Abstract
Background. The last case of polio associated with wild poliovirus (WPV) indigenous to the Democratic Republic of the Congo (DRC) was reported in 2001, marking a major milestone toward polio eradication in Africa. However, during 2006–2011, outbreaks associated with WPV type 1 (WPV1) were widespread in the DRC, with >250 reported cases.
Methods. WPV1 isolates obtained from patients with acute flaccid paralysis (AFP) were compared by nucleotide sequencing of the VP1 capsid region (906 nucleotides). VP1 sequence relationships among isolates from the DRC and other countries were visualized in phylogenetic trees, and isolates representing distinct lineage groups were mapped.
Results. Phylogenetic analysis indicated that WPV1 was imported twice in 2004–2005 and once in approximately 2006 from Uttar Pradesh, India (a major reservoir of endemicity for WPV1 and WPV3 until 2010–2011), into Angola. WPV1 from the first importation spread to the DRC in 2006, sparking a series of outbreaks that continued into 2011. WPV1 from the second importation was widely disseminated in the DRC and spread to the Congo in 2010–2011. VP1 sequence relationships revealed frequent transmission of WPV1 across the borders of Angola, the DRC, and the Congo. Long branches on the phylogenetic tree signaled prolonged gaps in AFP surveillance and a likely underreporting of polio cases.
Conclusions. The reestablishment of widespread and protracted WPV1 transmission in the DRC and Angola following long-range importations highlights the continuing risks of WPV spread until global eradication is achieved, and it further underscores the need for all countries to maintain high levels of poliovirus vaccine coverage and sensitive surveillance to protect their polio-free status.
Keywords: polio eradication, Democratic Republic of Congo, wild poliovirus, importation, molecular epidemiology
Since the launch of the Global Polio Eradication Initiative in 1988 [1], worldwide poliomyelitis incidence has declined by >99%. Four World Health Organization (WHO) regions have certified the eradication of indigenous WPVs: the Region of the Americas, in 1994 [2]; the Western Pacific Region, in 2000 [3]; the Europe Region, in 2002 [4]; and the South-East Asia Region (which includes India), in March 2014 [5]. WPV2 was last detected in Uttar Pradesh, India, in 1999 [6], and WPV3 was last detected in northern Nigeria, in November 2012 (updates are available at: http://www.polioeradication.org). In India, once the world's reservoir of the most intense polio endemicity, WPV was last detected in early 2011 [7]. Since 2011, only 3 countries have never interrupted indigenous WPV transmission [8]: Pakistan [9], Afghanistan [10], and Nigeria [11]. All WPV cases reported in 2013 were associated with only 2 WPV1 genotypes (isolates within a genotype share >85% VP1 sequence identity): the SOAS (South Asia) genotype, endemic to parts of northern Pakistan, with recent spread to Afghanistan and the Middle East [12]; and the WEAF-B (West Africa B) genotype, endemic to parts of northern Nigeria, with recent spread to the Horn of Africa [13].
The WHO African Region began polio eradication activities in 1995 [14]. The number of countries in AFR reporting WPV transmission declined from 25 in 1995 to only 1 (Nigeria) by 2008 [8], a milestone achieved through sustained efforts in (1) routine and supplementary immunization activities (SIAs; mass immunization campaigns) with oral poliovirus vaccine (OPV), (2) sensitive surveillance for cases of acute flaccid paralysis (AFP), and (3) laboratory-based poliovirus (PV) surveillance. The challenge of armed conflict in the DRC, Angola, and other African countries was met by Days of Tranquility, in which hostilities were suspended for the purpose of national immunization days [15]. The last cases associated with WPV indigenous to the DRC and neighboring Angola were reported in 2001 [14]. However, immunity gaps widened in many newly polio-free African countries, as OPV coverage rates declined and natural immunity from WPV infection no longer occurred. Starting in 2002, WPV1 and WPV3 spread from northern Nigeria in successive waves across West and Central Africa, and WPV1 and WPV3 were imported into Southern and Central Africa from India [13, 16–19]. The imported outbreak viruses were repeatedly rolled back in most African countries [8, 20], but sustained WPV transmission was reestablished in the DRC and Angola [20, 21]. The widespread outbreaks in the DRC prompted the launch of SIAs, reinforcement of routine immunization, retrospective assessments of age-specific population immunity [22], and intensified surveillance for AFP cases coupled with virologic characterization of PV isolates [21].
In this report, we describe reconstruction of the pathways of spread of the imported WPV1 from India to Angola, the DRC, and the Congo by phylogenetic analysis of the 906-nucleotide region encoding the major capsid protein, VP1. VP1 sequence relationships are routinely analyzed by virologists in the Global Polio Laboratory Network [23]. The rapid evolution of PV in nature (approximately 1% substitutions per site per year, equivalent to approximately 1–2 nucleotide substitutions per week over the entire genome) [24] permits precise reconstruction of genetic relationships between isolates, discrimination between PV-endemic source reservoirs and reinfected areas, and establishment of the timing of importation events. We document frequent WPV1 transmission across the borders of Angola, the DRC, and the Congo; the geographic distribution of multiple lineage groups within the DRC; and the detection of gaps in AFP surveillance by the appearance of orphan lineages.
MATERIALS AND METHODS
Virus Isolation and Identification
Viruses, isolated [25] from stool specimens collected from patients with AFP, were characterized by diagnostic reverse transcription polymerase chain reaction [26–28], enzyme-linked immunosorbent assay [29], and partial genomic sequencing at the National Institute for Communicable Diseases and the Centers for Disease Control and Prevention.
Sequence Analysis
The complete VP1 region (nucleotides 2480–3385) was sequenced as previously described [30], using cycle sequencing with the Big Dye Terminator Cycle sequencing kit version 3.1 on the Gene Amp 9700 (Applied Biosystems, Foster City, CA). The DNA sequence was determined using the ABI 3100 Genetic Analyzer, version 3.1 (Applied Biosystems). Raw data were edited using the Sequencher software package, version 4.1.4 (Gene Codes, Ann Arbor, MI).
Phylogenetic Analysis
VP1 sequences were aligned with Clustal X [31] and analyzed using the software package Geneious [32]. Phylogenetic trees were inferred using maximum likelihood methods as implemented in the PhyML [33] plug-in within Geneious. Genetic distances were estimated under models of evolution correcting for multiple substitutions at a site and for unequal transition and transversion rates. Estimation of the evolutionary rate of VP1 sequences was calculated using Bayesian Markov chain Monte Carlo methods, as implemented in BEAST v1.7.5 [34]. Onset dates were used for estimating the evolutionary rate under a strict molecular clock model. Two independent chains were run consisting of 20 000 000 steps each. Sample correlation was assessed by checking the effective size samples statistic in Tracer v1.5 (available at: http://beast.bio.ed.ac.uk). A maximum clade credibility (MCC) tree was inferred by BEAST, in which branch lengths were scaled to time according to the estimated mean substitution rate. The annotated tree file was visualized in Figtree v1.3 (available at: http://tree.bio.ed.ac.uk/software/figtree).
GenBank Accession Numbers
VP1 sequences of all isolates described here were deposited in the GenBank database under accession numbers KF992599-KF992623 and KJ502317-KJ502560.
RESULTS
Background
The DRC is one of the largest (area, 2.35 million km2) and most populous (population, approximately 75 million) countries in sub-Saharan Africa. It shares borders with 9 countries, and 2 borders (with Angola in the south and the Congo in the west) extend >2000 km. The DRC is administratively divided into 11 provinces (including the city-province of Kinshasa); each province is further divided into districts. The public health system is organized in approximately 515 health districts (Zones de Santé). All provinces except Kasai Oriental reported WPV1 cases during the 2006–2011 outbreaks. Concomitant with the outbreaks of WPV1 infection, the DRC reported >70 polio cases associated with circulating type 2 vaccine-derived PV (cVDPV2) [35] during 2004–2012. In addition, 4 polio cases associated with WPV3 originating from Uttar Pradesh, India, were reported in the southern provinces of the DRC (1 in 2008 and 3 in 2009) [17].
Two Separate WPV1 Importations From India Into Angola
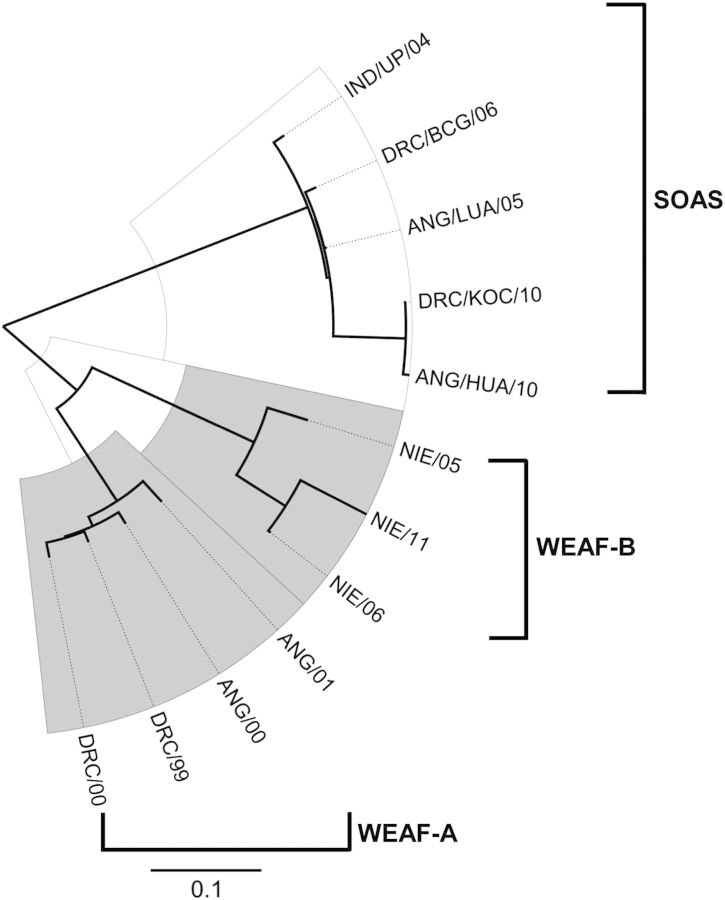
Before 2000, at least 5 distinct WPV1 genotypes circulated in sub-Saharan Africa [36]. In the DRC and Angola, the major indigenous genotype was WEAF-A, last detected in those countries in 2001 (Figure 1). A distantly related WPV1 genotype, WEAF-B, is indigenous to northern Nigeria and, after 2002, represented the last surviving WPV1 genotype indigenous to Africa [11, 37]. Quite distinct from the WEAF-A and WEAF-B genotypes is the SOAS genotype (Figure 1), previously indigenous to India and neighboring countries but whose remaining reservoirs of endemicity have been restricted to a few districts in Pakistan and Afghanistan [9, 10]. The last major reservoirs for the SOAS genotype in India were the large and populous northern states of Uttar Pradesh and Bihar [38].
Figure 1.
Maximum likelihood tree of VP1 region (906 nucleotides) sequence relationships among a survey of wild poliovirus type 1 isolates from cases in India (IND), the Democratic Republic of Congo (DRC), Angola (ANG), and Nigeria (NIE) during 1999–2011. Scale bar represents nucleotide substitutions per site.
Polio cases associated with WPV1 were again detected in Angola, in 2005, and in the DRC, in 2006. Sequence analysis showed that the case isolates were not related to the previously indigenous WEAF-A genotype, but to the SOAS genotype (Figure 1). The isolate most closely related (98.3% VP1 sequence match) to the first 2005 Angolan isolate (from Luanda) was a WPV1 from a 2004 case in Uttar Pradesh, India (Figures 1 and 2). The WPV1 from this importation spread from Angola to the DRC, where it circulated within the country until December 2011 (Figure 2).
Figure 2.
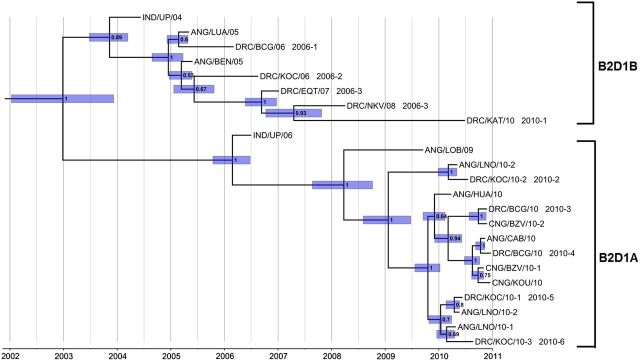
Maximum clade credibility tree based on the VP1 region of representative Democratic Republic of Congo (DRC) sequences from each inferred lineage, including closely related sequences from Angola (ANG), the Congo (CNG), and India (IND). Branch support at each interior node is represented by posterior values. Bars show the 95% credibility interval for estimated divergence times at each interior node.
A second importation from Uttar Pradesh occurred approximately 2006 and was associated with cases in Angola, the DRC, and the Congo from 2010 to 2011 (Figure 2 and Supplementary Figure 1). Viruses from the 2 importations were assigned to 2 separate genetic clusters (isolates within a cluster share >95% VP1 sequence identity), B2D1B (first importation), and B2D1A (second importation). We resolved 9 separate lineage groups (roughly corresponding to chains of transmission) in the DRC within the 2 clusters, which we designated 2006-1 to 2010-6 according to the dates of specimen collection for the earliest isolate of each lineage group (Table 1). Isolates of the different DRC lineage groups were color coded and mapped according to the location where onset of paralysis of the corresponding case occurred (Figure 3).
Table 1.
Independent Wild Poliovirus Type 1 (WPV1) Lineage Groups, Democratic Republic of the Congo (DRC), 2006–2011
| Lineage Group | WPV1 Isolates, Total No. | Latest Isolate Specimen Date | Importation Source | DRC Province(s) |
|---|---|---|---|---|
| 2006–1 | 12 | 17 May 2007 | Angola | BCG, BDD, EQT |
| 2006–2 | 5 | 18 Aug 2006 | Angola | KOC |
| 2006–3 | 41 | 13 Aug 2008 | Angola | EQT, SKV, ORT, NKV |
| 2010–1 | 23 | 5 Jan 2012a | DRC | KAT, MAN |
| 2010–2 | 1 | 18 Jul 2010 | Angola | KOC |
| 2010–3 | 1 | 24 Nov 2010 | Angola | BCG |
| 2010–4 | 63 | 24 Oct 2011 | Angola/Congo | BCG, KIN, BDD |
| 2010–5 | 79 | 16 May 2011 | Angola | KOC, BDD, KIN |
| 2010–6 | 25 | 3 Feb 2011 | Angola | KOC, BDD |
Abbreviations: BCG, Bas-Congo; BDD, Bandundu; EQT, Equateur; KAT, Katanga; KIN, Kinshasa; KOC, Kasai Occidental; MAN, Maniema; NKV, North Kivu; ORT, Orientale; SKV, South Kivu.
a Case onset date: 20 December 2011.
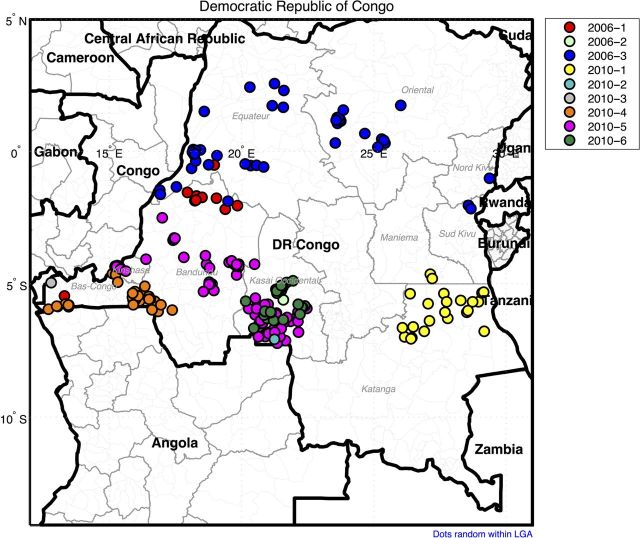
Figure 3.
Map of the Democratic Republic of the Congo, showing province and district boundaries. Locations of acute flaccid paralysis cases found to be associated with each separate wild poliovirus type 1 lineage group are shown in color-coded circles.
The dates of divergence of the 2 clusters and their component lineage groups can be estimated from the location of the nodes in the MCC trees (Supplementary Figure 1 and Figure 2).
Spread of the Imported WPV1 in the DRC
The first WPV1 case was reported in the southern province of Bas-Congo (BCG), with an onset date of 27 February 2006, and the last WPV1 case was reported in the eastern province of Maniema (MAN), with an onset date of 20 December 2011. A total of 252 cases associated with WPV1 isolates were confirmed in the DRC during 2006–2011: 13 cases in 2006, 41 in 2007, 4 in 2008, 0 in 2009, 100 in 2010, and 93 in 2011. The first importation (cluster B2D1B) was associated with 81 cases in the DRC, and the second importation (cluster B2D1A) was associated with 169 cases. Most of the reported cases associated with the first importation were detected in the northern provinces of Equateur and Oriental, the northern districts of Bandundu (BDD), and the eastern provinces of Nord Kivu, Sud Kivu, Katanga (KAT), and MAN (Figure 3). Reported cases associated with the second importation were from the primarily southern provinces bordering Angola (Kasai Occidental [KOC], BDD, BCG, and KAT), as well as Kinshasa.
There was frequent bidirectional cross-border transmission between Angola and the DRC (Supplementary Figure 1 and Figure 2), repeating a pattern seen with the indigenous WPV1 before 2001 (Figure 1; unpublished results). WPV1 from the second importation (lineage group 2010–5) spread northward from Angola or the DRC, sparking a large outbreak in the Congo (at least 445 cases) in 2010–2011 [39] and further cross-border transmission with the DRC (Supplementary Figure 1). WPV1 from cluster B2D1B spread from Angola to Namibia in 2006, causing an outbreak among adults with 19 virologically confirmed cases and 26 polio-compatible cases [40], and WPV1 from lineage group 2010–1 (cluster B2D1B) spread to the Central African Republic in 2008, resulting in 3 confirmed polio cases [41].
Individual WPV1 lineage groups tended to cluster geographically, reflecting intense local circulation upon introduction into the underimmunized population. Lineage group 2006–3 was most widely disseminated within the DRC, following major routes of river transport in 5 provinces during its spread. By contrast, lineage group 2010–6 was localized to KOC province (Figure 3). Lineage groups 2010–2 and 2010–3 were represented by single cases from independent importation events from nearby Angola (Figure 3).
Time Line of Importation and Dissemination Events
The MCC trees (Figure 2 and Supplementary Figure 1) were scaled to time based on an assumed VP1 substitution rate at all sites (KT) of 0.011 substitutions per site per year [24]. For clarity, the printed tree (Figure 2) was reconstructed only from sequences of the earliest isolates from each DRC lineage group, sequences of the most closely related preceding Indian isolates, and sequences of closely related isolates from Angola and CNG. The first importation from Uttar Pradesh was estimated to have occurred between November 2003 (95% highest posterior density [HPD], June 2003–March 2004) and December 2004 (95% HPD, August 2004–March 2005), and the second importation was estimated to have occurred between February 2006 (95% HPD, October 2005–June 2006) and April 2008 (95% HPD, October 2007–October 2008; Figure 2). The most recent common infection ancestral to all isolates was estimated to have occurred in December 2002 (95% HPD, January 2002 and December 2003). Unlike the earliest cluster B2D1B isolates (ANG/LUA/05 and ANG/BEN/05), which were from specimens collected within approximately 6 months of the original common African infection, cluster B2D1A viruses had diverged into 5 lineage groups by spring 2010, approximately 15 months after the most recent common African infection (Figure 2). Genetic diversity was greater for cluster B2D1B than for cluster B2D1A, reflecting the prolonged (>5 years) duration of circulation after the first importation from India.
Orphan Lineages
In areas with sensitive surveillance, PV isolates obtained by frequent sampling along a single chain of transmission are closely related (usually >99% VP1 sequence identity among the closest relatives). These closely related viruses are represented on phylogenetic trees as short branch connections between sequences. Long branch connections between isolate sequences indicate missing information. If the virus was imported, the missing information may be recovered from the sequence relationships among viruses from the source reservoir, as illustrated in Figures 1 and 2. However, when no closely related viruses can be found, the sequence gaps are indicative of surveillance gaps, and the long branches on phylogenetic trees represent orphan lineages. For example, the branch connecting IND/UP/06 and the B2D1A cluster isolates represents an orphan lineage, with >3 years of undetected circulation between the most closely linked cases, represented by the isolate pair IND/UP/06 and ANG/LOB/09, and >2 years of circulation between the inferred common ancestor in India and that in Africa (Figure 2). Therefore, the virologic history of that lineage is indeterminate. The most likely explanation for the absence of detection of circulating B2D1A viruses between 2006 and 2010 is surveillance gaps in Africa, because phylogenetic trees of WPV1 isolates from India during the same period had few orphan lineages (J. Deshpande, unpublished results). The frequent occurrence of orphan lineages (indicative of >1 year of undetected WPV1 circulation) within the B2D1B cluster is consistent with other surveillance gaps and suggests that the polio burden in the DRC (and Angola) during 2005–2011 was considerably higher than indicated by the number of virologically confirmed cases.
DISCUSSION
The reestablishment of widespread and prolonged WPV1 transmission in the DRC and Angola following long-range importations highlights the continuing risks of WPV spread until global eradication is achieved and further underscores the need for all countries to maintain high levels of PV vaccine coverage and sensitive surveillance to protect their polio-free status. Both the DRC and Angola had to surmount severe challenges, including armed conflict, to eliminate indigenous WPV transmission in 2001. However, the intensity of immunization [22] and surveillance activities [21] declined in both resource-poor countries. Consequently, immunity gaps widened more rapidly after 2001 than before because of the absence of natural immunity, thereby accounting for the elevated proportion (17%) of cases among patients ≥15 years of age [22].
The widespread dissemination of WPV1 far from the source reservoirs reinforces the point that geographic isolation from WPV-endemic areas does not ensure protection from polio outbreaks in underimmunized populations. Indeed, WPV3 also imported from India circulated in Angola and the DRC during 2008–2009) [17]. However, outbreaks associated with long-range importations are most commonly associated to WPV1 [42, 43], and include: (1) importation from the Middle East into South America, with circulation from approximately 1980 to 1991 [24, 43]; (2) a large outbreak in Tajikistan in 2010, with spread into neighboring countries, from WPV1 originating in Uttar Pradesh [18]; (3) importations from Pakistan to China, in 2011 [44, 45], and to the Middle East, in 2012–2013 [9]; and (4) repeated importation waves (during 2003–2006 and 2008–2013) of WEAF-B genotype virus from Nigeria across the importation belt of Africa [16, 17], reaching as far as Indonesia in 2005 [46]. Although the detected WPV3 importations tend to be of shorter range, WPV3 was imported from Turkey to Finland in 1984 [47] and from India-Netherlands-Canada in 1993 [48, 49]. Moreover, cVDPVs have emerged and spread in many countries with widening immunity gaps, including cVDPV2s in the DRC [50]. The recent outbreaks of WPV1 infection in the DRC underscore the broader risk of WPV and prolonged dissemination from secondary sites, which may persist after WPV circulation had stopped in the source reservoir [38].
Starting in 2010, the DRC raised PV vaccine coverage, targeting high-risk areas with SIAs. In 2011, several SIAs with monovalent OPV1 were implemented in BCG, BDD, KOC, and northern KAT. Surveillance, which was also intensified in tandem with improved vaccine coverage, documented fewer orphan lineages in 2010 and a declining genetic diversity of WPV1 after mid-2010. Because WPV1 remains endemic to northern Nigeria and parts of Pakistan, it is important that resource-poor countries maintain high levels of population immunity and sensitive surveillance until all PV circulation has ceased.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank staff at the Polio Working Group at the National Institute of Communicable Diseases (South Africa), the Centers for Disease Control and Prevention, and the National Institute for Biomedical Research (the DRC) for excellent technical assistance.
Financial support. This work was supported by the Poliomyelitis Research Foundation, South Africa (to N. G.); the National Institutes for Communicable Diseases (to M. V.); the Ministry of Public Health–Democratic Republic of the Congo (to J. J. M.-T. and Y. R.); the Indian Council of Medical Research (to J. D.); and the Centers for Disease Control and Prevention (to J. J., M. P., O. K., and C. B.).
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Assembly. Polio eradication by the year 2000. Geneva: World Health Organization; 1988. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Certification of poliomyelitis eradication—the Americas, 1994. MMWR Morbid Mortal Wkly Rep. 1994;43:720–2. [PubMed] [Google Scholar]
- 3.World Health Organization. Certification of poliomyelitis eradication: Western Pacific Region. Wkly Epidemiol Rec. 2000;75:399–400. [PubMed] [Google Scholar]
- 4.World Health Organization. Certification of poliomyelitis eradication, European region, June 2002. Wkly Epidemiol Rec. 2002;77:221–3. [Google Scholar]
- 5.World Health Organization. Polio-free certification of the World Health Organization South-East Asia Region. http://www.searo.who.int/entity/campaigns/polio-certification/en/ Accessed 10 July 2014. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Apparent global interruption of wild poliovirus type 2 transmission. MMWR Morbid Mortal Wkly Rep. 2001;50:222–4. [PubMed] [Google Scholar]
- 7.World Health Organization. Progress towards eradicating poliomyelitis: India, January 2010–September 2011. Wkly Epidemiol Rec. 2011;86:501–7. [PubMed] [Google Scholar]
- 8.World Health Organization. Progress towards global interruption of wild poliovirus transmission, January 2012–March 2013. Wkly Epidemiol Rec. 2013;88:181–7. [PubMed] [Google Scholar]
- 9.World Health Organization. Progress towards poliomyelitis eradication in Pakistan, January 2012– September 2013. Wkly Epidemiol Rec. 2013;88:501–7. [Google Scholar]
- 10.World Health Organization. Progress towards poliomyelitis eradication: Afghanistan, January 2012–August 2013. Wkly Epidemiol Rec. 2013;88:465–71. [PubMed] [Google Scholar]
- 11.World Health Organization. Progress towards poliomyelitis eradication in Nigeria, January 2012–September 2013. Wkly Epidemiol Rec. 2013;88:545–50. [PubMed] [Google Scholar]
- 12.World Health Organization. Polio in the Syrian Arab Republic -Update. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 13.Centers for Disease Control and Prevention. Notes from the field: outbreak of poliomyelitis—Somalia and Kenya, May 2013. MMWR Morbid Mortal Wkly Rep. 2013;62:484. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Angola, Democratic Republic of Congo, Ethiopia, and Nigeria, January 2000–July 2001. MMWR Morbid Mortal Wkly Rep. 2001;50:826–9. [PubMed] [Google Scholar]
- 15.Tangermann RH, Hull HF, Jafari H, Nkowane B, Everts H, Aylward RB. Eradication of poliomyelitis in countries affected by conflict. Bull World Health Organ. 2000;78:330–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 countries, 2002–2005. MMWR Morbid Mortal Wkly Rep. 2006;55:145–50. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Wild poliovirus type 1 and type 3 importations—15 countries, Africa, 2008–2009. MMWR Morbid Mortal Wkly Rep. 2009;58:357–62. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Outbreaks following wild poliovirus importations—Europe, Africa, and Asia, January 2009–September 2010. MMWR Morbid Mortal Wkly Rep. 2010;59:1393–9. [PubMed] [Google Scholar]
- 19.Kidd S, Goodson JL, Aramburu J, et al. Poliomyelitis outbreaks in Angola genetically linked to India: risk factors and implications for prevention of outbreaks due to wild poliovirus importations. Vaccine. 2011;29:3760–6. doi: 10.1016/j.vaccine.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission—Africa, 2009–2010. MMWR Morbid Mortal Wkly Rep. 2011;60:306–11. [PubMed] [Google Scholar]
- 21.Alleman M, Meyer S, Mulumba A, et al. Improved acute flaccid paralysis surveillance performance in the Democratic Republic of the Congo, 2010–2012. J Infect Dis. 2014;210(suppl 1):S50–61. doi: 10.1093/infdis/jit670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alleman MM, Wannemuehler KA, Weldon WC, et al. Factors contributing to wild poliovirus type 1 outbreaks involving persons aged ≥15 years in the Democratic Republic of Congo, 2010–2011, informed by a pre-outbreak polio immunity assessment. J Infect Dis. 2014;210(suppl 1):S62–73. doi: 10.1093/infdis/jiu282. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Laboratory surveillance for wild and vaccine-derived polioviruses—worldwide, January 2008–June 2009. MMWR Morbid Mortal Wkly Rep. 2009;58:950–4. [PubMed] [Google Scholar]
- 24.Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol. 2008;82:4429–40. doi: 10.1128/JVI.02354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Polio laboratory manual. 4th ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 26.Yang C-F, De L, Holloway BP, Pallansch MA, Kew OM. Detection and identification of vaccine-related polioviruses by the polymerase chain reaction. Virus Res. 1991;20:159–79. doi: 10.1016/0168-1702(91)90107-7. [DOI] [PubMed] [Google Scholar]
- 27.Kilpatrick DR, Nottay B, Yang C-F, et al. Group-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1996;34:2990–6. doi: 10.1128/jcm.34.12.2990-2996.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilpatrick DR, Nottay B, Yang C-F, et al. Serotype-specific identification of polioviruses by PCR using primers containing mixed-base or deoxyinosine residues at positions of codon degeneracy. J Clin Microbiol. 1998;36:352–7. doi: 10.1128/jcm.36.2.352-357.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Avoort HGAM, Hull BP, Hovi T, et al. A comparative study of five methods of intratypic differentiation of polioviruses. J Clin Microbiol. 1995;33:2562–6. doi: 10.1128/jcm.33.10.2562-2566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H-M, Zheng D-P, Zhang L-B, Oberste MS, Pallansch MA, Kew OM. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J Virol. 2000;74:11153–61. doi: 10.1128/jvi.74.23.11153-11161.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearse M, Moir R, Wilson A, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guindon S, Lethiec F, Duroux P, Gascuel O. PHYML Online--a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005;33:W557–9. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond A, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gumede N, Lentsoane O, Burns CC, et al. Emergence of vaccine-derived polioviruses, Democratic Republic of Congo, 2004–2011. Emerg Infect Dis. 2013;19:1583–9. doi: 10.3201/eid1910.130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chezzi C, Blackburn NK, Schoub BD. Molecular epidemiology of type 1 polioviruses from Africa. J Gen Virol. 1997;78:1017–24. doi: 10.1099/0022-1317-78-5-1017. [DOI] [PubMed] [Google Scholar]
- 37.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—India, January 2010–September 2011. MMWR Morbid Mortal Wkly Rep. 2011;60:1482–6. [PubMed] [Google Scholar]
- 39.Patel MK, Konde MK, Didi-Ngossaki BH, et al. An outbreak of wild poliovirus in the Republic of Congo, 2010–2011. Clin Infect Dis. 2012;55:1291–8. doi: 10.1093/cid/cis714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yusuf N, de Wee R, Foster N, et al. Outbreak of type 1 wild poliovirus in adults, Namibia, 2006. J Infect Dis. 2014;210(suppl 1):S353–60. doi: 10.1093/infdis/jiu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gouandjika-Vasilache I, Mazitchi A, Gumede N, et al. Wild poliovirus importation, Central African Republic. Emerg Infect Dis. 2013;19:1012–3. doi: 10.3201/eid1906.121821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patriarca PA, Sutter RW, Oostvogel PM. Outbreaks of paralytic poliomyelitis, 1976–1995. J Infect Dis. 1997;175(suppl 1):S165–72. doi: 10.1093/infdis/175.supplement_1.s165. [DOI] [PubMed] [Google Scholar]
- 43.Kew OM, Mulders MN, Lipskaya GY, da Silva EE, Pallansch MA. Molecular epidemiology of polioviruses. Semin Virol. 1995;6:401–14. [Google Scholar]
- 44.Centers for Disease Control and Prevention. Progress toward interruption of wild poliovirus transmission—worldwide, January 2011–March 2012. MMWR Morbid Mortal Wkly Rep. 2012;61:353–7. [PubMed] [Google Scholar]
- 45.Luo H-M, Zhang Y, Wang X-Q, et al. Identification and control of a poliomyelitis outbreak in Xinjiang, China. New Engl J Med. 2013;369:1981–90. doi: 10.1056/NEJMoa1303368. [DOI] [PubMed] [Google Scholar]
- 46.Estívariz C, Watkins MA, Handoko D, et al. A large vaccine-derived poliovirus outbreak on Madura Island—Indonesia, 2005. J Infect Dis. 2008;197:347–54. doi: 10.1086/525049. [DOI] [PubMed] [Google Scholar]
- 47.Pöyry T, Kinnunen L, Kapsenberg J, Kew O, Hovi T. Type 3 poliovirus /Finland/1984 is genetically related to common Mediterranean strains. J Gen Virol. 1990;71:2535–41. doi: 10.1099/0022-1317-71-11-2535. [DOI] [PubMed] [Google Scholar]
- 48.Oostvogel PM, van Wijngaarden JK, van der Avoort HGAM, et al. Poliomyelitis outbreak in an unvaccinated community in the Netherlands. Lancet. 1994;344:665–70. doi: 10.1016/s0140-6736(94)92091-5. [DOI] [PubMed] [Google Scholar]
- 49.Mulders MN, van Loon AM, van der Avoort HGAM, et al. Molecular characterization of the wild poliovirus type 3 epidemic in the Netherlands (1992–93) J Clin Microbiol. 1995;33:3252–6. doi: 10.1128/jcm.33.12.3252-3256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—worldwide, April 2011–June 2012. MMWR Morbid Mortal Wkly Rep. 2012;61:741–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.