Abstract
Mucopolysaccharidoses (MPS) are a group of lysosomal storage disorders caused by the deficiency of lysosomal enzymes. The enzymes are required to break down glycosaminoglycans (GAGs) that help build bone, cartilage, tendons, corneas, skin and connective tissue. In patients with MPS, a missing enzyme leads to the accumulation of GAGs in the cells, blood, connective tissues, and multiple organs. The consequence is permanent, with progressive cellular damage affecting patients’ appearance, physical abilities, organ and system function, and skeletal and mental development.
The measurement of each specific GAG in a variety of specimens is required to establish the correlation between GAGs and physiological status of patients and/or prognosis and pathogenesis of the disease and to separate the patients with MPS from the healthy controls.
We have developed a highly accurate, sensitive, and cost-effective liquid chromatography tandem mass spectrometry (LC-MS/MS) method for measurements of disaccharides derived from four specific GAGs [chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), and keratan sulfate (KS)]. Disaccharides were produced by specific enzyme digestion of each GAG, and subsequently, quantified by negative ion mode of multiple reaction monitoring. Subclasses of GAGs with the same molecular weights can be separated by liquid chromatography.
We have also developed another GAG assay by high-throughput mass spectrometry (HT-MS/MS). The HT-MS/MS consists of an integrated solid phase extraction robot that binds and de-salts samples from assay plates and directly injects them into a MS/MS detector, reducing sample processing time to within ten seconds. HT-MS/MS consequently yields much faster throughput than conventional LC-MS/MS-based methods; however, the HT-MS/MS system does not use a chromatographic step, and therefore, cannot separate GAGs that have the same molecular weights. Both techniques can be applied to the analysis of dried blood spots, blood, and urine specimens.
In this review, we describe the assay methods for GAGs and the application to newborn screening and diagnosis of MPS.
Keywords: tandem mass spectrometry, glycosaminoglycans, diagnosis, mucopolysaccharidoses, newborn screening
1. Introduction
The mucopolysaccharidoses (MPS) are a group of the lysosomal storage diseases (LSD), which includes more than 50 genetic disorders that result from malfunctions of the lysosome. The lysosome is commonly referred to as the recycling center of the cells because it processes undegraded materials into substances that the cell can reuse. Complex macromolecules are broken down in the lysosome by hydrolytic enzymes. LSDs, such as MPS, are triggered when a particular enzyme is deficient. There are 11 known enzyme deficiencies, leading to 7 distinct forms of MPS with a collective incidence of over 1 in 25,000 live births (Table 1). GAG accumulation results in a progressive damage of multiple tissues that may cause coarse facial features, mental retardation, recurrent ear and nose infections, inguinal and umbilical hernias, hepatosplenomegaly, and skeletal deformities. Clinical features related to bone lesions may include marked short stature, cervical stenosis, pectus carinatum, small lungs, joint rigidity, kyphoscoliosis, lumbar gibbus, and genu valgum. The clinical features and severity of MPS differ with each patient and its subtype of MPS, but the average life span of most patients is one to two decades if untreated. Most clinical signs and symptoms in patients with MPS are not apparent at birth, but appear and progress with age.
Table 1.
Classification of mucopolysaccharidoses and related GAG(s).
| Disorder | Deficient Enzyme | Trait | Chromosome | Primary storage GAG(s) |
KS elevation in blood* |
|---|---|---|---|---|---|
|
| |||||
| MPS I (Hurler) | α-L-Iduronidase (IDUA) | AR | 4p16.3 | DS, HS | ↑ ↑ ↑ |
| MPS II (Hunter) | Iduronate-2-sulfatase (IDS) | XR | Xq28 | DS, HS | ↑ ↑ ↑ |
| MPS IIIA (Sanfillipo A) | Heparan-N-sulfatase (SGSH) | AR | 17q25.3 | HS | ↑ |
| MPS IIIB (Sanfillipo B) | α-N-Acetylglucosaminidase (NAGLU) |
AR | 17q21 | HS | ↑ |
| MPS IIIC (Sanfillipo C) | α-Glucosaminidase acetyltransferase (HGSNAT) |
AR | 8p11-q13 | HS | ↑ |
| MPS IIID (Sanfillipo D) | N-Acetylglucosamine 6-sulfatase (GNS) |
AR | 12q14 | HS | NA |
| MPS IVA (Morquio A) | Galactose 6-sulfatase, N- acetylgalactosamine-6-sulfate sufatase (GALNS) |
AR | 16q24.3 | C6S, KS | ↑ ↑ ↑ |
| MPS IVB (Morquio B) | (β-Galactosidase (G1B1) | AR | 3p21.33 | KS | ↑ |
| MPS VI (Maroteaux- Lamy) |
N-Acetylgalactosamine-4- sulfatase (G4S) |
AR | 5q13.3 | C4S, DS | ↑ ↑ |
| MPS VII (Sly) | (β-D-Glucuronidase (GUSB) | AR | 7q21-q22 | C4, 6S, DS, HS | ↑ ↑ |
| MPS IX (Natowicz) | Hyaluronidase | AR | 3p21.31 | Hyaluronic acid | NA |
KS is elevated in the blood from several types of MPS, even when KS is not the primary storage GAG. AR: autosomal recessive, XR: X-linked recessive, C4S: chondroitin 4-sulfate, C6S: chondroitin 6-sulfate, DS: dermatan sulfate, HS: heparan sulfate, KS: keratan sulfate, NA: not available.
At present, enzyme replacement therapy (ERT), hematopoietic stem cell transplantation (HSCT), substrate reduction therapy (SRT), and gene therapy are clinically available or under clinical trials for patients with MPS. Initiating these treatments at birth or a very early stage provides the greatest therapeutic effect on the clinical course of the disease (1-8). To improve activities of daily living (ADL) for patients, early diagnosis and early treatment are critical. Accurate prognosis and monitoring of therapeutic efficacy are also important for providing optimal care for these patients.
Proteoglycans (PGs) contain a core protein covalently attached to GAGs. GAGs are polysaccharides composed of a linear chain of disaccharides. These disaccharides comprise one amino sugar (N-acetylglucos-amine or N-acetylgalactosamine) and either a uronic acid (glucuronic acid or iduronic acid) or galactose sugar unit. Hyaluronan is not covalently linked to a protein, but instead interacts non-covalently with proteins that contain a hyaluronan-binding motif. GAGs comprise chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and hyaluronan. These GAGs are found in various tissues and are major components of the extracellular matrix (ECM). PGs have various biological functions and play important roles in control of growth and differentiation. Particular sulfation patterns in the GAG chains allow interactions, normally of an ionic nature, with growth factors. The PG protein cores are not just scaffolds for GAGs: they contain domains that have particular biological activities (9). Many PGs are thus multifunctional molecules that engage in several different specific interactions simultaneously.
Newborn screening (NBS) is recognized as a vital, preventive public health process that enables early identification and treatment of inherited diseases that can affect long-term health. Tandem mass spectrometry (MS/MS) has evolved as a powerful tool to detect small molecules because it directly measures each compound based on the mass of the parent compound and a specific fragment. We developed a method that can assay CS, DS, HS, and KS simultaneously in blood and/or urine samples using high performance liquid chromatography tandem mass spectrometry (LC-MS/MS) for MPS (10-21). The LC-MS/MS method not only shows sensitivity and specificity for detecting all subtypes of MPS but is also able to monitor therapeutic efficacy in MPS patients and animal models (1, 8, 13, 14, 17-20, 23-25).
A limitation of the LC step is that it is time-consuming, limiting its utility for the mass screening of NBS samples. The use of a high-throughput mass spectrometry (HT-MS/MS; RapidFire) (Agilent Technologies Palo Alto, CA, USA) resolves the bottleneck of throughput, while keeping the quality and consistency of standard MS/MS read-outs. Samples are absorbed to a matrix to concentrate and desalt, and then, are eluted directly into the MS/MS without chromatographic separation. Each sample is analyzed in less than ten seconds, yielding much faster throughput than conventional LC-MS/MS. A single HT-MS/MS platform would be able to analyze over one million samples annually. However, it cannot separate samples with the same molecular weight.
In this review, we describe GAG assay methods using LC-MS/MS and HT-MS/MS, and discuss their potential application to newborn screening and diagnosis of MPS.
2. Methods and Materials
2.1. Standards and enzymes
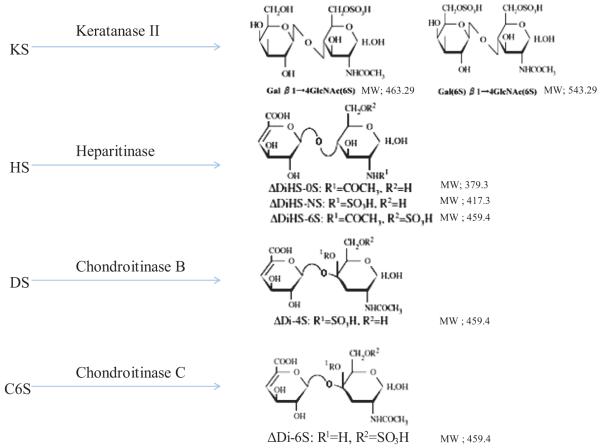
To digest “polymer” CS, DS, HS, and KS to disaccharides, the enzyme chondroitinase C was obtained from Sigma-Aldrich (St. Louis, MO) and the enzymes chondroitinase B, heparitinase, and keratanase II were obtained from Seikagaku Co. (Tokyo, Japan). Chondrosine (Seikagaku Co.) was used as an internal standard (IS), while unsaturated disaccharides, [ Δ DiHS-0S, 2-acetamido-2-deoxy-4-O-(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-D-glucose; ΔDiHS-NS, 2-deoxy-2-sulfamino-4-O(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-D-glucose; Δ Di-4S/6S (DS or CS), 2-acetamido-2-deoxy-4-O-(4-deoxy-a-L-threo-hex-4-enopyranosyluronic acid)-4 or 6-O-sulfo-D-glucose] were used to make standard curves. Stock solutions of ΔDi-6S (250 μg/ml), ΔDi-4S (250 μg/ml), ΔDiHS-0S (100 μg/ml), ΔDiHS-NS (100 μg/ml), ΔDiHS-6S (100 μg/ml), and IS (5 mg/ml) were prepared separately in ddH2O. A mixture of mono-sulfated KS [Galß1-4GlcNAc(6S)] and di-sulfated KS [Gal(6S)ß1-4GlcNAc (6S)] were made by digestion of bovine cornea KS with keratanase II. Mono-sulfated KS and di-sulfated KS (10 μg/ml) were also prepared separately (18-21, 25, 26).
For standard curves, dilutions of the stock solutions were prepared as follows: ΔDi-6S, ΔDi-4S, ΔDiHS-0S, ΔDiHS-NS, and ΔDiHS-6S (1, 5, 10, 50, 100, 500, and 1000 ng/ml), and digested KS (0.01, 0.05, 0.1, 0.5, 1, 2.5, 5, and 10 μg/ml), each containing IS solution (50 ng/ml). ΔDi-4S is composed of DS, and ΔDi-C6S derived from C6S while ΔDiHS-0S, ΔDiHS-NS, and ΔDiHS-6S are subclasses of HS. KS comprises mono-sulfated KS and di-sulfated KS (Fig. 1). ΔDi-4S, ΔDiHS-6S, and ΔDi-C6S have the same molecular weight.
Figure 1. Disaccharides after digestion of glycosaminoglycans by enzymes.
(adapted from Tomatsu et al. Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications. J Anal Bioanal Tech. 2014 Mar 1; 2014 (Suppl 2): 006). KS; Keratan sulfate, HS; heparan sulfate, DS; dermatan sulfate, CS; chondroitin sulfate.
2.2. Sample preparation
Samples were centrifuged to remove insoluble material. Ten μL of the supernatant (plasma/serum or urine) was mixed with 90 μL of 50 mM Tris-HCl Buffer (pH 7.0), and then, added to AcroPrep™ Advance 96-Well Filter Plates with Ultra filtration Omega 10K (PALL Co., NY, USA) (18-21, 25, 26). This was centrifuged at 2,500 g for 15 min to deplete low molecular compounds that could interfere with the assay. This step can be eliminated. The filters were transferred to fresh plates and 20 μL of internal standard (500 ng/mL chondrosine), 20 μL of 50 mM Tris-HCl buffer and 10 μL of each enzyme, chondroitinase C, chondroitinase B, heparitinase, and keratanase II, respectively, (each 2 mU in 50 mM Tris-HCl buffer) were added onto each filter. Samples were incubated with shaking at 37 °C for 15 hr to digest the GAGs. Filter plates were then centrifuged at 1,500 g for 1 hr. Samples were stored at −20 °C until injection to LC-MS/MS or HT-MS/MS.
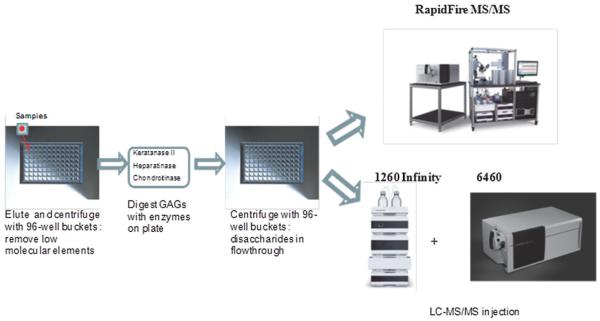
2.3. Equipment (Fig. 2)
Figure 2. Procedures of LC-MS/MS and RF-MS/MS.
(adapted from Tomatsu et al. Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications. J Anal Bioanal Tech. 2014 Mar 1; 2014 (Suppl 2): 006). The same samples were analyzed by both LC-MS/MS and HT-MS/MS.
Liquid chromatography tandem mass spectrometry (LC-MS/MS)
The equipment used for LC-MS/MS was a 1260 infinity LC /6460 Triple Quad (Agilent Technologies, Palo Alto, CA, USA). Samples were removed from the −20 °C and were allowed to thaw at an ambient temperature. Sample tubes were quickly centrifuged to collect all samples to the bottom of the plate. Twenty-five μL of each sample were transferred to a fresh 96 well polypropylene plate, and diluted with 25 μL of 50 mM Tris buffer (pH 7.5). The plate was centrifuged at 3,500 g for 5 min, and 2 μL was loaded into the 1260 infinity LC /6460 Triple Quad (18, 21, 25, 26).
High-throughput mass spectrometry (HT-MS/MS)
The samples purified by the filters as described above for LC-MS/MS were also analyzed using the HT-MS/MS platform (Agilent Technologies, Inc: Santa Clara, CA). Individual AUC (areas under the curve) for each analyte in each sample was determined. Each sample was prepared and analyzed in duplicate on separate days. All GAG levels were measured in triplicate and average levels of GAGs calculated using standard curves (18, 19, 25).
3. Results
3.1. LC-MS/MS: Disaccharide determination derived from GAGs
3.1.1. Chromatography
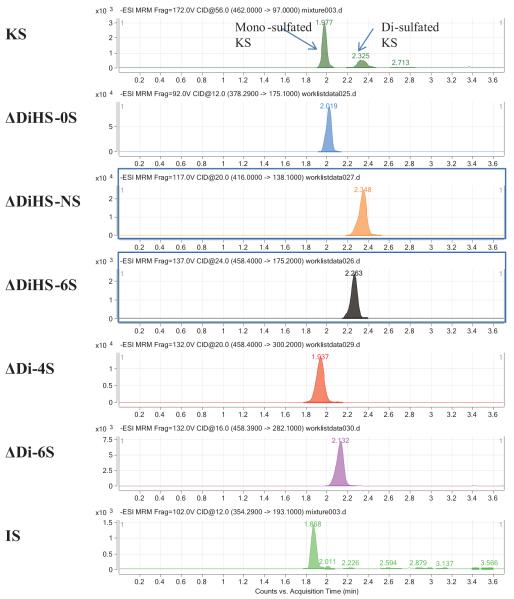
Disaccharides were separated on a Hypercarb (2.0 mm × 50 mm, 5 μm; Thermo Electron Corp.) column and eluted with an acetonitrile gradient of 0% to 100% in 5 mM ammonium acetate. Using purified preparations of ΔDiHS-0S, ΔDiHS-NS, ΔDiHS-6S, ΔDi-4S, and ΔDi-6S, we optimized MRMs for each sugar and obtained single peaks corresponding to the pure compound. All disaccharides were eluted in less than 3 min. Although ΔDiHS-6S, ΔDi-4S (DS), and ΔDi-6S (C6S) had the same molecular weights, they eluted at different times from the column and were quantified separately (21, 25). All disaccharides eluted consistently as seen for the corresponding standards. Mono-sulfated KS and di-sulfated KS, were also separated by chromatography (20, 25) (Fig. 3).
Figure 3. Chromatograms of disaccharides.
(adapted from Tomatsu et al. Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications. J Anal Bioanal Tech. 2014 Mar 1; 2014 (Suppl 2): 006). Chromatograms for disaccharides of mono-sulfated KS, di-sulfated KS, ΔDiHS-NS, ΔDiHS-0S, ΔDiHS-6S, ΔDi-4S (DS), ΔDi-6S (C6S), and chondrosine (IS). Equipment: 6460 Triple Quad MS/MS with 1260 infinity LC (Agilent Technologies). IS: internal standard.
3.2. HT-MS/MS: Disaccharide determination derived from GAGs
3.2.1. Chromatography
The HT system does not have a chromatographic separation step but rather samples are first bound to solid phase matrix for desalting and concentration and then eluted directly into an MS/MS system. Consequently, ΔDiHS-6S, ΔDi-4S, and ΔDi-6S were not distingished since they have the same molecular weight and provide the similar MRMs; so the MS/MS quantified the combination these three disaccharides and distinguished them from other disaccharides.
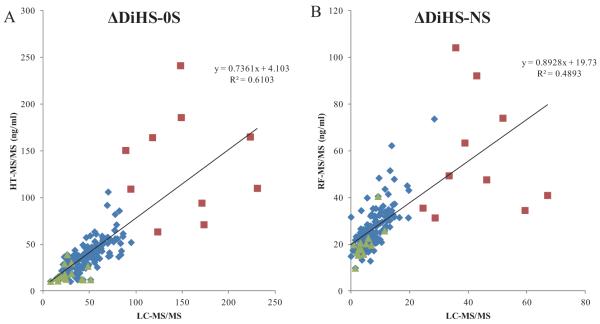
3.2.2. Correlation of GAG levels between LC-MS/MS and HT-MS/MS
Human blood samples were analyzed for DiHS-NS, DiHS-0S, Di-4S, and KS by both LC-MS/MS and HT-MS/MS. Correlation between LC-MS/MS and HT-MS/MS was evaluated using linear regression analysis. The moderate correlation in serum ΔDiHS-NS and ΔDiHS-0S measurements of control subjects between LC-MS/MS and HT-MS/MS indicates that results from each assay are comparable (Fig. 4). Measurements of KS also showed moderate correlation (data not shown).
Figure 4. Correlation in GAG concentration between LC-MS/MS and HT-MS/MS.
- ΔDiHS-NS concentration. Correlation in blood (plasma or serum) ΔDiHS-NS of human subjects between LC-MS/MS and HT-MS/MS is significant (Control n = 187, MPS IVA n = 17, MPS II n = 10, p<0.0001).
- ΔDiHS-0S concentration. Correlation in blood (plasma or serum) ΔDiHS-0S of human subjects between LC-MS/MS and HT-MS/MS is significant (Control n = 187, MPS IVA n = 17, MPS II n = 10, p<0.0001).
3.3. Analysis of Plasma and Serum Samples in MPS Patients
3.3.1. Chromatograms of GAG assays
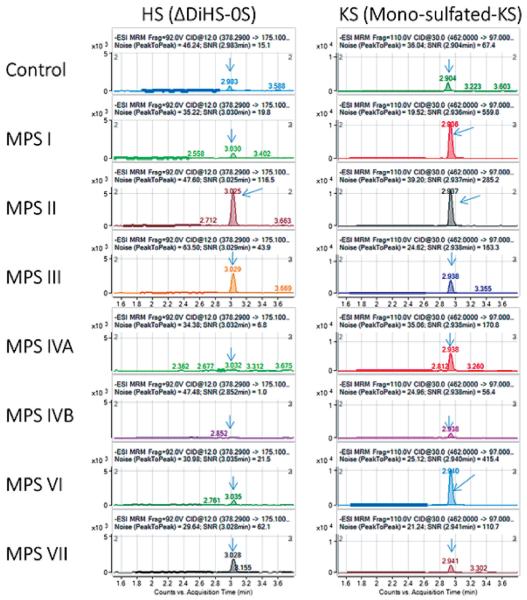
Disaccharide GAGs assayed by LC-MS/MS have been used for diagnosis, screening, prognosis of clinical severity, and assessment of therapeutic efficacy in human patients and animal models with MPS (15-17, 22-24, 26, 28-30). MRMs for ΔDiHS-0S and mono-sulfated KS patterns in MPS I-MPS VII are shown in Figure 5.
Figure 5. MRM of plasma samples in patients with MPS I-VII by LC-MS/MS.
(adapted from Tomatsu et al Establishment of Glycosaminoglycan Assays for Mucopolysaccharidoses. Metabolites 2014, 4, 655-679). ΔDiHS-0S (left) and mono-sulfated KS (right) are shown.
3.3.2. Levels of specific GAGs in blood
Each type of MPS has a different pattern of elevated GAG levels in the blood. We described the concentrations of DS-, HS-, and KS-derived disaccharides and their composition ratios in human control and MPS patients (10-12).
Chondroitin Sulfate (CS)
Chondroitin 6-sulfate (C6S) is distributed in the growth plates, aorta, and cornea, but the physiological function of C6S is not understood well. One of the limitations is that no rapid, precise quantitative method to measure C6S has been developed. MPS IVA and MPS VII result in accumulation of C6S and other GAG(s). C6S levels were measured in blood from control subjects and patients with MPS IVA and VII aged from 0 - 58 years of age. We also assayed KS levels in the same samples for comparison with C6S. Levels of C6S in blood decreased with age and were significantly elevated in patients with MPS IVA and VII, compared with age-matched controls. Levels of KS in patients with MPS IVA were also higher than those in age-matched controls, although differences were less pronounced that with C6S. Combining KS and C6S data, discriminated patients with MPS IVA from age-matched control subjects better than either C6S or KS level alone (21).
Keratan Sulfate (KS)
Blood KS concentrations were found to vary with age. In healthy control newborns, blood KS concentration was below 2.0 μg/mL, and it rose, reaching a peak between 0 and 2 years of age, and the concentrations stayed relatively constant until the individual reached 15 years of age. After 15 years of age, blood KS concentrations decreased and stabilized thereafter. When control subjects and MPS IVA patients in each age range were compared, the difference in KS levels between MPS IVA patients and age-matched controls was greatest in the younger children. KS levels in patients with MPS IVA were reduced with age and were almost at normal levels by the age of 15 years. Blood KS levels in a severe form of MPS IVA were elevated more than in the attenuated form, suggesting that KS level is associated with clinical phenotype. The level of blood KS was also compared between patients with other MPS and age-matched controls. Four out of 31 (12.9%) patients with MPS I had the plasma KS level more than 2SD above the mean of age-matched controls. Patients with MPS II had the highest mean KS in their blood among all types of MPS patients. Twenty-two out of 28 (78.6%) of MPS II patients had plasma KS values more than 2SD above the mean of age-matched controls. Five of 19 (26.3%) MPS III patients, 3 of 6 (50%) MPS VI patients, and 1 of 6 MPS VII patients had plasma KS levels more than 2SD above the mean of age-matched controls.
Levels of elevated KS detected by LC-MS/MS in plasma for MPS I, II, VI and VII are not equivalent to those determined using ELISA methods and may reflect differences in the two assays or differences in the patient populations studied. The amount of KS measured by LC-MS/MS was over 10 times higher than measured by the sandwich ELISA method, and the correlation between plasma KS levels assayed by both methods was not strong. The monoclonal antibody used for sandwich ELISA (Seikagaku, Tokyo, Japan) is specific for di-sulfated KS, Gal β 1(6S)→4GlcNAc(6S) (31, 32), and, therefore, this ELISA approach does not quantify total KS. Furthermore, the ELISA method cannot detect less than 2.5 ng/mL of KS, whereas LC-MS/MS can measure as little as 0.2 ng/mL of KS. As a result, in rodents that synthesize far less KS, blood levels are measurable by LC-MS/MS but not by standard ELISA.
Dermatan Sulfate (DS)
All MPS VI patients (n = 4) showed an elevation of plasma DS. MPS I (18/22, 81.8%), MPS II (26/27, 96.3%), and MPS VII (2/7, 28.6%) patients had a significant elevation of DS as well. These findings indicate that DS measurements by LC-MS/MS are applicable to the screening for most MPS I, II, and VI patients (18).
Heparan Sulfate (HS)
All MPS I, II, and III patients (n = 60) showed a significant elevation of plasma ΔDiHS-0S and ΔDiHS-NS. The group of MPS III patients comprised five IIIA, four IIIB, and two IIIC patients. Two out of 6 patients had a significant elevation of HS (18).
The results showed 1) that blood levels of ΔDiHS-NS and ΔDiHS-0S were significantly elevated in patients with MPS II and III, 2) that patients with a severe form of MPS II had a higher level of HS than those with an attenuated form, and 3) that reduction of blood HS was seen in MPS II patients treated with ERT or HSCT (18, 19).
Composition of DS and HS in Blood
The compositional ratio of ΔDiHS-0S, ΔDiHS-NS, and ΔDi-4S in blood samples of MPS patients was compared. The ratio of each GAG composition was expected to be affected by deficiency of a specific enzyme. In the normal control samples, the ratio of ΔDiHS-0S, ΔDiHS-NS, and ΔDi-4S was 40.4%, 7.7%, and 51.9%, respectively. The proportion of ΔDi-4S was higher in patients with MPS VI, compared to that in normal controls (mean; 80.6% vs. 51.9%). The proportion of ΔDiHS-0S was higher in patients with MPS III and VII, compared to that in normal controls (mean; 56.4% vs. 40.4%; 65.1% vs. 40.4%). The proportion of ΔDiHS-NS was also higher in MPS III patients compared to that in normal controls (mean; 19.7% vs. 7.7%). Other types of MPS did not provide any difference in ratios of DS and HS (18, 25).
3.4. Newborn MPS
It is critical to elucidate when GAGs start to accumulate in tissues of patients to determine feasibility of measuring GAGs for newborn screening for MPS. To evaluate whether the LC-MS/MS method can distinguish MPS newborns from healthy control newborns, we assayed DS and HS levels in DBS samples that had been obtained at birth from six individuals later diagnosed with MPS (four MPS I, one MPS II, and one MPS VII). All six cases showed elevations of DS and HS levels, compared with those of control newborns (26).
HT-MS/MS also demonstrated that the levels of ΔDiHS-0S and ΔDiHS-NS are elevated in DBS obtained at birth from 11 patients diagnosed with MPS I (n = 6) or MPS III (n = 5), when compared to control newborn DBS (19). The levels of ΔDiHS-0S and ΔDiHS-NS from DBS of a newborn with MPS II were 3 and 1.5 times higher than in DBS from control newborns. In this study ΔDiHS-0S was more discriminating than Δ DiHS-NS in separating patients from controls.
Ruijter et al. have also shown that DS and HS levels from newborn DBS of MPS I (11), MPS II (1), and MPS III (6) patients, ranging from severe to relatively attenuated forms, were elevated compared with those in newborn control DBS (15).
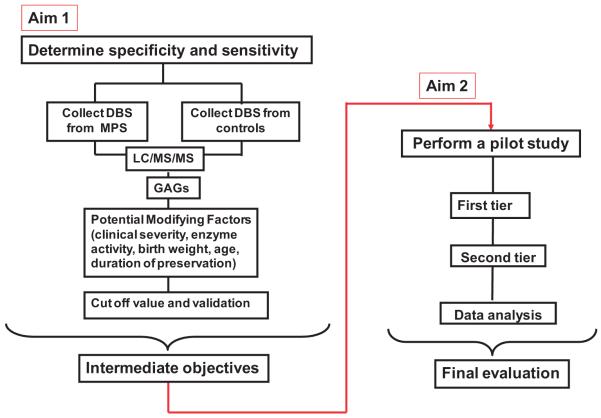
These findings demonstrate that DS and HS are a useful diagnostic and screening marker for MPS I, II, III, VI, and VII. We are collecting newborn DBS to evaluate a newborn screen for MPS (Fig. 6). We will screen 200,000 newborn DBS over the next 3 years.
Figure 6. Scheme for newborn screening and diagnosis of mucopolysaccharidoses.
Aim 1. Determine the specificity and sensitivity of a new LC-MS/MS method and evaluate specific GAGs as biomarkers for MPS. Aim 2. Perform a MPS newborn screening pilot study on de-identified newborn samples.
3.5. Secondary Elevation of KS
KS is synthesized primarily in cartilage, and the catabolized KS is secreted into the circulation and excreted in the urine. Blood KS levels in the normal population peak during the growth spurt and decreases when the growth plate closes. MPS IVA results from a deficiency of the enzyme involved in KS degradation pathway, leading to accumulation of undegraded KS in cartilage. The resulting accumulation of undegraded KS alters the structure of PGs and collagen fibers in cartilage causing abnormalities of the ECM and malfunction of chondrocytes. The undegraded KS is released into the body fluids. The degree of elevation of KS in blood and urine in patients with MPS IVA correlates with clinical phenotype and its prognosis at a progressive stage (22, 34, 35). However, KS levels in patients starts to decrease after 10 years of age, earlier than in the normal population, due to the main synthetic site of KS, cartilage, being destroyed and lost. Blood KS level in MPS IVA patients decline to that seen in age-matched controls in their teenage years (22, 34, 35).
Correlation between types of MPS and specific GAG(s) is seen based upon the degradative enzyme affected (33). Elevation of KS level in blood and urine specimens was originally thought to be a specific biomarker of MPS IV since the deficient enzyme is engaged in catabolism of KS. However, other MPS types were also associated with elevated KS levels in blood and urine, in addition to the specific GAGs expected due to the deficient enzyme (34, 35). KS elevation is more pronounced in blood than in urine, and patients with other MPS types can have as much blood KS as patients with MPS IVA.
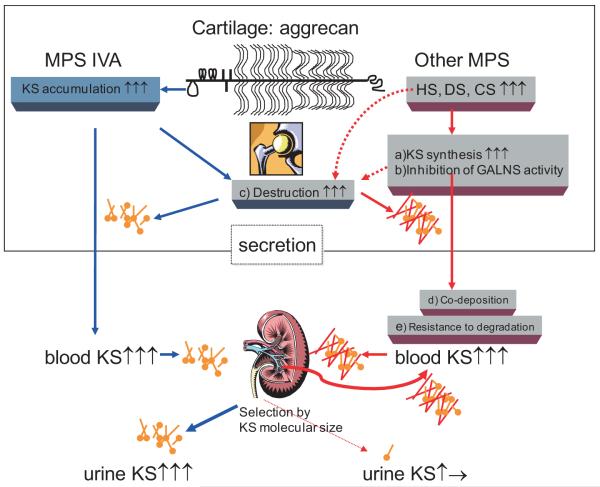
Several hypotheses have been proposed to explain the elevation of blood KS in patients with other types of MPS as follows (Fig. 7): a) Synthesis of KS is induced by accumulated GAGs and/or inflammation caused due to storage of other GAGs. b) GALNS activity is inhibited by the increased HS in patients with MPS (36). c) KS secretion into the circulation is due to damage of cartilage and its ECM caused by accumulation of other GAGs and its subsequent inflammation. The degradation of proteoglycans occurs early in joint damage, and their fragments are released into the synovial fluid and subsequently the blood (37, 38). d) Polymer KS is co-deposited with the other accumulated GAGs, hindering the interaction between KS and its catabolizing enzymes, e) Changes in the extent and distribution of fucosylation, sialylation, and sulfation on KS, making the altered KS resistant to degradation (39). One or a combination of these factors will contribute to a secondary elevation of KS.
Figure 7. Proposed mechanism of secondary elevation of blood KS in MPS.
a) Elevation of synthesis of KS by storage of other GAGs. b) Inhibition of GALNS activity by the increased concentrations of HS. c) KS secretion into the circulation due to damage of cartilage and its ECM. d) Co-deposition of KS with the other accumulated GAGs. e) Altered KS resistant to degradation.
Most MPS patients have involvement of skeletal dysplasia and cartilage damage. The elevated KS in blood of other MPS patients is associated with underlying bone disease, especially cartilage. Blood KS provides more specificity for the bone pathology of MPS disease than the primary substrates HS and/or DS. It is therefore of interest to monitor the KS levels in the blood as a biomarker for other MPS types. Whether KS level is elevated in selected newborn babies with MPS remains unanswered from the viewpoint of early detection. MPS are progressive disorders that often take years to present clinically. Nevertheless, there is considerable evidence from both human studies (40-42) and in animal models (43) that biochemical storage commences in the fetus.
4. Discussion
GAG is a useful biomarker for diagnosis, prognosis, screening, and monitoring of MPS. We have established HT-MS/MS and LC-MS/MS for GAG assay and applied the method to newborn screening and diagnosis of MPS.
The HT-MS/MS platform can identify the disaccharides of ΔDiHS-0S, ΔDiHS-NS, ΔDi-4S/6S, mono-sulfated KS, and di-sulfated KS with similar reproducibility, sensitivity, and specificity as LC-MS/MS (10-17), suggesting that the LC component may not be required for identification of these disaccharides. The correlation between LC-MS/MS and HT-MS/MS for detection of disaccharides demonstrates that non-specific interactions or signal quenching are not major concerns for the HT system for GAG assay. HT-MS/MS provides up to 100 fold faster throughput compared to conventional LC-MS/MS, and consequently may be more appropriate for mass screening applications. However, HT-MS/MS is not useful for all applications. As there is no separation step, interfering compounds will not be distinguishable from the compound under investigation. The significance of these false signals to this limited study requires further clarification, but the data emphasize the need to ensure that samples will not give excessive levels or numbers of false positive signals prior to establishment of a HT technique.
HT-MS/MS cannot separate ΔDi-4S, ΔDi-6S, and ΔDiHS-6S that have the same molecular weight, and, therefore, LC-MS/MS is required if quantities of specific disaccharides are sought.
The LC-MS/MS method is also required to search sulfation levels of HS and KS that lead to functional alterations of proteoglycans. HT-MS/MS is more valuable for situations where rapid throughput is necessary.
The physiological role of GAGs is dependent upon tissue and/or cell type. Sulfation level of GAG(s) also varies with age, tissue, and pathological status. For instance, KS is almost always sulfated on C (6) of GlcNAc, while C (6) of Gal is sulfated to a variable extent, depending on the tissue and age (44). KS chains of fibromodulin in articular cartilage are highly sulfated compared to that in cornea (45, 46), and the levels of sulfation in cornea and cartilage increase during normal aging (47, 48). The mechanism of alteration of their sulfation levels remains unknown. However, corneas in patients with macular corneal dystrophy (MCD) have been reported to synthesize low-sulfated KS, resulting in corneal clouding, and sulfate groups attached to KS play critical roles in maintaining corneal transparency (49).
GAGs are used as biomarkers not only for diagnosis, prognosis, and monitoring therapeutic effects for MPS but may also prove valuable for the study of other diseases and physiological roles.
The mechanism of alteration of GAGs levels and their sulfation levels remains unknown. Investigation of each GAG level and its sulfation level in each disease and physiological status should help elucidate the mechanism of secondary alteration of GAGs and their roles.
5. Conclusion
Both LC-MS/MS and HT-MS/MS methods provide a comparable sensitivity and accuracy for measurements of GAGs (CS, DS, HS, and KS). Identification of GAGs leads to the diagnosis, prognosis, monitoring the disease progression and therapeutic efficacy as well as screening for MPS. Advantage of use of LC-MS/MS is that ΔDi-6S (C6S), ΔDi-4S (DS), and DiHS-6S with the same molecular weight are distinguishable while it takes 3 min per sample to be analyzed. The HT-MS/MS platform should provide both high throughput and economic advantages as a screening system for MPS, compared with LC-MS/MS although this system cannot separate molecules with the same molecular weight.
Acknowledgement
This work was supported by grants from the Austrian MPS Society, International Morquio Organization (Carol Ann Foundation) and Delaware Bioscience Center for Advanced Technology. S.T. and R.W.M. were supported by National Institutes of Health grant P20GM103464. S.T. and A.M were supported by National Institutes of Health grant 1R01HD065767. F.K. was supported by Conselho National de Desenvolvimento Científico e Tecnológico from Brazil (CNPQ). The content of the article has not been influenced by the sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Abbreviations not common to the field
- CS
chondroitin sulfate
- CNS
central nervous system
- DBS
dried blood spot
- DS
dermatan sulfate
- GAG
glycosaminoglycans
- HS
heparan sulfate
- KS
keratan sulfate
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MPS
mucopolysaccharidoses
- MRM
multiple reaction monitoring
- NBS
newborn screening
- HT-MS/MS
high-throughput tandem mass spectrometry
References
- 1).Tomatsu S, Montaño AM, Oikawa H, et al. Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions? Mol. Genet. Metab. 2014 Jun 4;(14):S1096–7192. doi: 10.1016/j.ymgme.2014.05.013. 00185-1. doi: 10.1016/j.ymgme. 2014. 05. 013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Dierenfeld AD, McEntee MF, Vogler CA, et al. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci. Transl. Med. 2010;2:1–8. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S, Hopwood JJ. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J. Clin. Invest. 1997;99:651–662. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Auclair D, Hopwood JJ, Brooks DA, Lemontt JF, Crawley AC. Replacement therapy in Mucopolysaccharidosis type VI: advantages of early onset of therapy. Mol. Genet. Metab. 2003;78:163–174. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 5).Baldo G, Mayer FQ, Martinelli BZ, et al. Enzyme replacement therapy started at birth improves outcome in difficult-to-treat organs in mucopolysaccharidosis I mice. Mol. Genet. Metab. 2013;109:33–40. doi: 10.1016/j.ymgme.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 6).Sands MS, Vogler C, Kyle JW, et al. Enzyme replacement therapy for murine mucopolysaccharidosis type VII. J. Clin. Invest. 1994;93:2324–2331. doi: 10.1172/JCI117237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Vogler C, Sands MS, Levy B, Galvin N, Birkenmeier EH, Sly WS. Enzyme replacement with recombinant beta-glucuronidase in murine mucopolysaccharidosis type VII: impact of therapy during the first six weeks of life on subsequent lysosomal storage, growth, and survival. Pediatr. Res. 1996;39:1050–1054. doi: 10.1203/00006450-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 8).Pievani A, Azario I, Antolini L, et al. Neonatal bone marrow transplantation prevents bone pathology in a mouse model of mucopolysaccharidosis type I. Blood. 2014 Oct 8; doi: 10.1182/blood-2014-06-581207. [pii: blood-2014-06581207] [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 10).Oguma T, Tomatsu S, Montaño AM, Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal. Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 11).Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfates in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed. Chromatogr. 2007;21:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 12).Tomatsu S, Montaño AM, Oguma T, et al. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucolipidoses II and III by tandem mass spectrometry. Mol. Genet. Metab. 2010;99:124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13).Tomatsu S, Montaño AM, Oguma T, et al. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis I. J. Inherit. Metab. Dis. 2010;33:141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- 14).Tomatsu S, Montaño AM, Oguma T, et al. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. J. Inherit. Metab. Dis. 2010;33(Suppl 3):S35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 15).de Ruijter J, de Ru MH, Wagemans T, et al. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol. Genet. Metab. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 16).de Ruijter J, Ijlst L, Kulik W, et al. Heparan sulfate derived disaccharides in plasma and total urinary excretion of glycosaminoglycans correlate with disease severity in Sanfilippo disease. J. Inherit. Metab. Dis. 2013;36:271–279. doi: 10.1007/s10545-012-9535-5. [DOI] [PubMed] [Google Scholar]
- 17).de Ru MH, van der Tol L, van Vlies N, et al. Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans. J. Inherit. Metab. Dis. 2013;36:247–255. doi: 10.1007/s10545-012-9538-2. [DOI] [PubMed] [Google Scholar]
- 18).Tomatsu S, Shimada T, Mason RW, et al. Establishment of Glycosaminoglycan Assays for Mucopolysaccharidoses. Metabolites. 2014;4:655–679. doi: 10.3390/metabo4030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Shimada T, Kelly J, LaMarr WA, et al. Novel heparan sulfate assay by using automated high-throughput mass spectrometry: application to monitoring and screening for mucopolysaccharidoses. Mol. Genet. Metab. 2014;113:92–99. doi: 10.1016/j.ymgme.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Shimada T, Tomatsu S, Mason RW, et al. Di-sulfated keratan sulfate as a novel biomarker for mucopolysaccharidosis IVA. JIMD. Rep. 2014 doi: 10.1007/8904_2014_330. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Shimada T, Tomatsu S, Yasuda E, et al. Chondroitin 6-sulfate as a novel biomarker for mucopolysaccharidosis IVA and VII. JIMD. Rep. 2014;16:15–24. doi: 10.1007/8904_2014_311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Tomatsu S, Montaño AM, Dung VC, et al. Enhancement of drug delivery: enzyme-replacement therapy for murine Morquio A syndrome. Mol. Ther. 2010;18:1094–1102. doi: 10.1038/mt.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Montaño AM, Oikawa H, Tomatsu S, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolysaccharidosis type VII mice. Mol. Genet. Metab. 2008;94:178–189. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 24).Tomatsu S, Montaño AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum. Mol. Genet. 2008;17:815–824. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 25).Tomatsu S, Shimada T, Mason RW, et al. Assay for Glycosaminoglycans by Tandem Mass Spectrometry and its Applications. J. Anal. Bioanal. Tech. 2014;(Suppl 2):006. doi: 10.4172/2155-9872.S2-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Tomatsu S, Fujii T, Fukushi M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Mol. Genet. Metab. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Johnston I. I’ll give you a definite maybe: An introductory handbook on probability, statistics, and excel. 2000 [ https://records.viu.ca/~johnstoi/maybe/title.htm]
- 28).Hintze JP, Tomatsu S, Fujii T, et al. Comparison of liquid chromatography-tandem mass spectrometry and sandwich ELISA for determination of keratan sulfate in plasma and urine. Biomark. Insights. 2013;6:69–78. doi: 10.4137/BMI.S7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Martell LA, Cunico RL, Ohh J, Fulkerson W, Furneaux R, Foehr ED. Validation of an LC-MS/MS assay for detecting relevant disaccharides from keratan sulfate as a biomarker for Morquio A syndrome. Bioanalysis. 2011;3:1855–1866. doi: 10.4155/bio.11.172. [DOI] [PubMed] [Google Scholar]
- 30).Auray-Blais C, Lavoie P, Zhang H, et al. An improved method for glycosaminoglycan analysis by LC-MS/MS of urine samples collected on filter paper. Clin. Chim. Acta. 2012;413:771–778. doi: 10.1016/j.cca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 31).Caterson B, Christner JE, Baker JR. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J. Biol. Chem. 1983;258:8848–8854. [PubMed] [Google Scholar]
- 32).Mehmet H, Scudder P, Tang PW, Hounsell EF, Caterson B, Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly (N-acetyllactosamine) series. Eur. J. Biochem. 1986;157:385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- 33).Neufeld E, Muenzer J, The Mucopolysaccharidoses . In: The Metabolic and Molecular Bases of Inherited Disease. 8th ed Scriver CR, Beaudet AL, Sly WS, Valle D, editors. McGraw-Hill; New York: 2001. pp. 3421–3452. [Google Scholar]
- 34).Tomatsu S, Okamura K, Maeda H, et al. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. J. Inherit. Metab. Dis. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 35).Tomatsu S, Montaño AM, Oguma T, et al. Validation of keratan sulfate level in Mucopolysaccharidosis IVA by liquid tandem mass spectrometry method. J. Inherit. Metab. Dis. 2010;33:35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]
- 36).Rowan DJ, Tomatsu S, Grubb JH, Montaño AM, Sly WS. Assessment of bone dysplasia by micro-CT and glycosaminoglycan levels in mouse models for mucopolysaccharidosis type I, IIIA, IVA, and VII. J. Inherit. Metab. Dis. 2013;36:235–246. doi: 10.1007/s10545-012-9522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Dingle JT, Horsfield P, Fell HB, Barratt ME. Breakdown of proteoglycan and collagen induced in pig articular cartilage in organ culture. Ann. Rheum. Dis. 1975;34:303–311. doi: 10.1136/ard.34.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Ratcliffe A, Doherty M, Maini RN, Hardingham TE. Increased concentrations of proteoglycan components in the synovial fluids of patients with acute but not chronic joint disease. Ann. Rheum. Dis. 1988;47:826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Tai GH, Huckerby TN, Nieduszynski IA. 600 MHz 1H NMR study of a fucose-containing heptasaccharide derived from a keratanase digestion of bovine articular cartilage keratan sulphate. Carbohydr. Res. 1994;255:303–309. doi: 10.1016/s0008-6215(00)90987-x. [DOI] [PubMed] [Google Scholar]
- 40).Crow J, Gibbs DA, Cozens W, Spellacy E, Watts RW. Biochemical and histopathological studies on patients with mucopolysaccharidoses, two of whom had been treated by fibroblast transplantation. J. Clin. Pathol. 1983;36:415–430. doi: 10.1136/jcp.36.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Wiesmann UN, Spycher MA, Meier C, Liebaers I, Herschkowitz N. Prenatal mucopolysaccharidosis II (Hunter): A pathogenetic study. Pediatr. Res. 1980;14:749–756. doi: 10.1203/00006450-198005000-00008. [DOI] [PubMed] [Google Scholar]
- 42).Beck M, Braun S, Coerdt W, Merz E, Young E, Sewell A. Fetal presentation of Morquio disease type A. Prenat. Diagn. 1992;12:1019–1029. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]
- 43).Rowan DJ, Tomatsu S, Grubb JH, et al. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol. Genet. Metab. 2012;107:161–172. doi: 10.1016/j.ymgme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Bhavanandan VP, Meyer K. Studies on keratosulfates. methylation, desulfation, and acid hydrolysis studies on old human rib cartilage keratosulfate. J. Biol. Chem. 1968;243:1052–1059. [PubMed] [Google Scholar]
- 45).Lauder RM, Huckerby TN, Nieduszynski IA. The structure of the keratan sulphate chains attached to fibromodulin from human articular cartilage. Glycoconj. J. 1997;14:651–660. doi: 10.1023/a:1018552913584. [DOI] [PubMed] [Google Scholar]
- 46).Nieduszynski IA, Huckerby TN, Dickenson JM, et al. There are two major types of skeletal keratan sulphates. Biochem. J. 1990;271:243–245. doi: 10.1042/bj2710243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Liles M, Palka BP, Harris A, et al. Differential relative sulfation of Keratan sulfate glycosaminoglycan in the chick cornea during embryonic development. Invest. Ophthalmol. Vis. Sci. 2010;51:1365–1372. doi: 10.1167/iovs.09-4004. [DOI] [PubMed] [Google Scholar]
- 48).Brown GM, Huckerby TN, Bayliss MT, Nieduszynski IA. Human aggrecan keratan sulfate undergoes structural changes during adolescent development. J. Biol. Chem. 1998;273:26408–26414. doi: 10.1074/jbc.273.41.26408. [DOI] [PubMed] [Google Scholar]
- 49).Hasegawa N, Torii T, Kato T, et al. Decreased GlcNAc 6-O-sulfotransferase activity in the cornea with macular corneal dystrophy. Invest. Ophthalmol. Vis. Sci. 2000;41:3670–3677. [PubMed] [Google Scholar]