marrow failure that is seen in FA patients harboring mutation or deficiency in the FA pathway [32]. Of note, in the context of mTOR signaling, FANCD2 expression is differentially regulated by NF-κB in normal cells versus cancer cells. In normal cells (e.g. mouse hematopoietic stem and progenitor cells, human B lymphoblasts, mouse embryonic fibroblasts), we show that mTOR inhibition leads to an increased NF-κB activation, which in turn suppresses FANCD2 expression [68], whereas in cancer cells (e.g. T-ALL), mTOR inhibition leads to a decreased NF-κB activation and FANCD2 expression [53] (our unpublished data). Therefore, if severe side effects arise, we might adopt a salvage protocol by further addition of NF-κB inhibitor to alleviate cytotoxicity in normal cells.
Acknowledgments
This work is supported in part by a grant from the National Institutes of Health (GM 108661) to F.G.
Abbreviations
- mTOR
Mammalian Target of Rapamycin
- mTORC1
mTOR Complex 1
- mTORC2
mTOR Complex 2
- PI3K
Phosphatidylinositol-3-OH Kinase
- DDR
DNA Damage Response
- FA
Fanconi Anemia
- EGFR
Epidermal Growth Factor Receptor
- PTEN
Phosphatase and Tensin Homolog
- TSC
Tuberous Sclerosis Complex
- T-All
T-cell Acute Lymphocytic Leukemia
- FDA
Food and Drug Administration
- MM
Multiple Myeloma
- NF
Nuclear Factor
References
- 1.Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8:1003–1006. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracho-Valdés I, Moreno-Alvarez P, Valencia-Martínez I, Robles-Molina E, Chávez-Vargas L, et al. mTORC1- and mTORC2-interacting proteins keep their multifunctional partners focused. IUBMB Life. 2011;63:896–914. doi: 10.1002/iub.558. [DOI] [PubMed] [Google Scholar]
- 4.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, et al. Disruption of the mouse mTOR gene leads to early post implantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami M, Ichisaka T, Maeda M, Oshiro N, Hara K, et al. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–6718. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, et al. Ablation in mice of the mTORC component raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akti-FOXO and PKCα, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, et al. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi: 10.1016/j.cmet.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187:859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cybulski N, Polak P, Auwerx J, Róegg MA, Hall MN, et al. mTOR complex 2 in adipose tissue negatively controls whole-body growth. Proc Natl Acad Sci USA. 2009;106:9902–9907. doi: 10.1073/pnas.0811321106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 15.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between β-cell proliferation and cell size. Diabetes. 2011;60:827–837. doi: 10.2337/db10-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shende P, Plaisance I, Morandi C, Pellieux C, Berthonneche C, et al. Cardiac raptor ablation impairs adaptive hypertrophy, alters metabolic gene expression, and causes heart failure in mice. Circulation. 2011;123:1073–1082. doi: 10.1161/CIRCULATIONAHA.110.977066. [DOI] [PubMed] [Google Scholar]
- 19.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang F, Wu Q, Ikenoue T, Guan KL, Liu Y, et al. A critical role for rictor in T lymphopoiesis. J Immunol. 2012;189:1850–1857. doi: 10.4049/jimmunol.1201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K, Nam KT, Cho SH, Gudapati P, Hwang Y, et al. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med. 2012;209:713–728. doi: 10.1084/jem.20111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshii T, Tadokoro Y, Naka K, Ooshio T, Muraguchi T, et al. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J Clin Invest. 2012;122:2114–2129. doi: 10.1172/JCI62279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalaitzidis D, Sykes SM, Wang Z, Punt N, Tang Y, et al. mTOR complex 1 plays critical roles inhematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429–439. doi: 10.1016/j.stem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo F, Zhang S, Grogg M, Cancelas JA, Varney ME, et al. Mouse gene targeting reveals an essential role of mTOR in hematopoietic stem cell engraftment and hematopoiesis. Haematologica. 2013;98:1353–1358. doi: 10.3324/haematol.2012.080424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 26.Shen C, Lancaster CS, Shi B, Guo H, Thimmaiah P, et al. TOR signaling is a determinant of cell survival in response to DNA damage. Mol Cell Biol. 2007;27:7007–7017. doi: 10.1128/MCB.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, et al. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 28.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 29.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 30.Kee Y, D'Andrea AD. Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest. 2012;122:3799–3806. doi: 10.1172/JCI58321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitao H, Takata M. Fanconi anemia: a disorder defective in the DNA damage response. Int J Hematol. 2011;93:417–424. doi: 10.1007/s12185-011-0777-z. [DOI] [PubMed] [Google Scholar]
- 32.Leguit RJ, van den Tweel JG. The pathology of bone marrow failure. Histopathology. 2010;57:655–670. doi: 10.1111/j.1365-2559.2010.03612.x. [DOI] [PubMed] [Google Scholar]
- 33.Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11:63–76. doi: 10.1016/j.drup.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal SK, Quinn DI. Differentiating mTOR inhibitors in renal cell carcinoma. Cancer Treat Rev. 2013;39:709–719. doi: 10.1016/j.ctrv.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenone S, Brullo C, Musumeci F, Radi M, Botta M. ATP-competitive inhibitors of mTOR: an update. Curr Med Chem. 2011;18:2995–3014. doi: 10.2174/092986711796391651. [DOI] [PubMed] [Google Scholar]
- 36.Evangelisti C, Ricci F, Tazzari P, Tabellini G, Battistelli M, et al. Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia. 2011;25:781–791. doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- 37.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 38.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grolleau A, Wietzerbin J, Beretta L. Defect in the regulation of 4E-BP1 and 2, two repressors of translation initiation, in the retinoid acid resistant cell lines, NB4-R1 and NB4-R2. Leukemia. 2000;14:1909–1914. doi: 10.1038/sj.leu.2401904. [DOI] [PubMed] [Google Scholar]
- 40.Vanderweele DJ, Rudin CM. Mammalian target of rapamycin promotes vincristine resistance through multiple mechanisms independent of maintained glycolytic rate. Mol Cancer Res. 2005;3:635–644. doi: 10.1158/1541-7786.MCR-05-0063. [DOI] [PubMed] [Google Scholar]
- 41.Mungamuri SK, Yang X, Thor AD, Somasundaram K. Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Cancer Res. 2006;66:4715–4724. doi: 10.1158/0008-5472.CAN-05-3830. [DOI] [PubMed] [Google Scholar]
- 42.Presneau N, Shalaby A, Idowu B, Gikas P, Cannon SR, et al. Potential therapeutic targets for chordoma: PI3K/AKT/TSC1/TSC2/mTOR pathway. Br J Cancer. 2009;100:1406–1414. doi: 10.1038/sj.bjc.6605019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steelman LS, Navolanic P, Chappell WH, Abrams SL, Wong EW, et al. Involvement of Akt and mTOR in chemotherapeutic- and hormonal-based drug resistance and response to radiation in breast cancer cells. Cell Cycle. 2011;10:3003–3015. doi: 10.4161/cc.10.17.17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kagawa S, Takano S, Yoshitomi H, Kimura F, Satoh M, et al. Akt/mTOR signaling pathway is crucial for gemcitabine resistance induced by Annexin II in pancreatic cancer cells. J Surg Res. 2012;178:758–767. doi: 10.1016/j.jss.2012.05.065. [DOI] [PubMed] [Google Scholar]
- 45.Gravina GL, Marampon F, Petini F, Biordi L, Sherris D, et al. The TORC1/TORC2 inhibitor, Palomid 529, reduces tumor growth and sensitizes to docetaxel and cisplatin in aggressive and hormone-refractory prostate cancer cells. Endocr Relat Cancer. 2011;18:385–400. doi: 10.1530/ERC-11-0045. [DOI] [PubMed] [Google Scholar]
- 46.Gupta M, Hendrickson AE, Yun SS, Han JJ, Schneider PA, et al. Dual mTORC1/mTORC2 inhibition diminishes Akt activation and induces Puma-dependent apoptosis in lymphoid malignancies. Blood. 2012;119:476–487. doi: 10.1182/blood-2011-04-346601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 48.Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget. 2011;2:510–517. doi: 10.18632/oncotarget.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoang B, Frost P, Shi Y, Belanger E, Benavides A, et al. Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood. 2010;116:4560–4568. doi: 10.1182/blood-2010-05-285726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 52.Hoang B, Benavides A, Shi Y, Yang Y, Frost P, et al. The PP242 mammalian target of rapamycin (mTOR) inhibitor activates extracellular signal-regulated kinase (ERK) in multiple myeloma cells via a target of rapamycin complex 1 (TORC1)/eukaryotic translation initiation factor 4E (eIF-4E)/RAF pathway and activation is a mechanism of resistance. J BiolChem. 2012;287:21796–21805. doi: 10.1074/jbc.M111.304626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo F, Li J, Zhang S, Du W, Amarachintha S, et al. mTOR kinase inhibitor sensitizes T-cell lymphoblastic leukemia for chemotherapy-induced DNA damage via suppressing FANCD2 expression. Leukemia. 2014;28:203–206. doi: 10.1038/leu.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimaldi A, Balestrieri ML, D'Onofrio N, Di Domenico G, Nocera C, et al. The synergistic effect of everolimus and chloroquine on endothelial cell number reduction is paralleled by increased apoptosis and reduced autophagy occurrence. PLoS One. 2013;8:e79658. doi: 10.1371/journal.pone.0079658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deakyne JS, Mazin AV. Fanconi anemia: at the crossroads of DNA repair. Biochemistry (Mosc) 2011;76:36–48. doi: 10.1134/s0006297911010068. [DOI] [PubMed] [Google Scholar]
- 56.Røe OD, Anderssen E, Sandeck H, Christensen T, Larsson E, et al. Malignant pleural mesothelioma: genome-wide expression patterns reflecting general resistance mechanisms and a proposal of novel targets. Lung Cancer. 2010;67:57–68. doi: 10.1016/j.lungcan.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Chen Q, Van der Sluis PC, Boulware D, Hazlehurst LA, Dalton WS. The FA/BRCA pathway is involved in melphalan-induced DNA interstrand cross-link repair and accounts for melphalan resistance in multiple myeloma cells. Blood. 2005;106:698–705. doi: 10.1182/blood-2004-11-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, et al. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Res. 2003;63:7900–7906. [PubMed] [Google Scholar]
- 59.Yarde DN, Oliveira V, Mathews L, Wang X, Villagra A, et al. Targeting the Fanconi anemia/BRCA pathway circumvents drug resistance in multiple myeloma. Cancer Res. 2009;69:9367–9375. doi: 10.1158/0008-5472.CAN-09-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen CC, Taniguchi T, D'Andrea A. The Fanconi anemia (FA) pathway confers glioma resistance to DNA alkylating agents. J Mol Med (Berl) 2007;85:497–509. doi: 10.1007/s00109-006-0153-2. [DOI] [PubMed] [Google Scholar]
- 61.Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–574. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 62.Burkitt K, Ljungman M. Compromised Fanconi anemia response due to BRCA1 deficiency in cisplatin-sensitive head and neck cancer cell lines. Cancer Lett. 2007;253:131–137. doi: 10.1016/j.canlet.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Kachnic LA, Li L, Fournier L, Ferraiolo N, Dahm-Daphi J, et al. FANCD2 but not FANCA promotes cellular resistance to type II topoisomerase poisons. Cancer Lett. 2011;305:86–93. doi: 10.1016/j.canlet.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 64.Xiao H, Xiao Q, Zhang K, Zuo X, Shrestha UK. Reversal of multidrug resistance by curcumin through FA/BRCA pathway in multiple myeloma cell line MOLP-2/R. Ann Hematol. 2010;89:399–404. doi: 10.1007/s00277-009-0831-6. [DOI] [PubMed] [Google Scholar]
- 65.Seif AE. Pediatric leukemia predisposition syndromes: clues to understanding leukemogenesis. Cancer Genet. 2011;204:227–244. doi: 10.1016/j.cancergen.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Furgason JM, Bahassi el M. Targeting DNA repair mechanisms in cancer. Pharmacol Ther. 2013;137:298–308. doi: 10.1016/j.pharmthera.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 67.Ferrer M, Span SW, Vischioni B, Oudejans JJ, van Diest PJ, et al. FANCD2 expression in advanced non-small-cell lung cancer and response to platinum-based chemotherapy. Clin Lung Cancer. 2005;6:250–254. doi: 10.3816/CLC.2005.n.005. [DOI] [PubMed] [Google Scholar]
- 68.Guo F, Li J, Du W, Zhang S, O'Connor M, et al. mTOR regulates DNA damage response through NF-κB-mediated FANCD2 pathway in hematopoietic cells. Leukemia. 2013;27:2040–2046. doi: 10.1038/leu.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen C, Oswald D, Phelps D, Cam H, Pelloski CE, et al. Regulation of FANCD2 by the mTOR pathway contributes to the resistance of cancer cells to DNA double-strand breaks. Cancer Res. 2013;73:3393–3401. doi: 10.1158/0008-5472.CAN-12-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh M, Leasure JM, Chronowski C, Geier B, Bondra K, et al. FANCD2 is a Potential Therapeutic Target and Biomarker in Alveolar Rhabdomyosarcoma Harboring the PAX3/FOXO1 Fusion Gene. Clin Cancer Res. 2014;20:3884–3895. doi: 10.1158/1078-0432.CCR-13-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venkitaraman AR. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat Rev Cancer. 2004;4:266–276. doi: 10.1038/nrc1321. [DOI] [PubMed] [Google Scholar]
- 72.Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, et al. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]


