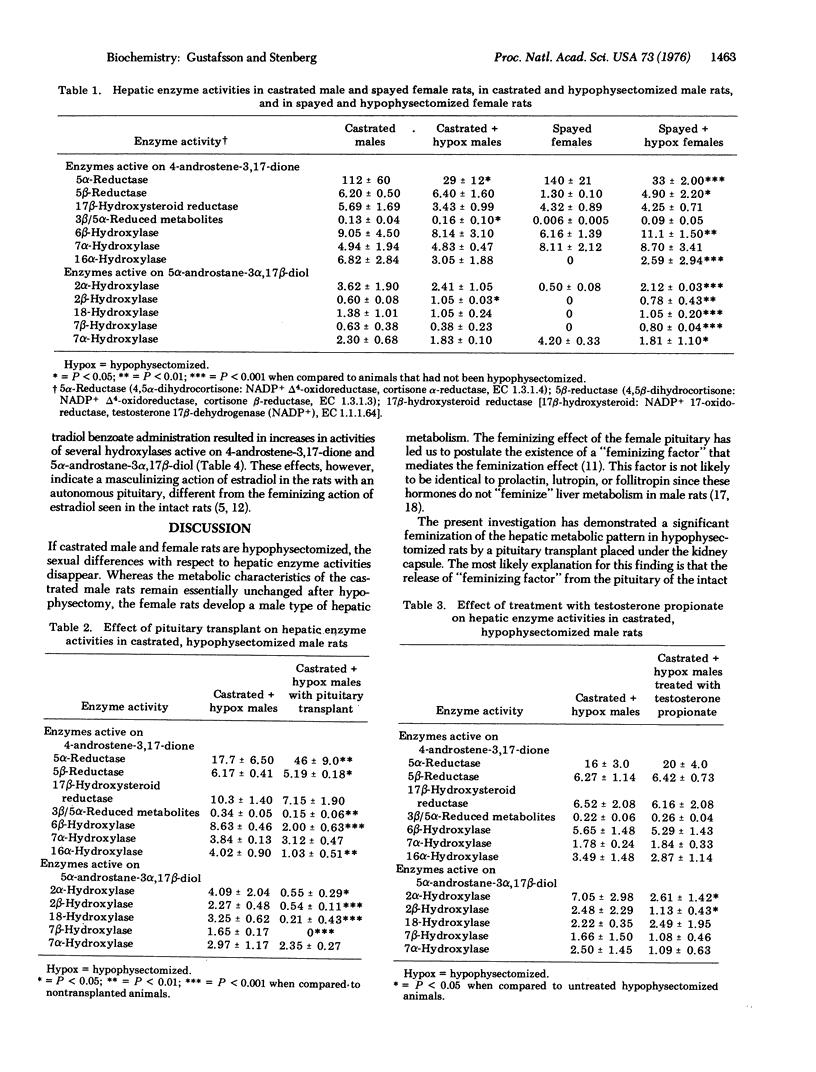
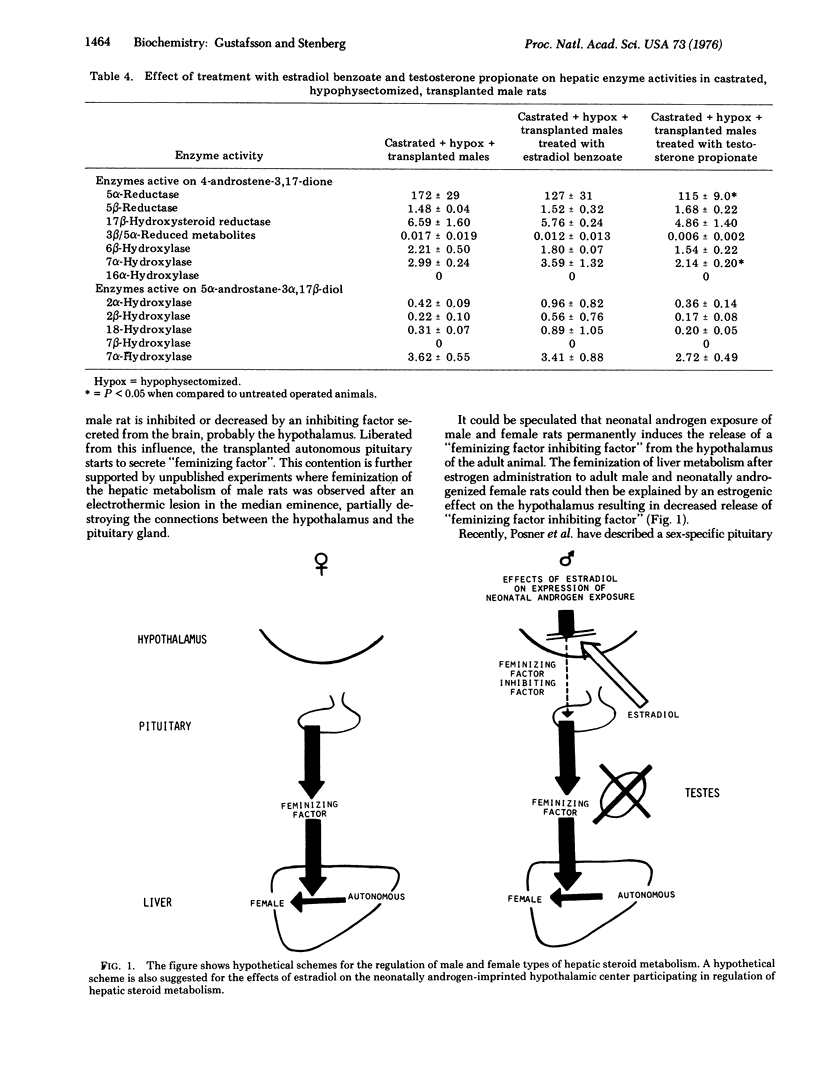
Abstract
The hepatic metabolism of 4-[4-14C]androstene-3,17-dione and 5alpha-[4-14C]androstane-3alpha,17beta-diol was studies in castrated and hypophysectomized male rats with a transplanted pituitary under the kidney capsule. The effects of testosterone propionate and estradiol benzoate on liver metabolism were also studied in these experimental animals. It was found that the autonomous pituitary secreted a "feminizing factor" that transformed the male type of steroid metabolism characteristic of hypophysectomized rats into a female type of metabolism Hypophysectomized rats were unresponsive towards androgen action on the liver and did not respond with feminized hepatic metabolism after treatment with estradiol benzoate. It is concluded that estrogenic action on liver enzymes is mediated via modulation of the secretion of a central (probably hypothalamic) "feminizing factor inhibiting factor" and the sex hormonal effects of hepatic metabolism only occur in the presence of a pituitary in situ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortz W. M. The pathogenesis of hypercholesterolemia. Ann Intern Med. 1974 Jun;80(6):738–746. doi: 10.7326/0003-4819-80-6-738. [DOI] [PubMed] [Google Scholar]
- CONNEY A. H., SCHNEIDMAN K., JACOBSON M., KUNTZMAN R. DRUG-INDUCED CHANGES IN STEROID METABOLISM. Ann N Y Acad Sci. 1965 Mar 12;123:98–109. doi: 10.1111/j.1749-6632.1965.tb12248.x. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Gustafsson J. A. Sexual differences in hepatic sulphurylation of deoxcorticosterone in rats. Eur J Biochem. 1973 Jul 2;36(1):172–177. doi: 10.1111/j.1432-1033.1973.tb02898.x. [DOI] [PubMed] [Google Scholar]
- DORFMAN R. I., FORCHIELLI E. Separation of delta 4-5 alpha-hydrogenases from rat liver homogenates. J Biol Chem. 1956 Nov;223(1):443–448. [PubMed] [Google Scholar]
- De Moor P., Denef C. The "puberty" of the rat liver. Feminine pattern of cortisol metabolism in male rats castrated at birth. Endocrinology. 1968 Mar;82(3):480–492. doi: 10.1210/endo-82-3-480. [DOI] [PubMed] [Google Scholar]
- Denef C. Effect of hypophysectomy and pituitary implants at puberty on the sexual differentiation of testosterone metabolism in rat liver. Endocrinology. 1974 Jun;94(6):1577–1582. doi: 10.1210/endo-94-6-1577. [DOI] [PubMed] [Google Scholar]
- Einarsson K., Gustafsson J. A., Stenberg A. Neonatal imprinting of liver microsomal hydroxylation and reduction of steroids. J Biol Chem. 1973 Jul 25;248(14):4987–4997. [PubMed] [Google Scholar]
- Engelhardt D., Eisenburg J., Unterburger P., Karl H. J. Untersuchungen über den Stoffwechsel von Testosteron und delta-4-Androstendion in der Leber des Menschen. Klin Wochenschr. 1971 Apr 1;49(7):439–440. doi: 10.1007/BF01485004. [DOI] [PubMed] [Google Scholar]
- FORCHIELLI E., BROWN-GRANT K., DORFMAN R. I. Steroid delta 4-hydrogenases of rat liver. Proc Soc Exp Biol Med. 1958 Dec;99(3):594–596. doi: 10.3181/00379727-99-24430. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A. Androgen responsiveness of the liver of the developing rat. Biochem J. 1974 Nov;144(2):225–229. doi: 10.1042/bj1440225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. A., Ingelman-Sundberg M. Regulation and properties of a sex-specific hydroxylase system in female rat liver microsomes active on steroid sulfates. I. General characteristics. J Biol Chem. 1974 Mar 25;249(6):1940–1945. [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Influence of prolactin on the metabolism of steroid hormones in rat liver and adrenals. Acta Endocrinol (Copenh) 1975 Mar;78(3):545–553. doi: 10.1530/acta.0.0780545. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Irreversible androgenic programming at birth of microsomal and soluble rat liver enzymes active on androstene-3,17-dione and 5alpha-androstane-3alpha,17beta-diol. J Biol Chem. 1974 Feb 10;249(3):711–718. [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Masculinization of rat liver enzyme activities following hypophysectomy. Endocrinology. 1974 Sep;95(3):891–896. doi: 10.1210/endo-95-3-891. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Neonatal programming of androgen responsiveness of liver of adult rats. J Biol Chem. 1974 Feb 10;249(3):719–723. [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Partial masculinization of rat liver enzyme activities following treatment with FSH. Endocrinology. 1975 Feb;96(2):501–504. doi: 10.1210/endo-96-2-501. [DOI] [PubMed] [Google Scholar]
- INMAN D. R., BANFIELD R. E., KING R. J. AUTORADIOGRAPHIC LOCALIZATION OF OESTROGEN IN RAT TISSUES. J Endocrinol. 1965 Apr;32:17–22. doi: 10.1677/joe.0.0320017. [DOI] [PubMed] [Google Scholar]
- Kraulis I., Clayton R. B. Sexual differentiation of testosterone metabolism exemplified by the accumulation of 3-beta, 17-alpha-dihydroxy-5-alpha-androstane 3-sulfate as a metabolite of testosterone in the castrated rat. J Biol Chem. 1968 Jun 25;243(12):3546–3547. [PubMed] [Google Scholar]
- Paul S., Bickers D. R., Levere R. D., Kappas A. Inhibited induction of hepatic delta-aminolevulinate synthetase in pregnancy. FEBS Lett. 1974 May 1;41(2):192–194. doi: 10.1016/0014-5793(74)81209-3. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Induction of a lactogenic receptor in rat liver: influence of estrogen and the pituitary. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2407–2410. doi: 10.1073/pnas.71.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind A. B., Sassa S., Merkatz I. R., Winchester R., Harber L., Kappas A. Stimulators and inhibitors of hepatic porphyrin formation in human sera. J Clin Invest. 1974 Apr;53(4):1167–1177. doi: 10.1172/JCI107655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YATES F. E., HERBST A. L., URQUHART J. Sex difference in rate of ring A reduction of delta 4-3-keto-steroids in vitro by rat liver. Endocrinology. 1958 Dec;63(6):887–902. doi: 10.1210/endo-63-6-887. [DOI] [PubMed] [Google Scholar]



