Abstract
Introduction.
The geriatric functional measures and syndromes collected 5 years apart in Waves 1 and 2 of the National Social Life, Health, and Aging Project (NSHAP) data set included: difficulty with activities of daily living and instrumental activities of daily living, the timed up and go, a 3-m timed walk, repeated chair stands, self-reported physical activity, accelerometry-assessed (in)activity, falls, fractures, and frailty. The purpose of this paper was to describe the data collection methods and report preliminary population estimates for each measures.
Method.
Frequencies, means, or medians were estimated for each measure stratified by age and gender, using the age-eligible samples in Wave 1 (n = 3,005) and Wave 2 (n = 3,196). An adapted phenotypic frailty scale was constructed in the sample common to both waves (n = 2,261). Changes over 5 years were reported for four measures common to both waves.
Results.
The functional measures worsened with age (p < .001). The syndromes were more prevalent with age except “all fractures” (p value range < .001–.03). Functional measures were worse among females than males except chair stand performance and the accelerometry-assessed (in)activity measures (p value range < .001–.01). The syndromes were more common among females than males except Wave 2 falls and Wave 2 hip fractures (p value range < .001–.03). Changes from Wave 1 to 2 revealed 11.5%–25.2% of individuals reported better health and 21.3%–44.7% reported worse health.
Discussion.
The NSHAP provides a comprehensive assessment of geriatric health. Our findings are consistent with the literature and support the construct of the study measures.
Key Words: Accelerometry, Falls, Fracture, Frailty, Functional assessment, Gait, Older adults, Physical activity.
Evaluating functional status and geriatric syndromes are critical to the health assessment of older adults and are often used to help predict their subsequent health outcomes. Both subjective and objective evaluations of geriatric functional measures (GFM) and geriatric syndromes (GS) exist. These measures may be used individually or collectively in indices. Older adults constitute a rapidly growing and increasingly heterogeneous population (Engelman, Canudas-Romo, & Agree, 2010), and accurate assessments of their functional status and the presence of geriatric syndromes are essential for efficient delivery of high-quality and cost-effective care. The utility and application of prognostic tests has, therefore, become a central issue in efforts to individualize and optimize medical care.
To inform these efforts, this paper describes several key functional and geriatric syndrome measures collected in Wave 1 (W1) and Wave 2 (W2) of the National Social Life, Health, and Aging Project (NSHAP). The GFM collected in W1 and/or W2 of the NSHAP data set include disability in activities of daily living (ADL) or instrumental ADL (IADL), timed up and go (TUG), timed walk (TW), timed chair stands (TCS), and both self-report and accelerometry-measured physical activity (Figure 1). The GS measured in W1 and W2 of NSHAP includes a history of falls, prior fracture, incontinence, depression, cognitive impairment, and pain (Figure 1). We also propose a frailty scale based on Fried’s frailty phenotype utilizing NSHAP’s available measures for use in W2. For each GFM and GS, excluding incontinence, depression, cognitive impairment, and pain which are presented in other papers in this issue (citation to follow), we describe the data collection and preliminary findings of the NSHAP by age and gender subgroups. We also report the change in these measures between W1 and W2 for the sample included in both waves. We conclude by reviewing some considerations for data use in future studies.
Figure 1.
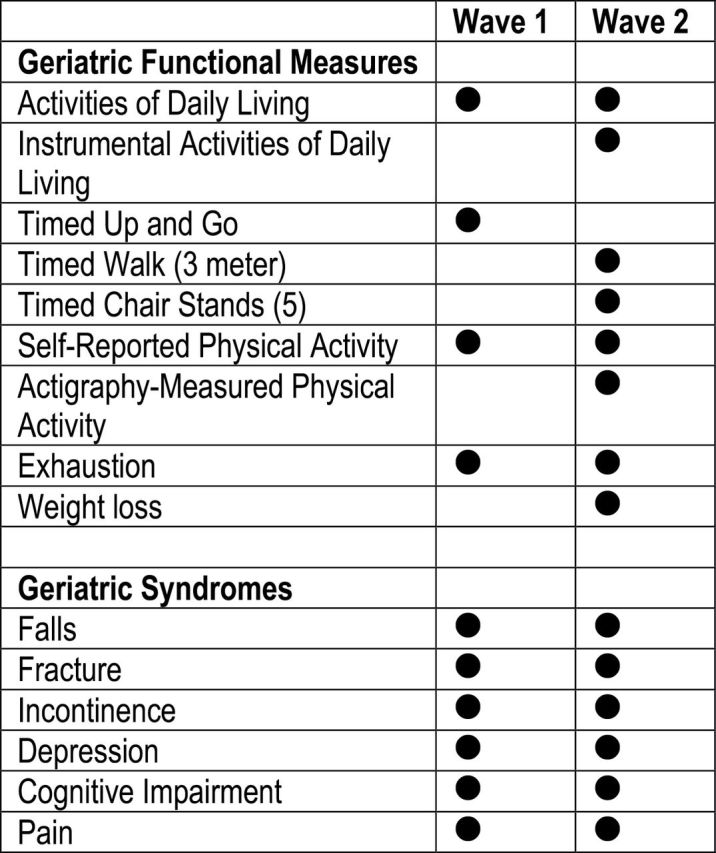
NSHAP geriatric functional measures and geriatric syndromes. NSHAP = National Social Life, Health, and Aging Project.
Method
NSHAP W1 and W2 Overview
NSHAP was designed to evaluate the health and well-being of older adults within the context of their romantic partners and social context. The NSHAP data set includes survey and biomeasure data collected from a nationally representative sample of community-dwelling older adults. Baseline data (W1) were collected in 2005–2006 (Waite, L. J., Laumann, E. O., Levinson, W., Lindau, S. T., & O’Muircheartaigh, C. A. National Social Life, Health, and Aging Project (NSHAP): Wave 1. ICPSR20541-v6. Ann arbor, MI: Inter-university Consortium for Political and Social Research [distributor], April 30, 2014. doi:10.3886/ICPSR20541.v6); 5-year follow-up data (W2) were collected in 2010–2011 (Waite, L. J., Cagney, K., Dale, W., Huang, E., Laumann, E. O., McClintock, M., O’Muircheartaigh, C. A., Schumm, L. P., and Cornwell, B. National Social Life, Health, and Aging Project (NSHAP): Wave 2 and Partner Data Collection. ICPSR34921-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], April 29, 2014. doi:10.3886/ICPSR34921.v1). All assessments were conducted in the home by trained NORC interviewers. In W1, 3,005 adults between the ages of 57 and 85 were interviewed. These W1 respondents were reinterviewed between 2010 and 2011 with their coresident spouses or partners in addition to newly recruited respondents and partners, for a total of 3,377 completed interviews; however, only a subset of partners were considered “age-eligible” and fell between the ages of 62 and 91 (O’Muirchaigtaigh et al., this issue). Excluding age-ineligible respondents, the total W2 sample was 3,196. Of the total age-eligible sample interviewed in W1 and W2, 2,261 individuals were included in both W1 and W2 assessments. Respondents were selected using a multistage area probability design, with oversampling among men and minorities. The detailed sampling design, respondent recruitment, and study methods have been reported elsewhere (O’Muircheartaigh, Eckman, & Smith, 2009).
W1 and W2 Evaluations
The W1 data collection contained two main parts: (a) the 1.5-hr in-person computer-assisted interview (CAPI) with a core biomeasure assessment, and (b) a leave-behind questionnaire (LBQ), a paper-and-pencil questionnaire completed by the respondent on their own time. In addition to these key portions, there were six substudies containing various combinations of additional questions and additional biomeasures. W1 respondents were randomly assigned a priori to complete one of six evaluation pathways which determined: (a) which additional questions and biomeasures were to be administered and (b) whether the additional questions were to be given in the CAPI or in the LBQ. These pathways have been previously described (Smith et al., 2009).
In W2, all respondents were also administered a CAPI with a core biomeasure assessment and LBQ. W2 had three additional biomeasure substudies which were administered in a subset of respondents following random assignment to an interview path (Jaszczak et al., this issue). In addition, a randomized subset of the W2 sample (n = 738) participated in an Actigraph Study which included the accelerometry protocol and a separate self-report sleep activity log. W2 pathways are described in detail elsewhere in this journal issue (Jaszczak et al., this issue).
Functional Assessment
ADL and IADL.
Difficulty with ADL and IADL were assessed by self-report in W1 and W2 in the CAPI portion of the interview. All W1 and W2 participants were asked to report degree of difficulty completing the following ADL activities: (a) walking one block, (b) walking across a room, (c) dressing, including putting on shoes and socks, (d) bathing or showering, (e) eating, such as cutting up food, (f) getting in or out of bed, and (g) using the toilet, including getting up and down. All W1 participants were asked to report degree of difficulty completing the following IADL activities: (a) driving a car during the day and (b) driving a car during the night. Only W2 participants were asked to report degree of difficulty completing the IADL activities: (a) preparing meals, (b) taking medications, (c) managing money such as writing checks and keeping track of bills, (d) shopping for groceries, (e) performing light housework such as dishes, light vacuuming, or dusting, (f) using a telephone, (g) driving a car during the day, and (h) driving a car during the night. Answer options for all ADL/IADL questions in both waves included (a) no difficulty, (b) some difficulty, (c) much difficulty, or (d) unable to do. In W2, interviewers also recorded whether the respondent stated they have never previously done the IADL.
For the current analysis, we created an ADL scale for both W1 and W2 and an IADL scale for W2 to measure functional ability. To create the scales, we assigned one point for each activity that was reportedly done without difficulty. We summed the points for each activity to give a total score. For the ADL scale, we included the following activities: walking a block, walking across the room, dressing, bathing, eating, transferring in and out of bed, and toileting. For the IADL scale, we included meal preparation, money management, shopping, light housework, medication administration, telephone use, driving during the day, and driving during the night. The ADL scales ranged from 0–7, a score of 7 indicating the best function. The IADL scale ranged from 0–8, a score of 8 indicating the best function. (Researchers may consider an alternative scale that consolidates the two walking ADL functions. For example, respondents able to “walk a block” should be able to “walk across a room” and could be assigned only 1 point; respondents only able to “walk across a room” could be assigned 0.5 point.)
Timed up and go.
In W1 only, approximately half of the respondents (N = 1,506) were randomly assigned to complete a 3-m TUG test (Podsiadlo & Richardson, 1991) ((Respondents who were wheelchair bound or were unsafe to complete the gait or chair stands exercise were also identified as “unable to do” and considered the group with the poorest functional status for that measure.). Using a chair from the home setting and a 3-m length of string placed on the floor starting at the chair legs and extending directly outward, the interviewer first demonstrated the activity and then asked the respondent to complete the task. Respondents were allowed to use walking assistive devices and were recommended to walk at a “comfortable and safe” pace. Time was recorded at the end of three intervals during the TUG test: (a) starting with respondent seated in the chair and ending when the respondent reached the end of the 3-m string, (b) starting with the respondent turn and ending when the respondent reached the chair, (c) starting with the respondent initiation of sit movement and ending with respondent fully seated. The total time to complete this test was calculated by summing the three intervals. Interviewers recorded any observed gait abnormalities (Gait abnormalities noted include: walked unsteadily; limped, shuffled or dragged a leg; had an unsteady turn; used a cane or walker; or stated the walk was painful). The TUG test was scored using previously published guidelines (Podsiadlo & Richardson, 1991). Respondents completing the task in 10 or fewer seconds were considered to have a “normal” performance; respondents requiring 11–20 s to complete the task were considered to have a “delayed” performance; respondents requiring more than 20 s to complete the task were considered to have an “impaired” performance.
Gait speed.
In W2 only, respondents completed a 3-m TW which was completed two times (Respondents who were wheelchair bound or were unsafe to complete the gait or chair stands exercise were also identified as “unable to do” and considered the group with the poorest functional status for that measure). The TW exercise was derived from the Short Physical Performance Battery (SPPB) (Guralnik et al., 1994). The interviewer first demonstrated the exercise for the respondent. The respondents were asked to stand at the beginning of a 3-m piece of string. When given a cue, they walked the length of the string at their “usual pace.” Walking devices such as a cane or walker were allowed during the task. Interviewers recorded the total time required to complete each walk. Exercise limitations were identified when present (Exercise limitations noted included: there was an equipment problem; the respondent tried but was unable to complete the task; the respondent could not walk unassisted; the walk was not attempted due to respondent or interviewer safety concerns; the respondent could not understand the instructions; or other reasons prevented completion of task). Interviewers also noted any observed gait abnormalities as described above. Scoring of the fastest TW was based on the gait speed score for the SPPB, which originally applied to a 4-m walk (Guralnik et al., 1994) but scoring was later provided for an 8-foot walk (Guralnik et al., 2000): 1 point if the time to walk 3 m was ≥5.7 s, 2 points if 4.1–5.6 s, 3 points if 3.2–4.0 s, and 4 points if ≤3.1 ss (Guralnik et al., 1994).
Chair stands.
TCS were measured in W2 only (Respondents who were wheelchair bound or were unsafe to complete the gait or chair stands exercise were also identified as “unable to do” and considered the group with the poorest functional status for that measure). The TCS exercise was adapted from the SPPB (Guralnik et al., 1994). Respondents were first asked to complete a single chair stand using a chair in the home setting without the use of their arms. The interviewer first demonstrated the activity: feet were planted firmly on the ground leaving approximately one hand’s width of space between the knee and the chair, arms were folded across the chest, and the person attempted to stand keeping the arms folded. At the end of the task, interviewers noted any exercise limitations as described above. If the single chair stand was successful, the respondent was asked to perform the TCS exercise, five serial chair stands, as quickly as possible. The time was recorded after the respondent completely stood up on the fifth stand. Exercise limitations were again identified as previously described. Scoring of the TCS was based on the original TCS score from the SPPB: 1 point if time to complete the exercise was ≥16.7 s, 2 points if the time was between 13.7 and 16.6 s, 3 points if time was between 11.2 and 13.6 s, and 4 points if time was ≤11.1 s (Guralnik et al., 1994).
Physical Activity
NSHAP measured physical activity in two ways: (a) self-report in both waves and (b) accelerometry in Wave 2. Accelerometers have been applied extensively in clinical and research settings to objectively measure physical activity (Cheung, Gray, & Karunanithi, 2011; Kowalski, Rhodes, Naylor, Tuokko, & Macdonald, 2012; Prince et al., 2008). NSHAP used Actiwatch Spectrum accelerometers to evaluate physical activity objectively in a subset of W2 study respondents.
Physical activity measurement: self-report.
In W1, all respondents were asked a single question about their exercise habits: “How often do you participate in physical activity such as walking, dancing, gardening, physical exercise, or sports?” Response options included: (a) 3 or more times per week, (b) 1–2 times per week, (c) 1–3 times per month, (d) less than 1 time per month, (e) never, (f) don’t know, or (g) refused.
In W2, self-reported physical activity was assessed using four questions. All respondents were asked to report their participation in vigorous physical activity with the following question: “On average over the last 12 months, how often have you participated in vigorous physical activity or exercise? By vigorous physical activity, we mean 30 MIN OR MORE of things like sports, exercise classes, heavy housework, or a job that involves physical labor.” Answer choices included (a) 5 or more times per week, (b) 3 or 4 times per week, (c) 1–2 times per week, (d) 1–3 times per month, (e) less than 1 time per month, or (f) never. In the current analysis, respondents reporting “3 or 4 activities per week” or “5 or more activities per week” were combined into a single category to be able to compare it to W1 data.
Additionally in W2, respondents in the Actigraph Study (n = 793 total; n = 738 age eligible) were given a take-home sleep activity log. Respondents were asked about the frequency of their involvement in three intensities of activity. First, “How often do you take part in sports or activities that are vigorous, such as running or jogging, swimming, cycling, aerobics or gym workout, tennis, or digging with a spade or shovel?” Second, “How often do you take part in sports or activities that are moderately energetic, such as gardening, cleaning the car, walking at a moderate pace, dancing, or floor or stretching exercises?” Third, “ How often do you take part in sports or activities that are mildly energetic, such as vacuuming, laundry, or home repairs?” Answer options were the same for each question: (a) more than once a week, (b) once a week, (c) one to three times a month, and (d) hardly ever or never.
Physical activity measurement: actigraphy.
A random sample (n = 738) of the W2 respondents was included in the Actigraph Study. The Actiwatch Spectrum model (Actiwatch Spectrum; Philips Respironics, Andover, MA), a uniaxial, omnidirectional, piezo-electric, waterproof accelerometer worn on the wrist (Respironics, 2013), was used in the NSHAP Actigraph Study to objectively measure sleep and physical activity patterns. It continuously collects acceleration/deceleration data at a rate of 32 Hz; the movements are then averaged in real time over 15-s intervals called “epochs” and recorded as an activity “count.” If no activity occurs during the epoch, such as during sleep or rest, 0 is recorded for that activity count. There are 5,760 activity counts recorded per day. The Actiwatch Spectrum is optimized to collect movement in the 0.35–7.5 Hz range. To help distinguish sleep and awake intervals, the Actiwatch Spectrum model also records ambient light exposure and wearers can record when they go to bed and when they awaken. The Actiwatch Spectrum is 48×37×14mm in size and weighs 30g. Data stored in the accelerometer can be downloaded and analyzed using the Actiware software available from the manufacturer (Respironics, 2013). Once the data are downloaded, rest and wake intervals can be identified using manufacturer-suggested guidelines, cues from respondent recordings, and the light data (Lauderdale et al., this issue). Several commonly used accelerometer measurements, including the rest and wake intervals, are also available in the public NSHAP W2 data set.
Respondents were given instructions during the interview on use of the accelerometer monitors. Then, a copy of the instructions was mailed with the monitors to the participating respondents. Respondents were asked to place the monitors on their nondominant wrist and to wear the monitors for 72hr (not removed during water activities like bathing.) Sleep activity booklets were also provided and respondents were asked to record daily events including date, time arising, number of minutes napped, and time to bed. Respondents also pushed a button on the monitors to indicate when they went to bed and when they got up from bed. After confirming the mailing address, interviewers called respondents to arrange a time of package delivery. After use, the monitors were returned to the project in a prepaid mailing box. When the accelerometers were returned, the data were downloaded using the Actiware software. Preliminary findings are reported for the following measures: mean count over the total wear time, peak count during total wear time, and percent of day spent at rest (count = 0). For each gender/age subgroup, we presented the 95th, 75th, 50th, 25th, and 5th percentile values.
Geriatric Syndromes
Falls.
Respondents’ falls history was collected via self-report in both W1 and W2 of NSHAP. In W1, respondents were first asked whether they had fallen in the past 12 months. If the respondents answered “yes,” they were then asked, “How many times have you fallen in the past 12 months?” Responses were recorded as integers. In W2, all respondents were asked if they had fallen 0, 1, or 2 or more times in the last year in the LBQ. For this analysis, we separately reported the percent of participants in W1 and W2 who reported falling once in the past year and the percent who reported two or more times in the last year.
Fracture.
History of fracture was assessed by self-report in W1 and W2. In W1, respondents were asked in their interview whether they had a fracture or broken a bone after the age of 45. For those answering “yes,” a follow-up question asked respondents to identify which bone was broken (hip, leg, wrist, vertebrae/backbone, or other). If respondents reported “other” bone, they were asked to specify. In W2, an interviewer asked all respondents if a doctor had ever told them they had a hip fracture. In the LBQ, respondents were also asked whether they have had a fracture or broken bone in the past 5 years (e.g., since the W1 survey). Those who responded yes were asked to identify which bone was fractured (hip, leg, wrist, vertebrae/backbone, nose, skull, or other). If respondents reported “other” bone, they were asked to specify. In this analysis, we reported results for the LBQ question.
Frailty.
Frailty measurement. Several proposed models for measuring frailty exist including the accumulated deficits index first proposed by Rockwood et al. (Mitnitski, Mogilner, & Rockwood, 2001), a set of phenotypic criteria proposed by Fried and colleagues (2001), and a physician index proposed by Studenski and colleagues (2004). Several frailty measurements could be constructed using the NSHAP data set. As an example, we proposed adapted frailty phenotypic criteria in W2 based on the original five criteria: (a) unintentional weight loss, (b) weak grip strength, (c) slow gait speed, (d) presence of exhaustion, and (e) low physical activity (Fried et al., 2001).
All five of the criteria were either directly measured or a surrogate measure was available in the subset of W2 respondents included in both waves. Presence of exhaustion was determined using two modified Center for Epidemiologic Studies Depression (CES-D) scale questions (Radloff, 1977). Respondents were asked how often over the last week they felt that everything was an effort and how often they felt that they could not get going. Answer options for both questions included: (a) rarely or none of the time, (b) some of the time, (c) occasionally, or (d) most of the time. A point for exhaustion was assigned to respondents who answered “occasionally” or “most of the time” to either of these questions. Gait speed was measured directly. A point for slow gait speed was given to individuals requiring ≥5.7 s to complete the TW and to individuals who were wheelchair bound or could not complete the exercise safely. Physical activity was assessed using a single self-reported question described above in W2. Respondents were given a point for low physical activity if they reported participating in physical activity “1–3 times per month,” “less than 1 time per month,” or “never.” TCS was used as a surrogate measure for grip strength. Respondents were assigned a point for weakness if they required ≥16.7 s to complete the exercise or were wheelchair bound or could not complete the task safely. Weight loss was calculated by determining the difference in measured weight between W1 and W2 for each respondent. A weight loss point was assigned to individuals losing 10% of their weight or more. A frailty score, ranging 0–5, was created by summing the points for each criteria. A similar but abbreviated frailty scale could be constructed in W1 using self-reported exhaustion, TUG performance in place of gait and chair stands, and self-reported physical activity participation; weight loss was not measured in W1.
Change Over Time
Several GFM and GS measures were available in both waves allowing comparison of trend over time: self-reported physical activity, gait/chair stand evaluation, ADL function, and falls. For these measures, we reported the following changes over time: (a) proportion of respondents who improved, (b) proportion of respondents who stayed the same, and (c) the proportion of respondents who declined over 5 years. In addition, the 5-year incidence was also calculated for the following measures: those reporting ≤3 vigorous activities per month, those declining to the slowest 25th percentile of the sample for gait and chair stand performance, those reporting dependence in at least one ADL, and those reporting two or more falls in the prior year.
Since gait and chair stand function were evaluated together using the TUG in W1 and separately in W2 using the TW and TCS, we summed the fastest walk time from the TW and the time to complete the TCS. Comparing the W1 TUG measure to the combined W2 measure yielded the best correlation factor of all attempted combinations of the variables: 0.29 (Figure 2). To indentify the proportion of the sample who performed better, worse, or stayed the same on their walk and chair stand performance, we calculated the change in quantile among respondents with non-missing values for both measures. To report the incidence of the poorest performers, we identified the proportion of respondents whose time to complete the exercises dropped into the lowest 25th percentile in W2
Figure 2.
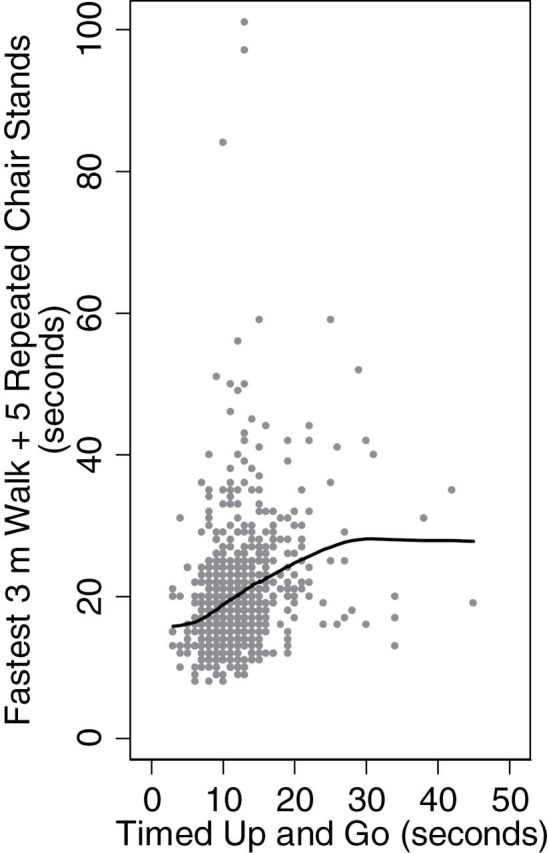
Lowess curve: relationship between timed up and go and timed walk + timed chair stands. Pearson’s correlation coefficient r = .29.
Statistical Analysis
Version 2.1 of the NSHAP data set was used for all analyses. Sample design weights were used in the analyses to account for the complex survey design and to obtain point estimates and standard errors that reflect the national population composition. Proportions, means, or medians were estimated for each functional measure and geriatric syndrome stratified by age/gender subgroups using the age-eligible respondents in W1 and W2. Standard errors are presented for all values. The significance of age and gender was assessed using linear (log TUG, log gait speed, log chair stands, mean activity count, peak activity count, percent time spent at rest) and logistic regression (physical activity: 0–2 vs. 3–4; ADL: 0–6 vs. 7; IADL: 0–7 vs. 8; falls – 0–1 vs. 2+; all fractures: no vs. yes; hip fractures: no vs. yes; osteoporotic fractures: no vs. yes; frailty: 0–2 vs. 3+) as appropriate for each functional measure and geriatric syndrome, though only p values for t tests are reported in the text. Stata 13 (StataCorp, 2011) was used for all analyses except to calculate medians and quantiles. TUG, TW, and TCS medians and quantile summaries of actigraphy data were obtained using the R 2.15 (R Core) (R Development Core Team, 2012) svyquantile function available in the “survey” package (Lumley, 2004, 2012).
Results
Functional Assessment
ADLs and IADLs.
Using the ADL scale in W1, mean ADL functional ability was 6.2 (SE = 0.04) for the total sample. ADL function significantly declined with age (p < .001) and was worse among females (p < .001). Therefore, the oldest age groups had the worst ADL function (men = 5.9, SE = 0.2; women = 5.7, SE = 0.1). Using the ADL scale in W2, mean ADL functional ability was 6.2 (SE = 0.05) for the total sample. Difficulty with ADL functions was more common with advancing age (p < .001) and among females (p = .001). The oldest age groups has the worst ADL function (men = 5.7, SE = 0.1; women = 5.7, SE = 0.1). Using the W2 IADL scale, mean IADL functional ability was 7.0 (SE = 0.6) for the total sample. Difficulty with IADL functions was also more common with advancing age (p < .001) and among females (p < .001). The oldest groups had the worst IADL function (men = 6.6, SE = 0.2; women = 6.0, SE = 0.2).
TUG.
In the W1, 1,506 respondents were randomized to complete the TUG test (Table 1). The median time to complete the TUG for the entire sample was 10.8 s (range 3–118 s). TUG performance was significantly slower among women than among men (p = .003) and worsened with age (p < .001). Of the total sample, 7.1% (SE = 1) took greater than 20 s to complete the exercise, whereas 41.3% (SE = 2) completed the exercise within 10 s, the fastest performance category.
Table 1.
NSHAP Wave 1 Geriatric Syndromes and Functional Measures
| Wave 1 | Men | Women | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 57–64 | 65–74 | 75–85 | 57–64 | 65–74 | 75–85 | 57–85 | ||||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | |
| Computer-assisted interview | n = 528 | n = 547 | n = 379 | n = 492 | n = 545 | n = 514 | n = 3,005 | |||||||
| Regulara physical activity—self-reportb | ||||||||||||||
| 3+ times/week | 66 | 1.9 | 69.3 | 2.4 | 69 | 2.4 | 57.7 | 2.6 | 60 | 2 | 55.3 | 3.1 | 62.7 | 1 |
| 1–2 times/week | 17.5 | 1.6 | 14.7 | 1.9 | 13.6 | 2.3 | 17.9 | 1.9 | 15.7 | 1.4 | 13.8 | 2 | 15.9 | 0.7 |
| 1–3 times/month | 5.5 | 1.1 | 5 | 1 | 3.5 | 1 | 8.3 | 1.4 | 6.4 | 1.4 | 7.6 | 0.9 | 6.2 | 0.4 |
| < 1 time/month | 6.3 | 1.5 | 4.5 | 0.9 | 5.4 | 1.3 | 8.6 | 1.3 | 7.6 | 1.3 | 5.7 | 1.1 | 6.5 | 0.6 |
| Never | 4.7 | 0.9 | 6.5 | 1.1 | 8.6 | 1.5 | 7.4 | 1.2 | 10.2 | 1.4 | 17.6 | 2.2 | 8.7 | 0.6 |
| Activites of Daily Living Function scale | ||||||||||||||
| Mean (range) | 6.4 | 0.1 | 6.4 | 0.06 | 5.9 | 0.2 | 6.2 | 0.07 | 6.2 | 0.08 | 5.7 | 0.1 | 6.2 | 0.04 |
| Leave-behind questionnairec | n = 433 | n = 465 | n = 310 | n = 423 | n = 463 | n = 430 | n = 2,524 | |||||||
| Fallsb | ||||||||||||||
| 1 Fall only, 12 months | 5.5 | 1 | 7.1 | 1.4 | 10.6 | 1.9 | 9.1 | 1.6 | 14.5 | 1.8 | 11.6 | 1.9 | 9.4 | 0.6 |
| 2+ Falls, 12 months | 7.4 | 1.8 | 12 | 2 | 11.5 | 2.3 | 12.9 | 2.1 | 12 | 1.7 | 14.7 | 2 | 11.5 | 0.8 |
| Fracture | ||||||||||||||
| All fracture (after 45)b | 16.3 | 1.9 | 15 | 1.7 | 23 | 2.3 | 21.3 | 2.1 | 29 | 2.5 | 39.7 | 3.2 | 23.1 | 0.9 |
| Hip fracture (after 45)b | 0.9 | 0.5 | 1.7 | 0.8 | 0.9 | 0.8 | 0.2 | 0.2 | 0.6 | 0.4 | 3.5 | 1.3 | 1.2 | 0.3 |
| Osteoporotic fractures (vertebral, hip, wrist; after 45) | 2.8 | 0.8 | 3.4 | 0.9 | 6.3 | 1.4 | 5.5 | 1.5 | 6.6 | 1.4 | 13.8 | 2.6 | 5.9 | 0.6 |
| Biomeasure modulesc | n = 275 | n = 280 | n = 192 | n = 253 | n = 256 | n = 250 | n = 1,506 | |||||||
| Gaitb | ||||||||||||||
| Median time to complete get up and go in seconds (range)b | 9.4 | (3–104) | 10.4 | (4–34) | 12.1 | (5–48) | 10.6 | (3–58) | 11.2 | (3–118) | 12.7 | (4–90) | 10.8 | (3–118) |
| Unable to complete get up and go (wheelchair bound or tried but unable)b | 0.9 | 0.6 | 0.2 | 0.2 | 2.9 | 1.4 | 1 | 0.7 | 0.7 | 0.5 | 2.4 | 1.2 | 1.2 | 0.4 |
| Time to complete get up and go = ≥20 s | 3.6 | 1.1 | 6.2 | 1.4 | 5.6 | 2 | 6.2 | 2.1 | 9.4 | 1.8 | 14 | 2.8 | 7.1 | 1 |
| Time to complete get up and go = >10 to <20 s | 36.5 | 3.3 | 48.3 | 3 | 64.4 | 3.9 | 51.3 | 4 | 52.8 | 4.4 | 59.3 | 4.6 | 50.4 | 1.9 |
| Time to complete get up and go = ≤10 s | 59.1 | 3.8 | 45.4 | 3.3 | 27.1 | 3.7 | 41.5 | 3.9 | 37.1 | 4.3 | 24.4 | 4.1 | 41.3 | 2 |
Notes. LBQ = leave-behind questionnaire; NSHAP = National Social Life, Health, and Aging Project.
aRegular exercise (walking, dancing, gardening, physical exercise).
bW1 regular physical activity missing = 5, W1 falls missing = 138, W1 all fracture missing = 112, W1 hip fracture missing = 158, W1 timed get up and go missing = 140.
cW1 LBQ not returned = 481, W1 did not receive biomeasure testing = 1,499.
Gait speed.
All of the age-eligible respondents in W2 were asked to complete two, 3-m TWs (n = 3,196). One walk time was recorded as 0 s. Since 0 s is not physiologically possible, this value was recoded as missing. Excluding this respondent, the median gait speed for the total sample was 4.1 s (range 2–60 s). Gait speed significantly declined with age (p < .001) and was generally slower among women (p = .01). Of the faster of the two walks, approximately 29.6% (SE = 1.5) of the age-eligible sample took 5.7 s or longer to walk 3 m, whereas 16.2% (SE = 1.2) were able to walk the same distance in less than or equal to 3.1 s.
Chair stands.
All of the W2 age-eligible respondents were asked to complete the TCS (n = 3,196). One TCS time was recorded as 1 s. Since 1 s is not physiologically possible, this value was recoded as missing. The median time to complete the five repeated chair stands was 12.6 s (range 1–76 s). Time to complete the TCS significantly increased with age (p < .001) and was generally higher among women though this was not a significant trend (p = .09). In this sample, 20% (SE = 1.4) took 16.7 s or longer to complete the exercise, whereas 29.1% (SE = 1.6) completed the exercise in ≤ 11.1 s, the fastest category.
Physical activity.
In W1, 62.7% (SE = 1) of respondents reported participating in physical activity three or more times per week, a percentage thought to be much higher than actual physical activity participation. Frequency of reported physical activity declined with age (p = .02) and was lower among females (p < .001). Of the 3,196 age-eligible respondents in W2, 41.9% (SE = 1.3) reported participating in vigorous activities three or more times per week. Frequency of reported physical activity declined with age (p < .001) and was lower among females (p < .001). The variation in reported activities between W1 and W2 is likely due, in part, to differences in question wording.
Actigraphy data was collected in 793 W2 respondents; only 738 of them were age-eligible (Table 3). The mean activity count and the peak activity count significantly declined with age (p < .001 for both), while percentage of time spent at rest increased with age (p = .005). The mean activity count was significantly higher among females than males (p < .001); peak activity count and percentage of time spent at rest did not vary by gender (peak activity count p = .59, percentage time spent at rest p = .26). The 5th percentile of the mean activity count for this sample was 16.3 counts (SE = 0.7); the 95th percentile of the mean activity count was 63.0 counts per day (SE = 2.4). The median percent of time spent at rest was 50.2% (SE = 0.9), but this measure varied widely: its 5th percentile was 38% of the day (SE = 0.9), while its 95th percentile was 65.2% of the day (SE = 0.7).
Table 3.
NSHAP Actigraph Study Functional Measures
| Wave 2 age eligible participants | Men | Women | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 62–69 | 70–79 | 80–90a | 62–69 | 70–79 | 80–90* | 62–90 | ||||||||
| Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE | |
| Accelerometry Study | n = 137 | n = 128 | n = 82 | n = 169 | n = 147 | n = 75 | n = 738 | |||||||
| Distribution of mean activity over entire wear time (counts) | ||||||||||||||
| 5th percentile | 16.7 | 2.8 | 14.0 | 2.4 | 12.5 | 3.9 | 20.0 | 2.1 | 15.9 | 1.4 | 11.9 | 1.7 | 16.3 | 0.7 |
| 25th percentile | 26.4 | 1.0 | 22.1 | 1.6 | 20.4 | 1.0 | 31.3 | 1.9 | 22.3 | 1.9 | 21.1 | 1.5 | 25.1 | 0.8 |
| 50th percentile | 33.4 | 1.1 | 30.8 | 1.9 | 24.6 | 1.6 | 41.0 | 1.7 | 34.3 | 2.6 | 26.9 | 2.5 | 33.3 | 0.8 |
| 75th percentile | 40.8 | 2.4 | 37.8 | 2.0 | 30.7 | 2.5 | 49.8 | 1.7 | 47.1 | 3.0 | 36.4 | 3.2 | 42.8 | 1.4 |
| 95th percentile | 64.2 | 7.0 | 46.3 | 4.0 | 41.5 | 8.1 | 64.7 | 3.3 | 64.0 | 20.1 | 50.3 | 5.3 | 63.0 | 2.4 |
| Distribution of peak activity over entire wear time (counts) | ||||||||||||||
| 5th percentile | 389.3 | 34.0 | 361.0 | 65.4 | 267.5 | 32.4 | 384.9 | 38.1 | 343.8 | 14.3 | 297.0 | 60.9 | 358.8 | 16.8 |
| 25th percentile | 559.1 | 32.3 | 480.0 | 16.6 | 440.4 | 34.5 | 606.0 | 18.5 | 498.0 | 17.2 | 435.3 | 31.8 | 508.4 | 10.0 |
| 50th percentile | 730.8 | 35.4 | 582.0 | 27.2 | 511.4 | 45.1 | 744.6 | 33.4 | 616.5 | 23.8 | 529.2 | 25.8 | 648.8 | 15.2 |
| 75th percentile | 954.5 | 52.8 | 765.6 | 50.5 | 684.3 | 105.7 | 884.2 | 29.7 | 805.5 | 38.4 | 672.9 | 59.1 | 844.2 | 16.1 |
| 95th percentile | 1,574.1 | 171.0 | 1,160.2 | 126.4 | 1,032.3 | 115.1 | 1,278.2 | 105.9 | 1,181.3 | 116.8 | 958.9 | 263.7 | 1,273.4 | 41.7 |
| Distribution of the percent of time with 0 activity count (%)a | ||||||||||||||
| 5th percentile | 38.7 | 3.3 | 40.9 | 1.1 | 40.0 | 3.7 | 35.8 | 4.9 | 38.5 | 0.5 | 38.9 | 8.3 | 38.0 | 0.9 |
| 25th percentile | 45.4 | 1.3 | 46.4 | 1.4 | 48.4 | 2.5 | 44.2 | 1.5 | 44.0 | 1.5 | 47.5 | 2.6 | 45.2 | 0.6 |
| 50th percentile | 50.3 | 1.0 | 49.6 | 1.4 | 53.2 | 1.2 | 48.7 | 1.0 | 49.5 | 1.1 | 52.9 | 1.3 | 50.2 | 0.5 |
| 75th percentile | 55.0 | 1.1 | 56.8 | 1.3 | 56.8 | 1.1 | 53.3 | 0.7 | 58.0 | 1.0 | 58.5 | 2.0 | 56.2 | 0.6 |
| 95th percentile | 65.1 | 2.7 | 64.7 | 2.6 | 66.4 | 7.8 | 62.5 | 2.4 | 65.8 | 4.1 | 66.9 | 4.2 | 65.2 | 0.7 |
Notes. NSHAP = National Social Life, Health, and Aging Project.
aLower “Proportion of Time with 0 Activity Count” corresponds to higher activity level.
Geriatric Syndromes
Falls.
In W1, 9.4% (SE = 0.6) and 11.5% (SE = 0.8) of the respondents had experienced one and two or more falls, respectively, in the previous year (Table 1). In W2, 17.4% (SE = 0.8) and 12.1% (SE = 0.8) of respondents reported having experienced one and two or more falls, respectively, in the previous year (Table 2). Reporting two or more falls was significantly more common with advancing age (W1 p = .002, W2 p < .001) and among females in W1 (W1 p = .002, W2 p = .06) such that the oldest women were the most likely to have reported two or more falls in both W1 and W2 (W1: 14.7%, SE = 2; W2: 16.7%, SE = 2.1).
Table 2.
NSHAP Wave 2 Geriatric Syndromes and Functional Measures
| Wave 2 age eligible participants | Men | Women | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 62–69 | 70–79 | 80–90a | 62–69 | 70–79 | 80–90a | 62–90 | ||||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | |
| Computer-assisted interview | n = 566 | n = 610 | n = 338 | n = 655 | n = 633 | n = 394 | n = 3,196 | |||||||
| Vigorousb physical activity last 12 months—self-reporta | ||||||||||||||
| 3–5+ times/week | 50 | 3.4 | 48.1 | 2.6 | 31.6 | 2.7 | 42.4 | 2.2 | 38.6 | 2.4 | 28.3 | 3.2 | 41.9 | 1.3 |
| 1–2 times/week | 20 | 2.2 | 14.8 | 2.1 | 16.6 | 2.9 | 18.5 | 1.9 | 13.7 | 2 | 13.9 | 2 | 16.6 | 0.9 |
| 1–3 times/month | 6.7 | 1 | 8.8 | 1.3 | 8.3 | 2.2 | 9.8 | 1.7 | 8.2 | 1.5 | 6.2 | 1.5 | 8.2 | 0.6 |
| <1 time/month | 8.7 | 1.2 | 8.4 | 1.2 | 6.1 | 2.4 | 9.3 | 1.4 | 10 | 1.7 | 9.3 | 1.6 | 8.9 | 0.6 |
| Never | 14.6 | 2.3 | 19.8 | 2.6 | 37.4 | 5.1 | 20 | 2.1 | 29.5 | 2.6 | 42.3 | 3.4 | 24.5 | 1.4 |
| Activities of Daily Living Function scale | ||||||||||||||
| Mean (range) | 6.4 | 0.1 | 6.4 | 0.07 | 5.7 | 0.1 | 6.2 | 0.1 | 6.1 | 0.07 | 5.7 | 0.1 | 6.2 | 0.05 |
| Instrumental Activities of Daily Living Function scale | ||||||||||||||
| Mean (range) | 7.3 | 0.1 | 7.2 | 0.07 | 6.6 | 0.2 | 7.1 | 0.1 | 6.7 | 0.09 | 6.0 | 0.2 | 7 | 0.6 |
| Leave-behind questionnairec | n = 492 | n = 535 | n = 293 | n = 581 | n = 571 | n = 327 | n = 2,799 | |||||||
| Fallsa | ||||||||||||||
| 1 Fall only, 12 months | 12.3 | 1.5 | 16 | 1.7 | 22.9 | 3.1 | 17.5 | 1.8 | 19.3 | 2.7 | 22.8 | 3.1 | 17.4 | 0.8 |
| 2+ Falls, 12 months | 9.3 | 1.7 | 13.5 | 1.8 | 12.7 | 2.6 | 11 | 1.6 | 12.8 | 2.2 | 16.7 | 2.1 | 12.1 | 0.8 |
| Fracture | ||||||||||||||
| All fracture (last 5 years)a | 6.6 | 1.2 | 10.8 | 2.1 | 9.1 | 2.7 | 14.8 | 2 | 19.7 | 2.7 | 13.7 | 2.1 | 12.7 | 1 |
| Hip fracture (last 5 years)a | 0.1 | 0.1 | 2.5 | 1.1 | 2.2 | 1 | 0.4 | 0.2 | 1.6 | 0.5 | 0.6 | 0.3 | 1.1 | 0.2 |
| Osteoporotic fractures (vertebral, hip, wrist; last 5 years) | 0.75 | 0.3 | 3.2 | 1.2 | 4.6 | 2.6 | 3.9 | 1.4 | 7.9 | 1.8 | 4.7 | 1.3 | 3.9 | 0.6 |
| Biomeasure core | n = 566 | n = 610 | n = 338 | n = 655 | n = 633 | n = 394 | n = 3,196 | |||||||
| Gaita | ||||||||||||||
| Median time to complete fastest 3-m walk in seconds (range) | 3.6 | (2–48) | 4.0 | (2–60) | 5.0 | (2–52) | 3.8 | (2–52) | 4.3 | (2–48) | 5.4 | (2–41) | 4.1 | (2–60) |
| Unable to do (wheelchair bound, tried but unable, too unsafe) | 1.5 | 0.6 | 2.4 | 0.9 | 2.8 | 1 | 1.4 | 0.6 | 3.5 | 1.1 | 5.1 | 1.3 | 2.5 | 0.4 |
| Time to complete fastest 3-m walk ≥ 5.7 s | 20.3 | 2.2 | 24.2 | 2.9 | 48.6 | 3.9 | 22.2 | 2.5 | 31.1 | 2.3 | 55.4 | 3.7 | 29.6 | 1.5 |
| Time to complete fastest 3-m walk = 4.1–5.6 s | 15.4 | 1.6 | 25.5 | 3.1 | 20.3 | 2.3 | 21.1 | 2.5 | 25.2 | 2.5 | 19.4 | 2.7 | 21.2 | 1.1 |
| Time to complete fastest 3-m walk = 3.2–4.0 s | 35.9 | 2.5 | 32.4 | 3.5 | 22.7 | 3.2 | 35.2 | 2.4 | 28.2 | 2.1 | 16.9 | 2.6 | 30.5 | 1.1 |
| Time to complete fastest 3-m walk ≤ 3.1 s | 26.9 | 2.4 | 15.4 | 3.2 | 5.8 | 1.2 | 20.1 | 2.1 | 12.1 | 1.9 | 3.3 | 0.7 | 16.2 | 1.2 |
| Chair standsa | ||||||||||||||
| Median time to complete five repeated chair stand in seconds (range) | 11.8 | (5–48) | 12.8 | (1–48) | 13.6 | (5–58) | 12.1 | (3–56) | 12.9 | (4–76) | 14.0 | (5–54) | 12.6 | (1–76) |
| Unable to do (wheelchair bound, tried but unable, too unsafe) | 5.3 | 1.8 | 8 | 1.5 | 23.3 | 3.9 | 6.3 | 1.2 | 10.7 | 2.6 | 22.3 | 2.6 | 10.3 | 1 |
| Time to complete five repeated chair stands ≥ 16.7 s | 15.8 | 1.9 | 20.5 | 3 | 21.8 | 3 | 17.9 | 2.6 | 22.8 | 1.9 | 26 | 4.1 | 20 | 1.4 |
| Time to complete five repeated chair stands = 13.7–16.6 s | 18.6 | 2.4 | 23.1 | 1.8 | 21.1 | 3.2 | 22 | 2.5 | 21.2 | 2.4 | 20.7 | 3.3 | 21.1 | 1 |
| Time to complete five repeated chair stands = 11.2–13.6 s | 22 | 2.4 | 22 | 2.9 | 15.9 | 2.4 | 20.1 | 2.5 | 17.3 | 2.1 | 16.4 | 2.6 | 19.6 | 1.2 |
| Time to complete five repeated chair stands ≤ 11.1 s | 38.3 | 3.4 | 26.3 | 2.4 | 17.9 | 3 | 33.9 | 3 | 28 | 2.7 | 14.6 | 3.2 | 29.1 | 1.6 |
Notes. LBQ = leave-behind questionnaire; NSHAP = National Social Life, Health, and Aging Project.
aW2 vigorous physical activity missing = 4, W2 falls missing = 102, W2 all fracture missing = 217, W2 3-m gait missing = 127, W2 chair stands missing = 215, W2 hip fracture missing = 216.
bVigorous activities (sports, exercise, heavy housework, physical labor).
cW2 LBQ not returned = 397.
Fractures.
Prevalence of all fractures after the age of 45 was 23.1% (SE = 0.9) in the entire W1 group; it was higher among females than males (p < .001) in all age groups and increased with age (p < .001) (Table 1). Frequency of reported osteoporotic fractures (hip, vertebral, wrist) was 5.9% (SE = 0.6). Overall prevalence of hip fracture since age 45 in W1 was 1.2% (SE = 0.3). In W2, prevalence of all fractures in the prior 5 years in the total sample was 12.7% (SE = 1) (Table 2). Prevalence was significantly higher among women than men (p < .001) and was highest among the 70–79 year olds for both gender groups (women: 19.7%, SE = 2.7; men: 10.9%, SE = 2.1) rather than in the oldest age category (trend with age p = .08), a finding perhaps related to fracture-related institutionalization. In the 5-year interval between W1 and W2, 3.9% (SE = 0.6) of W2 respondents reported having an osteoporotic fracture. In W2, 1.1% (SE = 0.2) reported having a hip fracture since W1. Both hip and osteoporotic fractures were significantly more common with advancing age (hip p < .001, osteoporotic p = .03). Osteoporotic fractures but not hip fractures were significantly more common among females (hip p = .34, osteoporotic p = .03).
Frailty.
All five frailty criteria were calculated in the subsample of W2 respondents who were also included in W1 (n = 2,261) (Table 4). In this group, 11.5% (SE = 1.1) were categorized as having significant weight loss, 29.1% (SE = 1.5) were categorized as having slow gait speed, 28.6 (SE = 1.9) of the W2 respondents were categorized as having slow chair stands, 23.5% (SE = 1.4) were categorized as having exhaustion, and 38.4% (SE = 1.7) were categorized as having low physical activity. Combining these findings into a 5-point frailty scale for W2 respondents classified 1.7% (SE = 0.5), 5.8% (SE = 0.6), and 11.4% (SE = 1.0) as frail (5, 4, and 3 points, respectively); 19.0% (SE = 1.2) and 29.7% (SE = 1.1) as pre-frail (2 and 1 points, respectively); and 33% (SE = 1.8) as nonfrail (0 points). Frail status was significantly associated with older age (p < .001) and female gender (p < .001).
Table 4.
NSHAP Frailty Scale for Wave 2
| WAVE 2, participants in both waves onlya | Men | Women | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 62–69 | 70–79 | 80–90b | 62–69 | 70–79 | 80–90b | 62–90 | ||||||||
| % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | |
| n = 429 | n = 408 | n = 246 | n = 414 | n = 446 | n = 318 | n = 2,261 | ||||||||
| Frailty criteriab | ||||||||||||||
| ≥10% Weight loss over 5 years | 9.0 | 2.5 | 6.3 | 1.3 | 14.6 | 2.9 | 8.0 | 2.1 | 14.0 | 1.7 | 26.8 | 3.1 | 11.5 | 1.1 |
| Slow gait speed: time to complete fastest 3-m walk ≥ 5.7 s or unable to do | 18.8 | 2.8 | 25.7 | 3.0 | 45.7 | 4.5 | 23.0 | 3.0 | 30.6 | 2.4 | 55.4 | 3.6 | 29.1 | 1.5 |
| Slow chair stands: time to complete five repeated chair stands ≥ 16.7 s or unable to do | 20.0 | 2.7 | 27.4 | 2.6 | 42.6 | 4.4 | 24.6 | 3.8 | 30.4 | 2.7 | 44.2 | 4.1 | 28.6 | 1.9 |
| Exhaustion: occasional or moderate difficulty getting going or everything was an effort | 17.8 | 2.7 | 23.1 | 2.8 | 28.6 | 3.7 | 19.0 | 2.2 | 28.0 | 2.9 | 34.5 | 4.2 | 23.5 | 1.4 |
| Low physical activity: ≤ 3 vigorous activities per month | 31.5 | 2.4 | 33.0 | 3.0 | 41.0 | 3.4 | 37.3 | 2.9 | 44.2 | 3.2 | 53.5 | 4.8 | 38.4 | 1.7 |
| Frailty score (0–5) | ||||||||||||||
| 0 Nonfrail | 45.5 | 3.2 | 35.6 | 4.0 | 18.7 | 3.0 | 38.6 | 3.0 | 26.8 | 2.2 | 9.9 | 2.2 | 32.9 | 1.8 |
| 1 Prefrail | 31.4 | 2.9 | 30.2 | 3.2 | 25.8 | 3.9 | 29.6 | 2.5 | 32 | 2.5 | 23.8 | 3.5 | 29.7 | 1.0 |
| 2 Prefrail | 12.3 | 2.2 | 22.0 | 2.1 | 28.0 | 3.2 | 17.7 | 2.4 | 17.7 | 2.2 | 27.1 | 3.7 | 19.0 | 1.2 |
| 3 Frail | 4.8 | 1.2 | 8.2 | 1.7 | 19.1 | 2.8 | 9.7 | 2.0 | 14.9 | 2.5 | 22.9 | 2.8 | 11.4 | 1.0 |
| 4 Frail | 3.6 | 1.1 | 3.5 | 1.1 | 8.4 | 2.3 | 4.1 | 1.1 | 7.5 | 1.2 | 13.7 | 2.2 | 5.8 | 0.6 |
| 5 Frail | 2.4 | 2.3 | 0.5 | 0.4 | 0 | 0 | 0.3 | 0.3 | 1.0 | 0.5 | 2.6 | 1.3 | 1.7 | 0.5 |
Notes. NSHAP = National Social Life, Health, and Aging Project.
aIncludes only respondents with nonmissing answers to all frailty criteria.
bW2 participants in both waves missing = 335.
Functional Change Between Wave 1 and Wave 2
Change in the GFM and GS measures were compared among the common.
W1/W2 study sample. Those who were included in W1 but not W2 were significantly different from those who were included in both waves (Table 5). While the common sample was slightly older (Age: W1 only = 71.2, W1-2 = 72.2, p value = .03), they were more educated (<High school education: W1 only = 28.0%, W1-2 = 15.8%, p value <.001) and had overall better function than the sample included only in Wave 1. They reported more physical activity (3+ activities/week: W1 only = 51.7%, W1-2 = 65.9%, p value <.001), had a higher level of ADL independence (ADL score: W1 only = 5.6, W1-2 = 6.3, p value <.001), fewer had two or more falls in the prior year (≥2 falls: W1 only = 19.4%, W1-2 = 9.5%, p value <.001), and fewer had impaired gait on the TUG at baseline (≥20 s: W1 only = 15.1%, W1-2 = 4.9%, p value <.001).
Table 5.
Comparing Baseline Characteristics of Study Participants Included Only in Wave 1 to Participants Included in Both Waves 1 and 2
| Wave 1 only (n = 744) | Both Wave 1 and Wave 2 (n = 2,261) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SE) | 71.2 (0.42) | 72.2 (0.20) | .03 |
| Female | 49.2% | 52.2% | .19 |
| African American | 11.4% | 9.6% | .35 |
| < High school education | 28.0% | 15.8% | <.001 |
| Regular physical activity—self-report | <.001 | ||
| 3+ times/week | 51.7% | 65.9% | |
| 1–2 times/week | 15.0% | 16.2% | |
| 1–3 times/month | 6.6% | 6.1% | |
| <1 time/month | 9.8% | 5.6% | |
| Never | 17.0% | 6.3% | |
| Activities of Daily Living Function scale | |||
| Mean (SE) | 5.6 (0.09) | 6.3 (0.04) | <.001 |
| Falls | (n = 518)a | (n = 1,868)a | <.001 |
| 0 Falls, 12 months | 70.7% | 81.2% | |
| 1 Fall only, 12 months | 9.9% | 9.3% | |
| 2+ Falls, 12 months | 19.4% | 9.5% | |
| Gait | (n = 312)b | (n = 1,054)b | <0.001 |
| Unable to complete get up and go (wheelchair bound or tried but unable)* | 3.1% | 0.7% | |
| Time to complete get up and go = ≥20 s | 15.1% | 4.9% | |
Notes. aWave 1 missing values = 226, Wave 1 + Wave 2 missing values = 393.
bWave 1 missing values = 432, Wave 1 + Wave 2 missing values = 1,027.
Comparing the change in GFM and GS measures among the common W1/W2 study sample revealed that 44.7% (SE = 1.2) of the respondents reported less physical activity, the gait and chair stand performance rank dropped by 10 percentiles or more in 44.7% (SE = 1.5) of the sample, 21.3% (SE = 1.1) had fewer independent activities, and 22.7% (SE = 1.1) reported more falls at W2 (Table 6). In contrast, 11.5% (SE = 0.8) reported more physical activity, the gait and chair stand performance rank improved by 10 percentiles or more in 25.2% (SE = 1.5) of the sample, 14% (SE = 0.8) reported more independent activities, and 11.6% (SE = 0.9) reported fewer falls in W2. Five-year incidence of low physical activity was 28.6% (SE = 1.1), of those declining to the slowest 25th percentile of gait and chair stand performance was 17.2% (SE = 1.6), of loss of complete independence was 13.6% (SE = 0.9), and of two or more falls in the prior year was 9.2% (SE = 0.9).
Table 6.
Change in Geriatric Functional Measures and Geriatric Syndromes Among Respondents Included in Both Wave 1 and Wave 2
| % | SE | |
|---|---|---|
| Change in W1 physical activity to W2 physical activity | n = 2,254 | |
| Reported less physical activity in W2 | 44.7 | 1.2 |
| Reported the same physical activity in W2 | 43.8 | 1.3 |
| Reported more physical activity in W2 | 11.5 | 0.8 |
| Incidence very low physical activity: ≤3 times/month | 28.6 | 1.1 |
| Change in W1 timed up and go to W2 gait speed + chair standsa | n = 886 | |
| Rank declined by 10 percentiles in W2 | 44.7 | 1.5 |
| Rank within 10 percentiles in W2 | 30.1 | 2.1 |
| Rank improved by 10 percentiles in W2 | 25.2 | 1.5 |
| Incidence dropping to slowest 25th percentile | 17.2 | 1.6 |
| Change in W1 ADL function to W2 ADL functionb | n = 2,246 | |
| Reported fewer independent activities in W2 | 21.3 | 1.1 |
| Reported the same independent activities in W2 | 64.7 | 1.3 |
| Reported more independent activities in W2 | 14 | 0.8 |
| Incidence of loss of complete independence | 13.6 | 0.9 |
| Change in W1 falls to W2 falls | n = 1,642 | |
| Reported more falls in W2 | 22.7 | 1.1 |
| Reported the same falls in W2 | 65.7 | 1.2 |
| Reported fewer falls in W2 | 11.6 | 0.9 |
| Incidence 2+ falls in prior year | 9.2 | 0.9 |
Notes. aSum of fastest timed walk (seconds) and total timed chair stands (seconds).
bADL = activities of daily living.
Discussion
The NSHAP data set provides rich subjective and objective functional data in a large, nationally representative sample of community-dwelling older adults. The consistent application of objective functional measures like TCS, TW, and accelerometry across several studies (e.g., Study of Osteoporotic Fractures; Health, Aging, and Body Composition; Minority Aging Project) allows for comparison between the NSHAP cohort and other large, American cohorts. NSHAP’s baseline and 5-year follow-up data furthermore allow the evaluation of functional status trends over time. Below we highlight several ways in which the NSHAP data could be used in future research and note issues that researchers should consider when using the data.
The NSHAP GFM and GS are appropriate for use as either primary outcome variables or covariates. For example, two or more falls could be used as an outcome in studies investigating fall risk factors. In contrast, studies comparing the size of social networks among subgroups might consider the effect of a prior fall on socialization. Declines in functional status can be considered both a consequence of disease and an indicator of disease burden, depending on the study. Slow gait speed, for example, may occur as a result of worsening heart failure (an outcome of the disease) and can also help identify people with more severe stages of heart failure (disease burden). The NSHAP functional measures allow for these important comparisons in older adult health studies.
Self-reported measures have been used widely among nationally representative datasets given its relative brevity and ease of administration (e.g., National Health and Nutrition Evaluation Survey, Health and Retirement Study). Among older adults—particularly those with cognitive impairment—there is concern regarding accuracy of self-reported data (Kuczmarski, Kuczmarski, & Najjar, 2001; van Uffelen, Heesch, Hill, & Brown, 2011; Yong & Saito, 2012). While NSHAP excluded people with a history of dementia, it is not uncommon for early cognitive impairment to go undiagnosed in the community (Bradford, Kunik, Schulz, Williams, & Singh, 2009) and, indeed, varying levels of cognitive functioning were identified in this population (Kotwal et al., this issue). NSHAP uniquely offers several objective measures in this national data set providing for numerous opportunities to evaluate subjective versus objective survey measurements.
Actigraphy-measured physical activity is a distinctive feature of the NSHAP data set; however, several points should be considered when using the actigraphy data. The Actiwatch Spectrum is one of several Actiwatch models (Actiwatch 64, Actiwatch 2, Actiwatch Spectrum) manufactured by Philips Respironics (Respironics, 2013). Each Actiwatch model has unique features; the Actiwatch 2 and Actiwatch Spectrum have a more durable accelerometer than the Actiwatch 64. Data output between these models, however, is highly correlated (“Equivalence of Activity Recordings and Derived Sleep Statistics: Actiwatch-64, Actiwatch-2, and Actiwatch Spectrum,” 2008). Older Actiwatch models have been used to validate the accelerometer against indirect calorimetry (Chen et al., 2003; Van Remoortel et al., 2012). The output (activity counts) from the Actiwatch models are moderately correlated (r = .53) with minute-to-minute metabolic equivalents (kcal/kg × hr) measured by indirect calorimetry in older, chronic obstructive pulmonary disease adults performing a set of lab-based exercises mimicking typical day-to-day activities (Van Remoortel et al., 2012). Activity counts are also significantly correlated (0.65±0.09) with indirect calorimeter-measured energy expenditure in kilocalories among young to middle-aged, healthy but sedentary women (Chen et al., 2003). The value of individual activity counts can, therefore, be used to help identify periods of higher or lower intensity activity or inactivity.
A few Actiwatch Spectrum limitations are also worth noting. The Actiwatch is worn on the wrist and does not readily detect (a) the increase of intensity when activities are performed on an incline or (b) acceleration when activities are performed on stationary units (i.e., ergometers). For the NSHAP data set, activities such as stair-climbing or stationary bicycle use may be underrepresented. Previous researchers have published an equation to predict kilocalorie energy expenditure from the Actiwatch activity counts (Chen et al., 2003). Predicted energy expenditure is only modestly correlated with measured energy expenditure and typically underestimates measured kilocalories, particularly at higher intensity activity (Chen et al., 2003).
Frailty, defined as a state of increased vulnerability and loss of ability to maintain homeostasis (Rockwood, Fox, Stolee, Robertson, & Beattie, 1994), is an important clinical concept carrying significant prognostic weight, predicting subsequent disability, falls, and mortality. A number of indexes of frailty for older adults have been suggested, each with advantages and disadvantages. While we proposed adapted frailty phenotype criteria, other frailty measures could easily be constructed using the NSHAP data set and studied (Fried et al., 2001; Mitnitski et al., 2001).
The modifications to the frailty phenotype criteria raise several notable considerations. Respondents may be more likely to meet the frailty criteria for exhaustion using the NSHAP alterations to the wording in the CES-D (“occasionally” replaced “occasionally or a moderate amount of time.”). In the Cardiovascular Health Study, Fried and colleagues (2001) noted that 17% of the total sample had self-reported exhaustion, while the NSHAP study found 24.9% in W1 and 24.3% in W2. In addition, depressed respondents may be classified as prefrail using the proposed frailty scale based on exhaustion as a symptom of their depression rather than a symptom of their physical decline.
The TW is not adjusted for height or gender like the original 15-foot walk in the frailty phenotype (Fried et al., 2001). Not accounting for these factors could conceivably overestimate the presence of slow gait speed among those with short stature or among women compared with men. In addition, the W1 and W2 gait exercises differed. Comparing gait performance in W1 to W2 may be best done through change in quantile for each respondent.
Measured weight loss is calculated over the course of the 5-year follow-up from W1 to W2. While the original frailty phenotype criteria used 5% or more of unintentional weight loss over 1 year, extrapolations of this criteria have been applied over longer intervals (Xue, Bandeen-Roche, Varadhan, Zhou, & Fried, 2008). We suggest using 10% or more weight loss as the adapted criteria; however, investigators may have reasons for choosing an alternative cutoff. NSHAP did not distinguish intentional from unintentional weight loss and may consequently overestimate frailty, particularly among the respondents who were actively trying to lose weight during this time frame. However, given the sedentary lifestyle of most U.S. adults (Li et al., 2011) as well as the difficulty sustaining weight loss for 5 years once achieved through lifestyle modifications (Kraschnewski et al., 2010), this type of overestimation is probably rare.
Finally, using chair stands as a surrogate for grip strength may additionally introduce an estimation of balance and proprioception into what should be strictly a sarcopenia measure. Since loss of balance and proprioception have also been shown to correlate with frailty measures, substituting chair stands for grip strength is reasonable (Deshpande, Metter, & Ferrucci, 2010; Martínez-Ramírez et al., 2011).
Studying change over time in the function and geriatric syndrome measures creates a unique opportunity to study, not only decline in health, but also improvement in health as we have shown. Of particular importance in our aging society is studying the social environment predictors of improvement in health. While we attempt to prevent any avoidable circumstances that might precipitate functional loss, some acute events such as hospitalization will inevitably occur among elders. NSHAP can be used to help study the factors that promote function recovery. It should be noted that some of the functional measures were similar but not identical between waves. In particular, W1 used the TUG while W2 used a separate TW and TCS. Caution is strongly advised when comparing these measures across waves. It should also be noted that the sample participants who dropped out of the study after W1 were less educated and more functionally impaired at baseline. Studying trends over time will not reflect this group and should be considered a limitation in future studies.
In conclusion, functional measures are some of the most important indicators of health status and tools for prognosis in older adults. NSHAP provides a rich resource for studying functional status among a nationally-representative sample of older community-dwelling adults. These important clinical measures combined with the detailed data on social networks, disability, cognitive function, medication use, mental health, comorbidity, and mortality provide a key resource for investigating trends in health status among older adults in the United States.
Key Points .
Functional measures are critical to understanding the health status of older adults and helping to determine prognoses.
The NSHAP data set provides rich functional data in a large, nationally representative sample of community dwelling older adults. Measures include timed walk, repeated chair stands, falls, fracture, and accelerometry. Indicators of frailty status are also available.
The NSHAP functional measures combined with the detailed data on social network, disability, cognitive function, medication use, mental health, comorbidity, and mortality collected at baseline and at 5-year follow-up.
Funding
The National Social Life, Health, and Aging Project is supported by the National Institutes of Health, including the National Institute on Aging (R37AG030481; R01AG033903); the Office of Women’s Health Research, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (R01AG021487); and by NORC which was responsible for the data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or NORC.
Author Contributions
M. Huisingh-Scheetz: planned the study, performed statistical analysis, wrote the paper; M. Kocherginsky: performed statistical analysis, revised paper; P. Schumm: planned the study, performed statistical analysis; M. Engelman: planned the study, revised paper; M. McClintock: planned the study, revised the paper; W. Dale: planned the study, revised the paper; E. Magett: planned the study, revised the paper; P. Rush: planned the study, revised the paper; L. Waite: planned the study, revised the paper.
References
- Bradford A., Kunik M. E., Schulz P., Williams S. P., Singh H. (2009). Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Disease and Associated Disorders, 23, 306–314. 10.1097/WAD.0b013e3181a6bebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Y., Acra S. A., Majchrzak K., Donahue C. L., Baker L., Clemens L., … Buchowski M. S. (2003). Predicting energy expenditure of physical activity using hip- and wrist-worn accelerometers. Diabetes Technology & Therapeutics, 5, 1023–1033. 10.1089/152091503322641088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V. H., Gray L., Karunanithi M. (2011). Review of accelerometry for determining daily activity among elderly patients. Archives of Physical Medicine and Rehabilitation, 92, 998–1014. S0003-9993(11)00045-1 [pii] 10.1016/j.apmr.2010.12.040 [DOI] [PubMed] [Google Scholar]
- Deshpande N., Metter E. J., Ferrucci L. (2010). Validity of clinically derived cumulative somatosensory impairment index. Archives of Physical Medicine and Rehabilitation, 91, 226–232. S0003-9993(09)00869-7 [pii] 10.1016/j.apmr.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman M., Canudas-Romo V., Agree E. M. (2010). The implications of increased survivorship for mortality variation in aging populations. Population and Development Review, 36, 511–539 doi:10.1111/j.1728-4457.2010.00344.x [DOI] [PubMed] [Google Scholar]
- Equivalence of Activity Recordings and Derived Sleep Statistics: Actiwatch-64, Actiwatch-2, and Actiwatch Spectrum. (2008). In Respironics P. (Ed.), (pp. 1–4). Koninkligke Philips Electronics N.V. [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., … McBurnie M. A; Cardiovascular Health Study Collaborative Research Group. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 56, M146–M156. [DOI] [PubMed] [Google Scholar]
- Guralnik J. M., Ferrucci L., Pieper C. F., Leveille S. G., Markides K. S., Ostir G. V., … Wallace R. B. (2000). Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 55, M221–M231. [DOI] [PubMed] [Google Scholar]
- Guralnik J. M., Simonsick E. M., Ferrucci L., Glynn R. J., Berkman L. F., Blazer D. G., … Wallace R. B. (1994). A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology, 49, M85–M94. [DOI] [PubMed] [Google Scholar]
- Kowalski K., Rhodes R., Naylor P. J., Tuokko H., Macdonald S. (2012). Direct and indirect measurement of physical activity in older adults: A systematic review of the literature. The International Journal of Behavioral Nutrition and Physical Activity, 9, 148. 1479-5868-9-148 [pii] 10.1186/1479-5868-9-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraschnewski J. L., Boan J., Esposito J., Sherwood N. E., Lehman E. B., Kephart D. K., Sciamanna C. N. (2010). Long-term weight loss maintenance in the United States. International Journal of Obesity (2005), 34, 1644–1654. ijo201094 [pii] 10.1038/ijo.2010.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski M. F., Kuczmarski R. J., Najjar M. (2001). Effects of age on validity of self-reported height, weight, and body mass index: Findings from the Third National Health and Nutrition Examination Survey, 1988-1994. Journal of the American Dietetic Association, 101, 28–34; quiz 35. S0002-8223(01)00008-6 [pii] 10.1016/S0002-8223(01)00008-6 [DOI] [PubMed] [Google Scholar]
- Li C., Balluz L. S., Okoro C. A., Strine T. W., Lin J. M., Town M., … Valluru B; Centers for Disease Control and Prevention (CDC). (2011). Surveillance of certain health behaviors and conditions among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C.: 2002), 60, 1–250. ss6009a1 [pii] [PubMed] [Google Scholar]
- Lumley T. (2004). Analysis of complex survey samples. Journal of Statistical Software, 9, 1–19. [Google Scholar]
- Lumley T. (2012). Survey: analysis of complex survey samples (Version R package version 3.28-2).
- Martínez-Ramírez A., Lecumberri P., Gómez M., Rodriguez-Mañas L., García F. J., Izquierdo M. (2011). Frailty assessment based on wavelet analysis during quiet standing balance test. Journal of Biomechanics, 44, 2213–2220. S0021-9290(11)00442-8 [pii] 10.1016/j.jbiomech.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Mitnitski A. B., Mogilner A. J., Rockwood K. (2001). Accumulation of deficits as a proxy measure of aging. The Scientific World Journal, 1, 323–336. 10.1100/tsw.2001.58 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Muircheartaigh C., Eckman S., Smith S. (2009). Statistical design and estimation for the national social life, health, and aging project. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 64(Suppl. 1), i12–i19. gbp045 [pii] 10.1093/geronb/gbp045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsiadlo D., Richardson S. (1991). The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society, 39, 142–148. [DOI] [PubMed] [Google Scholar]
- Prince S. A., Adamo K. B., Hamel M. E., Hardt J., Connor Gorber S., Tremblay M. (2008). A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. The International Journal of Behavioral Nutrition and Physical Activity, 5, 56. 1479-5868-5-56 [pii] 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2012). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from http://www.R-project.org. [Google Scholar]
- Radloff L. S. (1977). The CES-D Scale: A Self-Report Depression Scale for research in the general population. Applied Psychological Measurement, 1, 385–401 doi:10.1177/014662167700100306 [Google Scholar]
- Respironics P. (2013). Actiwatch. Retrieved February 19, 2013, from http://www.healthcare.philips.com/main/homehealth/sleep/actiwatch/default.wpd#&&/wEXAQUOY3VycmVudFRhYlBhdGgFCUVkdWNhdGlvbrs7D4d8dwFrxbRmM0TsUP60b3xr.
- Rockwood K., Fox R. A., Stolee P., Robertson D., Beattie B. L. (1994). Frailty in elderly people: An evolving concept. CMAJ: Canadian Medical Association Journal = journal de l’Association medicale canadienne, 150, 489–495. [PMC free article] [PubMed] [Google Scholar]
- Smith S., Jaszczak A., Graber J., Lundeen K., Leitsch S., Wargo E., O’Muircheartaigh C. (2009). Instrument development, study design implementation, and survey conduct for the national social life, health, and aging project. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 64(Suppl. 1), i20–i29. gbn013 [pii] 10.1093/geronb/gbn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. (2011). Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. [Google Scholar]
- Studenski S., Hayes R. P., Leibowitz R. Q., Bode R., Lavery L., Walston J., … Perera S. (2004). ). Clinical global impression of change in physical frailty: Development of a measure based on clinical judgment. Journal of the American Geriatrics Society, 52, 1560–1566. 10.1111/j.1532-5415.2004.52423.x [doi] JGS52423 [pii] [DOI] [PubMed] [Google Scholar]
- Van Remoortel H., Raste Y., Louvaris Z., Giavedoni S., Burtin C., Langer D., … Troosters T; PROactive consortium. (2012). Validity of six activity monitors in chronic obstructive pulmonary disease: A comparison with indirect calorimetry. PLoS One, 7, e39198. 10.1371/journal.pone.0039198 [doi] PONE-D-11-24879 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uffelen J. G., Heesch K. C., Hill R. L., Brown W. J. (2011). A qualitative study of older adults’ responses to sitting-time questions: Do we get the information we want? BMC Public Health, 11, 458. 1471-2458-11-458 [pii] 10.1186/1471-2458-11-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q. L., Bandeen-Roche K., Varadhan R., Zhou J., Fried L. P. (2008). Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63, 984–990. 63/9/984 [pii] [DOI] [PubMed] [Google Scholar]
- Yong V., Saito Y. (2012). How accurate are self-reported height, weight, and BMI among community-dwelling elderly Japanese?: Evidence from a national population-based study. Geriatrics & Gerontology International, 12, 247–256. 10.1111/j.1447-0594.2011.00759.x [DOI] [PubMed] [Google Scholar]


