Abstract
Objectives.
We present the novel urine collection method used during in-home interviews of a large population representative of older adults in the United States (aged 62–91, the National Social Life, Health and Aging Project). We also present a novel assay method for accurately measuring urinary peptides oxytocin (OT) and vasopressin (AVP), hormones that regulate social behaviors, stress, and kidney function.
Method.
Respondents in a randomized substudy (N = 1,882) used airtight containers to provide urine specimens that were aliquoted, stored under frozen refrigerant packs and mailed overnight for frozen storage (−80 °C). Assays for OT, AVP, and creatinine, including freeze-thaw cycles, were refined and validated. Weighted values estimated levels in the older U.S. population.
Results.
Older adults had lower OT, but higher AVP, without the marked gender differences seen in young adults. Mild dehydration, indicated by creatinine, specific gravity, acidity, and AVP, produced concentrated urine that interfered with the OT assay, yielding falsely high values (18% of OT). Creatinine levels (≥1.4mg/ml) identified such specimens that were diluted to solve the problem. In contrast, the standard AVP assay was unaffected (97% interpretable) and urine acidity predicted specimens with low OT concentrations. OT and AVP assays tolerated 2 freeze-thaw cycles, making this protocol useful in a variety of field conditions.
Discussion.
These novel protocols yielded interpretable urinary OT and AVP values, with sufficient variation for analyzing their social and physiological associations. The problem of mild dehydration is also likely common in animal field studies, which may also benefit from these collection and assay protocols.
Key Words: Urinary oxytocin assay, Vasopressin, Creatinine, Dehydration, Social behavior, Stress.
Oxytocin (OT) and vasopressin (AVP) are two closely related peptides with well-known physiological functions, including contractile and antidiuretic properties. They play a key role in regulating blood pressure and in facilitating the allostatic response to external stressors (Gimpl & Fahrenholz, 2001; Szczepanska-Sadowska, 2008). Importantly, for the National Social Life and Aging Project (NSHAP), both of these hormones, but in particular OT, have also been shown to play an important role in mediating and modulating social behaviors such as pair-bonding, attachment, sexuality, and care-taking (Reyes & Mateo, 2008; Wismer Fries, Ziegler, Kurian, Jacoris, & Pollak, 2005). The social role of OT and AVP in humans has been examined primarily in small convenience samples due to the special challenges associated with protein hormone measurement.
There is no single ideal experimental protocol for discerning the relationships between these neuropeptides, physiology, and behavior among the everyday lives of people living in their homes. Here, we present a method for gathering data from a large representative population of community-dwelling older adults living in the United States. For reasons detailed below, this is a conservative method, risking failing to detect associations between social peptides, health, and social life. Thus, if analysts do detect such relationships, their work will call for further study in more controlled laboratory settings designed to reveal underlying mechanisms.
Studies of central action of these social peptides, including brain binding and production, require highly invasive measurements of cerebrospinal fluid or post-mortem brain analysis. Animal models are optimal. Novel radiolabeled ligands in positron emission tomography could be developed. In these approaches, the neuroendocrinology is sophisticated and focus on where the peptides are having their immediate effects, but the behavior and physiology is far removed from the natural variation of everyday life and the human subjects who participate are not representative of the larger population.
Measuring peripheral levels of OT and AVP affords better behavioral measurement, yet does not directly measure at the site of action. OT and AVP are released from the posterior pituitary into the blood stream, from where they diffuse in saliva or pool in urine. There is evidence, albeit currently debated, that peripheral release is coordinated with central release of OT and that peripheral measures correlate to endocrine expression in the brain, although this is not necessarily the case for AVP (Carter et al., 2007; Wotjak et al., 1998).
Moreover, peripheral measures pose their own challenges. Neuropeptides are delicate hormones with a short half-life. Blood draws provide both the most acute and most invasive peripheral measurement, and the sample must be immediately treated with an anticoagulant, centrifuged cold, and frozen for storage, impractical in our large survey with field interviewers who are not medical personnel. Saliva is perhaps the least invasive measurement, but quantities of OT are very low, provide the most challenging results, and great care must be taken to immediately freeze the sample to protect the peptides. Although saliva reflects OT released across several minutes, it correlates closely to OT measured in plasma (Hoffman, Brownley, Hamer, & Bulik, 2012).
Urine provides a biological sample that integrates neuropeptide concentrations pooled across a longer time-span of at least 1hr, the time frame of our home interview. Urine is less invasive than blood draws thus lowering the barrier to participation and has less challenging handling requirements since the pH environment of the urine helps to stabilize the protein (Anestis, 2010).
Urinary OT and AVP have been successfully used to measure effects of social interactions on peptide secretion in laboratory settings (Moses & Steciak, 1986; Polito, Goldstein, Sanchez, Cool, & Morris, 2006; Seltzer & Ziegler, 2007; Seltzer, Ziegler, & Pollak, 2010). Urine specimens are therefore a useful noninvasive method of gathering biomeasures during a field study of psychosocial measures and health, especially large-scale population surveys in the home.
However, the short half-life, challenging storage requirements, including immediate and sustained low temperatures pose special challenges for handling in the field. A method to measure OT and AVP in large populations in the field is needed to test and advance the understanding of the social and health properties of these peptides.
Levels of any urinary hormone must be standardized based on creatinine levels to control for variation in urine concentration. Creatinine is secreted at a steady rate from creatine degradation in muscle tissue, and its levels directly reflect urine concentration (Barr et al., 2004; Cuthbertson, 1944). In addition to altered kidney function, the thirst response decreases as individuals age, leading to reduced fluid consumption and greater risk of dehydration among older adults. For example, dehydration is a leading cause of hospitalization for individuals older than 65 years (Lavizzo-Mourey, 1987; Sheehy, Perry, & Cromwell, 1999).
We describe here a field method for collecting urine pooled during highly standardized home interviews of older adults and measuring urinary OT, AVP, and creatinine. In addition to standardizing urine concentration, we show that creatinine, a marker of mild dehydration in this home dwelling population, can be used to solve issues of assay interference. Finally, we determined the effects of two freeze-thaw cycles on the assayed levels of these peptides.
Method
Urine Sample Collection
We established a noninvasive field method for collecting urine and measuring OT, AVP, and creatinine that was successfully used in our sample representative of the US population born between 1920 and 1947, 62 and 91 years of age during the Wave 2 home interview of the National Social Life, Health, and Aging Project (NSHAP; for an overview of all biomarker collection, see O’Doherty et al., 2014 and for sample design and field methods, see Jaszczak et al., 2014). Wave 2 urine specimens were collected from a randomly selected two-thirds of respondents. During a 120-min in-home interview, respondents were asked to describe in detail their social and family relationships, household living arrangements, measures of psychosocial well-being, and reply to a battery of questions detailing respondent health and medication. The standardized interview provided a controlled environment for OT and AVP to pool in respondents’ urine in the context of a deep discussion with the field interviewer of their social relationships, sexuality, stress, and work as well as providing health measures such as body weight and height, strength and mobility, heart rate and blood pressure.
At the same point in the standardized interview, respondents were asked to provide biosamples including a clean-catch urine specimen into a sealable container with a needle port on its cap. As soon as the respondent returned with the urine container, the field interviewer connected Vacutainers to its port creating three aliquots within a sealed system. The aliquots were immediately placed under frozen foam brick refrigerant packs keeping them at 40–50 °F while they were mailed overnight to the McClintock Survey Biomeasures Laboratory and frozen for long-term storage (−80 °C; for detailed methods of urine collection and shipping, see O’Doherty et al., 2014).
To establish feasibility of measuring social peptides in urine, assays were run on urine aliquots from three substudies: Wave 2 pretest (N = 115), the first set of samples collected from Wave 2 respondents (N = 216), and a confirmatory substudy of freeze-thaw cycles and dehydration markers among an additional 18 Wave 2 samples, as follows.
Assay Procedures
OT and AVP were extracted with solid phase extraction (SPE) columns to purify the urine and remove interference with the assay (Seltzer et al., 2010) and assayed with Assay Design ELISA kits, which have been validated for urine as well as blood assays (Oxytocin EIA Kit, #910-153; Enzo Life Sciences, Ann Arbor, MI and Arg8-Vasopressin EIA Kit, #901-017; Enzo Life Sciences, Ann Arbor, MI), per kit instructions as described previously (Bick & Dozier, 2010; Gray, Parkin, & Samms-Vaughan, 2007; Seltzer et al., 2010; Wismer Fries et al., 2005). Creatinine was assayed in microtiter plates as described previously (Ziegler, Scheffler, & Snowdon, 1995)
Variability of mean values across multiple assay plates is measured by the interassay coefficient of variation (CV), while variability of values across duplicates in a single assay is measured by the intraassay CV. Standards and controls were run on each plate of assays. The NSHAP data set provides the plate and duplicate number for analysts wishing to control for plate and duplicate effects to improve the precision of their statistical models.
OT had a 10.99 intraassay and 18.03 interassay CV. AVP had a 2.02 intraassay and 27.65 interassay CV, estimated across multiple low and high control runs over a several month period, values similar to others laboratories (Taylor, Saphire-Bernstein, & Seeman, 2010). Creatinine had an 8.9 interassay and 2.02 intraassay CV (duplicate r = .99, p < .001).
Each hormone assay relies on a standard curve derived from a set of samples that have a standard range of known hormone concentrations and measuring the direct signal being used in the assay, in this case absorbance per unit of measured protein hormone. A curvilinear regression line is plotted through these standard sample data points to determine the standard curve, which is used to determine the unknown concentration of specimen measured based on the absorbance directly measured for that sample. Samples with values not on the standard curve are typically rerun at a different dilution to bring the measures in range.
Specimens from the Wave 2 pretest (N = 115) and first set of samples from Wave 2 respondents (N = 216) were initially assayed for OT and AVP at 444 µl/well dilution and 18% were not on the standard curve. These samples were thawed and reassayed at a 222 µl/well dilution. For AVP, first Wave 2 respondent specimens (N = 216) yielded 87% of AVP values on the standard curve and as a cross-check were reassayed at 222 µl/well dilution, with 97.7% of values now on the standard curve indicating no assay interference. All creatinine values fell on its standard curve.
Freeze-Thaw and Dehydration Marker Study
We then designed a freeze-thaw/dehydration study to verify that the OT assay was not affected by the second freeze-thaw cycle necessitated by reassaying to correct the concentration. We also sought to validate creatinine as a measure of mild or potential dehydration and concentrated urine, since all specimens with OT above the standard curve at first assay also showed high creatinine and AVP levels. To test the mild dehydration hypothesis, we measured AVP, specific gravity, and acidity of urine, all additional markers of concentrated urine.
Day 1: 18 additional specimens were randomly selected from Wave 2 and assayed for OT, AVP, and creatinine, measuring specific gravity, and pH. All specimens for the OT freeze-thaw study were thawed at room temperature, then re-aliquoted into two 1ml and two 0.5ml aliquots—for a total of four aliquots per specimen. One of the 1 and 0.5ml aliquots for each specimen were frozen, while the others were immediately run through SPE tubes (Seltzer et al., 2010). Day 2: the 1 and 0.5ml aliquots frozen the day prior were thawed at room temperature and run through the SPE tubes. Day 3: all four tubes were set up for OT assay, then incubated overnight. Day 4: OT assays were finished and calculated, followed by AVP and creatinine assays. OT was run at both 444 µl/well dilution (using the 1ml aliquot) and 222 µl/well dilution (using the 0.5ml aliquot), with either a single or a double freeze-thaw cycle preceding the assay. The Day 1 specimens experienced one freeze-thaw cycle, while the Day 2 specimens experienced two freeze-thaw cycles.
Duplicate OT values were more highly correlated after a second freeze-thaw cycle: first thaw at 444 µl/well dilution (r = .79, p < .01), first thaw at 222 µl/well dilution (r = .86, p < .001), second thaw and 444 µl/well dilution (r = .93, p < .001), and second thaw and 222 µl/well dilution (r = .96, p < .001). AVP duplicates were highly correlated (r = .94, p < .001) as were creatinine duplicates (r = .99, p < .001).
Across all three substudies, Wave 2 pretest, first Wave 2 respondents, and freeze-thaw/dehydration, a total of 63 of the OT specimens fell above the standard curve and were thawed and diluted for reassay at a 222 µl/well dilution per kit instruction or as described above.
Remaining Wave 2 specimens, for a total of N = 1,838 OT values, and N = 1,882 AVP values, were assayed as described above.
Data Availability
Data for NSHAP Wave 2 are publicly available (NSHAP Wave 2: Waite, Linda J., Kathleen Cagney, William Dale, Elbert Huang, Edward O. Laumann, Martha K. McClintock, Colm A. O’Muircheartaigh, L. Phillip Schumm, and Benjamin Cornwell. National Social Life, Health, and Aging Project (NSHAP): Wave 2 and Partner Data Collection. ICPSR34921-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2014-04-29. doi:10.3886/ICPSR34921.v1.).
Results
Distribution of Valid Creatinine-Corrected Levels of Urinary Peptides
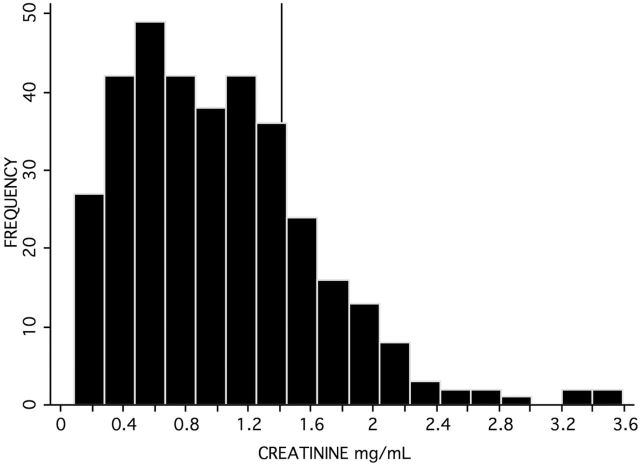
Fully 18% of older adults in the United States had creatinine values indicative of mild dehydration and concentrated urine that interfered with the OT ELISA assay (see Figure 1). To control for such variation in urine concentration and volume at the time of specimen collection, creatinine must always be used to standardize hormone levels across individuals. Creatinine is chosen to standardize urinary values because it is secreted at a steady rate from muscle, and so its level in a urine specimen is a marker for urine concentration. Standardized OT and AVP values are always expressed as their ratio with creatinine in mg/ml.
Figure 1.
Distribution of creatinine levels in all three studies combined (N = 349), with the 1.4mg/ml reference line indicating mild dehydration and concentrated urine requiring dilution to prevent interference with the oxytocin assay.
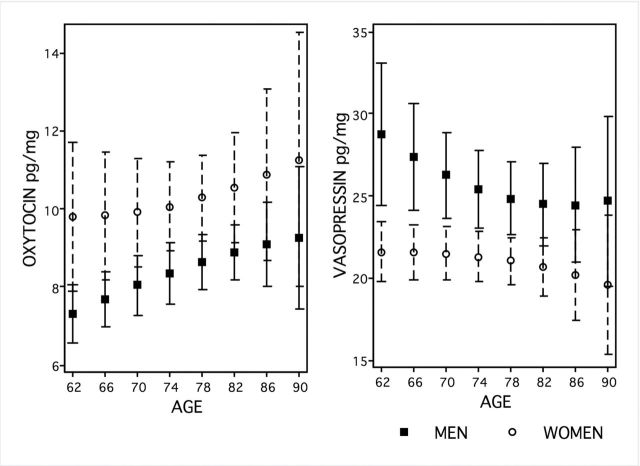
After refining our assay methods (detailed in the following sections), we determined the distributions of urinary OT and AVP (creatinine corrected) characteristic of community-dwelling older adults in the United States (born between 1920 and 1947, aged 62–91). The wide range in each distribution provides the opportunity to detect their associations with physical health and psychosocial variables. As a means of describing the data, we conducted a simple analysis of age and the gender differences expected from differences reported for younger adults (Weisman, Zagoory-Sharon, Schneiderman, Gordon, & Feldman, 2013), revealing interesting patterns warranting further evaluation in a richer model (see Figure 2).
Figure 2.
Adjusted predictions of creatinine-corrected oxytocin (OT) and vasopressin (AVP) during the NSHAP Wave 2 home interview, plotted by age and gender with 95% CI and weighted to represent the U.S. population aged 62–91.
OT was higher at older ages (β = 0.082, t(48) = 2.42, p < .019, multiple linear regression). As in younger adults, postreproductive women appeared graphically to have higher levels than men, but not significantly so in this simple analysis adjusting only for age (β = 7.213, t(48) = 1.31, p < .197). There were also no gender differences in the association of higher chronological age and higher OT levels (β = −0.07, t(48) = −0.96, p < .427). AVP was not associated with either age or gender in this simple analysis. However, as with reproductive aged adults, older men were graphically higher than women.
As an example that richer models are needed to reveal associations with social peptides, such as the expected lower levels of AVP at older ages, we introduced “happiness” as an exemplary psychosocial mood variable (a 5-point Likert style scale ranging from usually unhappy to extremely unhappy, see Payne et al., 2014). The lower values of AVP at older ages evident graphically in Figure 2 were indeed statistically significant (β = −2.153, t(32)= −4.24, p < .001), with neither gender differences overall (β = 16.034, t(32) = 0.23, p < .823) nor gender differences in the negative association between chronological age and AVP levels (β = −0.098, t(32) = −0.10, p < .924). Moreover, AVP was higher at every level of happiness than it was among those who were “usually unhappy”: extremely happy (β = 1.872, t(32) = 2.82, p < .007), very happy (β = 2.242, t(32) = 3.36, p < .002), pretty happy (β = 1.719, t(32) = 3.22, p < .002), and unhappy sometimes (β = 1.849, t(32) = 2.50, p < .016), relative to usually unhappy. There was no significant interaction between happiness and either gender or gender and age.
Valid versus Out of Range Values
Vasopressin.
Almost all AVP values were valid, falling on the linear portion of the standard curve used to convert an assay machine reading into biological levels of hormones. Moreover, although 13% of values were initially above the curve at the 444 µl dilution (high out of range [OOR]), the standard practice of diluting these saturated samples to bring values down onto the standard curve was successful. When diluted by half and assayed at 222 µl of urine per well, only 2.3% of values were above the curve. Moreover, initial and diluted reassayed AVP values significantly correlated (r = .51, p < .01) also demonstrating assay validity.
Oxytocin.
In contrast, 18% of OT values were highly saturated and above the standard curve (Table 1). This high incidence of uninterpretable, highly saturated OT values was replicated across the three substudies, indicating a problem with the OT assay for nearly one-fifth of the samples, as shown by a continuity-corrected homogeneity of proportions test (Pearson χ2(2) = 0.0891, p < .956).
Table 1.
Oxytocin Assay Values After One Freeze-Thaw Cycle
| Group | Uninterpretable values > 225 pg/ml |
|---|---|
| Pretest (N = 115) | 17.4% |
| Wave 2, first 216 samples | 18.5% |
| Freeze-thaw study (N = 18) | 16.7% |
| Total | 18.1% |
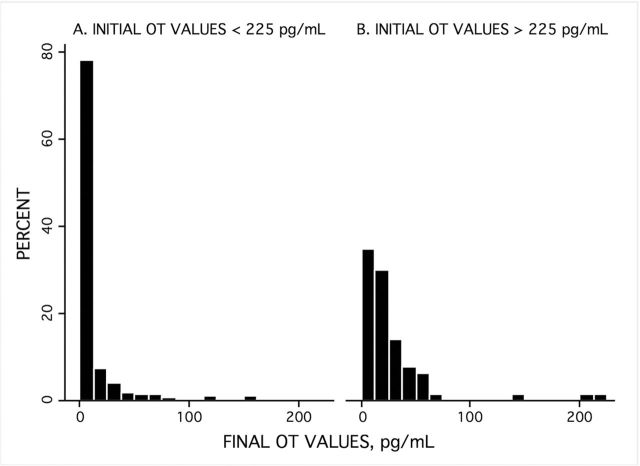
Following the standard practice for adjusting for saturated samples, specimens with OT values above the curve were also diluted by half to bring their OT concentrations down onto the standard curve during reassay. Surprisingly, these specimens did not actually have high OT values but were in the same range as the specimens not needing dilution (Figure 3).
Figure 3.
Final OT value distributions for undiluted specimens that were interpretable initially (<225 pg/ml, A, on the left) and those specimens that were highly saturated and uninterpretable initially (>225 pg/ml, B, on the right; combining all three studies (N = 349). After dilution and reassay, specimens that initially registered as highly saturated values (B) now fell within the same normal range seen in the undiluted interpretable specimens (A), rather than having high values above the range of the standard curve for OT, oxytocin.
In other words, the true values of the specimens were actually on the curve, but appeared, spuriously, to be above the curve when assayed in more concentrated urine. This indicated to us that there were likely other compounds in the concentrated urine that saturated the assay’s OT antibody, yielding inaccurately high values (known as assay interference). Dilution to reduce the concentration of interfering molecules solved this problem.
Effects of Dehydration
Why is the incidence of inaccurate above-curve values so high in our population of older individuals? Our working hypothesis is that mild dehydration typical among this older population yields more concentrated urine, and the molecular composition of this urine binds with this assay’s OT antibody. Barring a urine osmolality test for dehydration, urine concentration can be ascertained through an examination of creatinine, levels of AVP, specific gravity, and acidity (Barr et al., 2004; Eggleton, 1947; Kassirer, 1971). Consistent with our dehydration hypothesis, specimens with higher creatinine were more acidic (r(15) = −.50, p < .04), had higher specific gravity (r(16) = .62, p < .01), and higher AVP (r(16) = .76, p < .0003) (Figure 4).
Figure 4.
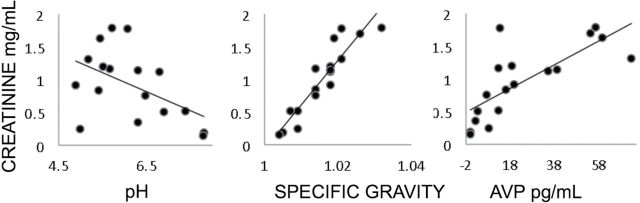
Scatter plots showing correlations between creatinine levels and three dehydration markers: pH (β = 0.29, p < .05), specific gravity (β = 68.1, p < .001), and vasopressin (β = 0.018, p < .001). Data from the freeze-thaw/dehydration study (N = 18, one pH value missing). AVP, vasopressin.
Indeed, creatinine predicted which specimens yield invalid over-saturated assay values, further supporting our hypothesis that concentrated urine interferes with the OT assay. Specimens that initially had OT values above the standard curve also had higher creatinine levels than specimens with OT initially on or below the curve (F(1,343) = 30.67, p < .000, analysis of variance). All three substudies had a similar prevalence of high creatinine indicating mild dehydration (effect of study F(2,343) = 0.58, p < .5621, analysis of variance).
Optimal Cutpoints for Identifying Concentrated Specimens Requiring Dilution
Next, to anticipate this interference problem and reduce the number of specimens requiring reassay, we asked what would be the optimal creatinine cutpoint for identifying specimens requiring more dilution than the standard 222 µl of urine per well. This avoids diluting all specimens, which would lower all values, and problematically increase the number of specimens with undetectable levels below the standard curve. Table 2 provides the trade-offs between the benefit of diluting specimens likely to be too concentrated, and the cost of over-diluting others and increasing the OOR low values. We recommend the creatinine concentration of more than or equal to 1.4mg/ml (based on concordance across substudies: Wave 2 pretest, 1.3 or 1.4mg/ml and Wave 2 first 216 specimens, 1.42mg/ml) to minimize the number of specimens uninterpretably above the curve.
Table 2.
Trade-offs Between the Number of Low, Interpretable And High OT Values Resulting From Each Potential Creatinine Cut Point for Selecting Samples to Dilute Prior to OT Assay.
| Possible creatinine cut points for diluting at 222 µl/well Prior To OT Assay | Low OOR values < 3 pg/ml (± OOR without dilution) | Interpretable values (± low OOR, high OOR) | High OOR values > 225 pg/ml (±OOR without dilution) |
|---|---|---|---|
| Wave 2 pretest data (N = 115) | |||
| 444 µl/well (no dilution) | 26 | 69 | 20 |
| 0.995mg/ml | 40 (+14) | 71 (−14,+16) | 4 (−16) |
| 1.04mg/ml | 40 (+14) | 71 (−14,+16) | 5 (−15) |
| 1.28mg/ml | 31 (+5) | 78 (−5,+14) | 6 (−14) |
| 1.31mg/ml | 29 (+3) | 79 (−3,+13) | 7 (−13) |
| 1.34mg/ml | 28 (+2) | 79 (−2,+12) | 8 (−12) |
| 1.41mg/ml | 27 (+1) | 79 (−1,+11) | 9 (−11) |
| 1.43mg/ml | 27 (+1) | 78 (−1,+10) | 10 (−10) |
| 1.62mg/ml | 26 (+0) | 78 (−0,+9) | 11 (−9) |
| 1.63mg/ml | 26 (+0) | 77 (−0,+8) | 12 (−8) |
| Wave 2 first 216 samples data | |||
| 444 µl/well (no dilution) | 50 | 126 | 40 |
| 1.2mg/ml | 65 (+15) | 139 (−15,+28) | 12 (−28) |
| 1.3mg/ml | 58 (+8) | 142 (−8,+24) | 16 (−24) |
| 1.4mg/ml | 55 (+5) | 144 (−5,+23) | 17 (−23) |
| 1.41mg/ml | 55 (+5) | 144 (−5,+23) | 17 (−23) |
| 1.42mg/ml | 52 (+2) | 147 (−2,+23) | 17 (−23) |
| 1.43mg/ml | 52 (+2) | 146 (−2,+22) | 18 (−22) |
| 1.49mg/ml | 51 (+1) | 146 (−1,+21) | 19 (−21) |
| 1.54mg/ml | 51 (+1) | 146 (−1,+21) | 19 (−21) |
| 1.61mg/ml | 51 (+1) | 145 (−1,+20) | 20 (−20) |
| Note. ±OOR indicates the increase or decrease in number of out of range values (OOR) relative to A 444 μl/well OT assay (no dilution). Confirmation of the optimal cut point (1.41 mg/ml creatinine) by comparing data distributions from Wave 2 pretest and Wave 2 first 216 samples. | |||
Effects of Freeze-Thaw Cycle, Dilution, and Acidity
Procedural differences in the handling of specimens did not introduce a systematic bias or error. A direct test of the effects of one or two freeze-thaw cycles on the results of the assay found no evidence for degradation of OT (Figure 5; r = .9, p < .0001). Acidity of the urine acts as a preservant of OT, preventing it from degrading prior to assay. Indeed, low acidity was associated with OT values below 3 pg/ml (and low OOR F(1,17) = 9.66, p < .007, a one-way analysis of variance of specimens with values <3 or >3 pg/ml).
Figure 5.
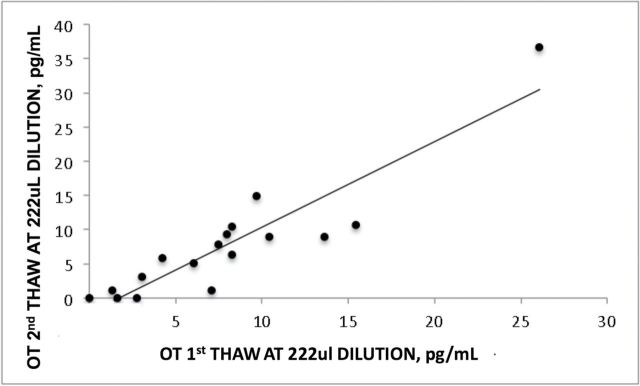
OT values are significantly correlated between the first and second freeze-thaw cycles (r = .9, p < .000, excluding the high outlier; 222 µl dilution). OT, oxytocin.
Discussion
These analyses demonstrate that OT, AVP, and creatinine can be measured using urine specimens collected in the home by nonmedical interviewers, permitting collection in both representative population samples and challenging field settings for both human and nonhuman animals. There are ample individual differences in these hormone levels among older adults (aged 62–91) living in communities of the United States, inviting analysts to determine their role in the links between social interactions, stress, and kidney function in older adults.
Concerns about possible confounds in the assay values reported can be addressed with the many variables included in the NSHAP data set. Plate and duplicate number can be used to model and control for plate effects, although the large sample size makes this only of interest to the specialist. Geocoding and date of interview can be used to evaluate seasonal effects.
The most important control, and one essential for interpreting the findings, was the interview itself. The field interviewers followed a computer-programmed script, so that the social interactions of the interview were rigorously controlled when urine was pooling in the hour before collection. The interview survey questions followed the same script across all participants and focused on social networks, sexuality, and intimate relationships prior to urine collection. Also during this time, participants engaged in measures of body type, cardiovascular function, mobility, and strength, again in a highly standardized protocol (see O’Doherty et al., 2014).
OT and AVP have been implicated in a growing number of studies examining social behaviors, cognition, bonding, and, in the extreme, social deficit disorders such as autism. AVP and creatinine can also be used to examine kidney function and dehydration in older adults. Finally, both OT and AVP release can be triggered in response to social and physical stressors (Heinrichs, Baumgartner, Kirschbaum, & Ehlert, 2003; Taylor et al., 2000; Wotjak et al., 1998). However, due to the challenges associated with urine collection and peptide measurement, these studies of necessity have examined a limited convenience sample or relied on pharmacological manipulation (De Dreu, 2012; Guastella, Graustella, & MacLeod, 2012; Modi & Young, 2012). NSHAP provides a large sample representative of older adults in the United States.
Thereby, NSHAP provides valuable information on the distribution of OT and AVP in older adults. We encourage richer models than presented here, which will profit by including multiple variables that are of interest in themselves and also potential controls: medication use, diagnosis of hypertension, race, and other demographic variables. Future analysts can also use such models to test the effects of psychosocial variables known to affect OT and AVP. Not only was the interview itself a highly standardized social interaction, but in the hour when urine was pooling, it asked participants about their social and sexual lives. Thus, variation in OT and AVP levels not only reflect differences in baseline, but also differences in response to the same social interview. Taken together, NSHAP data provide an exciting opportunity to examine the association between OT and AVP and social networks, health, and bonding in the aging U.S. population.
Key Points .
High creatinine levels (≥1.4mg/ml), high pH, and other established markers of mild dehydration predict interference with the OT assay, yielding artificially high results, which can be avoided with sample dilution.
Urine acidity predicts dilute urine with low OT concentrations for which the assay can be adjusted.
Valid AVP values can be obtained at a standard 222 µl dilution, without considering urine concentration or pH.
OT and AVP do not degrade after a second freeze-thaw cycle.
These techniques may also be useful for animal field studies where mild dehydration can also occur.
Funding
Funding graciously provided through the National Social Life, Health, and Aging Project, which is supported by by the National Institutes of Health, including the National Institute on Aging (R37AG030481; R01AG033903), the Office of Women’s Health Research, the Office of AIDS Research, and the Office of Behavioral and Social Sciences Research (R01AG021487), and by NORC which was responsible for the data collection. The project described was supported by the Clinical and Translational Science Award (CTSA) program , through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) (UL1TR000427), and the Wisconsin National Research Primate Center (WNPRC) for laboratory resources NIH (NCRR000167).
Acknowledgments
We gratefully acknowledge David Kern, Institute for Mind and Biology, University of Chicago, for all of his assistance with NSHAP biomeasure preparation, and Dan Witter, ICTR Core Laboratory, University of Wisconsin-Madison, for conducting the hormone assays.
References
- Anestis S. F. (2010). Hormones and social behavior in primates. Evolutionary Anthropology: Issues, News, and Reviews, 19, 66–78. 10.1002/evan.20253 [Google Scholar]
- Barr D. B., Wilder L. C., Caudill S. P., Gonzalez A. J., Needham L. L., Pirkle J. L. (2004). Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environmental Health Perspectives, 113, 192–200. 10.1289/ehp.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Dozier M. (2010). Mothers’ and children’s concentrations of oxytocin following close, physical interactions with biological and non-biological children. Developmental Psychobiology, 52, 100–107. 10.1002/dev.20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S., Pournajafi-Nazarloo H., Kramer K. M., Ziegler T. E., White-Traut R., Bello D., Schwertz D. (2007). Oxytocin: Behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences, 1098, 312–322. 10.1196/annals.1384.006 [DOI] [PubMed] [Google Scholar]
- Cuthbertson D. P. (1944). Creatine and creatinine metabolism. Nature, 154, 564–565. 10.1038/154564a0 [Google Scholar]
- De Dreu C. K. W. (2012). Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Hormones and Behavior, 61, 419–428. 10.1016/j.yhbeh.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Eggleton M. G. (1947). Some factors affecting the acidity of urine in man. The Journal of Physiology, 106, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G., Fahrenholz F. (2001). The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews, 81, 629–683. [DOI] [PubMed] [Google Scholar]
- Gray P. B., Parkin J. C., Samms-Vaughan M. E. (2007). Hormonal correlates of human paternal interactions: A hospital-based investigation in urban Jamaica. Hormones and Behavior, 52, 499–507. 10.1016/j.yhbeh.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Guastella A. J., Graustella A. J., MacLeod C. (2012). A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Hormones and Behavior, 61, 410–418. 10.1016/j.yhbeh.2012.01.002 [DOI] [PubMed] [Google Scholar]
- Heinrichs M., Baumgartner T., Kirschbaum C., Ehlert U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry, 54, 1389–1398. 10.1016/S0006-3223(03)00465-7 [DOI] [PubMed] [Google Scholar]
- Hoffman E. R., Brownley K. A., Hamer R. M., Bulik C. M. (2012). Plasma, salivary, and urinary oxytocin in anorexia nervosa: a pilot study. Eating Behaviors, 13, 256–259. 10.1016/j.eatbeh.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaszczak A., O’Doherty K., Colicchia M., Satorius J., McPhillips J., Czaplewski M., Imhof L., Smith S. (2014). Continuity and Innovation in the Data Collection Protocols of the Second Wave of the National Social Life, Health, and Aging Project. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassirer J. P. (1971). Clinical evaluation of kidney function–tubular function. The New England Journal of Medicine, 285, 499–502. 10.1056/NEJM197108262850906 [DOI] [PubMed] [Google Scholar]
- Lavizzo-Mourey R. J. (1987). Dehydration in the elderly: A short review. Journal of the National Medical Association, 79, 1033–1038. [PMC free article] [PubMed] [Google Scholar]
- Modi M. E., Young L. J. (2012). The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Hormones and Behavior, 61, 340–350. 10.1016/j.yhbeh.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses A. M., Steciak E. (1986). Urinary and metabolic clearances of arginine vasopressin in normal subjects. The American Journal of Physiology, 251, R365–R370. [DOI] [PubMed] [Google Scholar]
- O’Doherty K., Jaszczak A., Hoffmann J. N., You H. M., Kern D. W., Pagel K., McPhillips J., Schumm L. P., Dale W., Huang E. S., McClintock M. K. (2014). Survey field methods for expanded biospecimen and biomeasure collection in NSHAP Wave 2. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, S27–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C., Hedberg E., Kozloski M., Dale W., McClintock M. K. (2014). Using and interpreting mental health measures from the National Social Life, Health, and Aging Project. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, S99–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A. B., 3rd, Goldstein D. L., Sanchez L., Cool D. R., Morris M. (2006). Urinary oxytocin as a non-invasive biomarker for neurohypophyseal hormone secretion. Peptides, 27, 2877–2884. 10.1016/j.peptides.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Reyes T. L., Mateo J. M. (2008). Oxytocin and cooperation: Cooperation with non-kin associated with mechanisms for affiliation. Journal of Social, Evolutionary, and Cultural Psychology, 2, 90–102. [Google Scholar]
- Seltzer L. J., Ziegler T. E. (2007). Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Hormones and Behavior, 51, 436–442. 10.1016/j.yhbeh.2006.12.012 [DOI] [PubMed] [Google Scholar]
- Seltzer L. J., Ziegler T. E., Pollak S. D. (2010). Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences, 277, 2661–2666. 10.1098/rspb.2010.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy C. M., Perry P. A., Cromwell S. L. (1999). Dehydration: Biological considerations, age-related changes, and risk factors in older adults. Biological Research for Nursing, 1, 30–37. 10.1177/109980049900100105 [DOI] [PubMed] [Google Scholar]
- Szczepanska-Sadowska E. (2008). Role of neuropeptides in central control of cardiovascular responses to stress. Journal of Physiology and Pharmacology, 59(Suppl. 8), 61–89. [PubMed] [Google Scholar]
- Taylor S. E., Klein L. C., Lewis B. P., Gruenewald T. L., Gurung R. A., Updegraff J. A. (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review, 107, 411–429. 10.1037/0033-295X.107.3.411 [DOI] [PubMed] [Google Scholar]
- Taylor S. E., Saphire-Bernstein S., Seeman T. E. (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science, 21, 3–7. 10.1177/0956797609356507 [DOI] [PubMed] [Google Scholar]
- Weisman O., Zagoory-Sharon O., Schneiderman I., Gordon I., Feldman R. (2013). Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology, 38, 694–701. 10.1016/j.psyneuen.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Wismer Fries A. B., Ziegler T. E., Kurian J. R., Jacoris S., Pollak S. D. (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America, 102, 17237–17240. 10.1073/pnas.0504767102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak C. T., Ganster J., Kohl G., Holsboer F., Landgraf R., Engelmann M. (1998). Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience, 85, 1209–1222. 10.1016/S0306-4522(97)00683-0 [DOI] [PubMed] [Google Scholar]
- Ziegler T. E., Scheffler G., Snowdon C. T. (1995). The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus . Hormones and Behavior, 29, 407–424. 10.1006/hbeh.1995.1028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for NSHAP Wave 2 are publicly available (NSHAP Wave 2: Waite, Linda J., Kathleen Cagney, William Dale, Elbert Huang, Edward O. Laumann, Martha K. McClintock, Colm A. O’Muircheartaigh, L. Phillip Schumm, and Benjamin Cornwell. National Social Life, Health, and Aging Project (NSHAP): Wave 2 and Partner Data Collection. ICPSR34921-v1. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor], 2014-04-29. doi:10.3886/ICPSR34921.v1.).