Abstract
As part of the screening program for anticancer agents from natural sources, the sesquiterpene lactone goyazensolide (GZL) was identified as a potent NF-κB inhibitor. The hollow-fiber assay was used to evaluate the anti-tumor efficacy of GZL in vivo. The mechanistic effects of GZL were evaluated in the HT-29 colonic cell line to reveal the pathway through which GZL exerts its effects. NF-κB subunits p65 and p50 were inhibited, and the upstream mediator IκB kinase (IKKβ) was downregulated in a dose-dependent manner. Apoptosis was mediated by caspase-3, and cell cycle arrest was detected in G1-phase. Consequently, 96% of the cell population was in sub G1-phase after treatment with GZL (10 μM).The antitumor effect of GZL was observed at a dose of 12.5 mg/kg. Cell adhesion was affected as a result of NF-κB inhibition. GZL appears to selectively target the transcription factor NF-κB. In summary, GZL sensitizes HT-29 colon cancer cells to apoptosis and cell death in a dose-dependent manner both in vivo and in vitro, through NF-κB inhibition (IC50 = 3.8 μM). Thus, it is a new potent lead compound for further development into a new effective chemotherapeutic agent.
Keywords: Goyazensolide, NF-κB, apoptosis, caspase-3, ROS, adhesion
INTRODUCTION
Goyazensolide (GZL) is a naturally occurring heliangolide discovered mainly from plants of the tribe Vernonieae of the family Asteraceae. This compound was first isolated from Eremanthus goyazensis as part of a research program to find schistosomicidal agents(Vichnewski et al., 1976), and was obtained subsequently from Eremanthus mattogrossensis (Lunardello et al., 1995), Lychnophora granmongolense (Grael et al. 2000), Lychnophora pohlii (Grael et al., 2005a),Camchaya calcarea (Vongvanich et al., 2006), Centratherum punctatum (Valdes et al., 1998), and Piptocoma rufescens (Ren et al., 2012). GZL, isolated previously from Piptocoma rufescens in our research program, exhibits multiple bioactivities, including schistosomicidal, trypanocidal (Grael et al., 2005b; Vichnewski et al., 1976; Grael et al., 2005a Borkosky et al., 2009), antimycobacterial (Vongvanich et al., 2006), cytotoxic (Dos Santos et al., 2004; Vongvanich et al., 2006; Ren et al., 2012) and genotoxic (Mantovani et al., 1993) effects. It has also been reported by our research group that this compound shows nuclear factor kappa B (NF-κB) inhibitory activity (Rungeler et al., 1999; Siedle et al., 2004; Wagner et al., 2006; Ren et al., 2012).
Nuclear factor κB, a light-chain-enhancer of activated B cells, is a protein complex composed of subunits p50 and p65 that controls the transcription of DNA and is involved in the regulation of many diseases including cancer (Nakanishi and Toi, 2005). When inactive, NF-κB is bound to inhibitor of κB (IκB)_located in the cytosol (Nakanishi and Toi, 2005). A variety of intracellular or extracellular signals, including tumor necrosis factor (TNFα)(Fitzgerald et al., 2007), reactive oxygen species (ROS) (Gloire et al., 2006) and epidermal growth factors (EGF) can cause activation of NF-κB by phosphorylation of IκB and then dissociation of IκB from NF-κB (Nakanishi and Toi, 2005). The activated NF-κB is translocated into the nucleus and binds to promoter regions of the DNA to activate transcription of a wide range of target genes involved in apoptosis inhibition, cell adhesion, and cell mediation (Nakanishi and Toi, 2005). Such effects protect tumor cells from apoptosis. The IKKα (inhibitor of nuclear factor kappa-B kinase subunit α) and IKKβ are kinases that dissociate IκBα from NF-κB. Inhibition of IKKα and IKKβ results in the suppression of NF-κB activation and blocks tumor cell proliferation (Nakanishi and Toi, 2005). Therefore, IKKα, IKKβ, and NF-κB are widely used as molecular targets for the discovery new anticancer drugs (Li et al., 2011; Wu and Zhou, 2010). Recently, several natural products have been reported to exhibit potential antitumor activity through inhibition of NF-κB activation and some are under clinical development, such as parthenolide (Kishida et al., 2007; Oka et al., 2007; Idris et al., 2009).
As part of a search for new anticancer agents from diverse organisms (Kinghorn et al., 2009), GZL showed both cytotoxic and NF-κB p65 inhibitory activities in vitro (Ren et al., 2012). In the present study, the antitumor efficacy of GZL was evaluated using a mice model. The animal model, using immune deficient NCr nu/nu mice and hollow fiber, allow different cancer cell lines to be used in vivo. The mechanism through which this compound exerts inhibition of NF-κB activation and induction of apoptosis was also investigated.
MATERIALS AND METHODS
Plant material
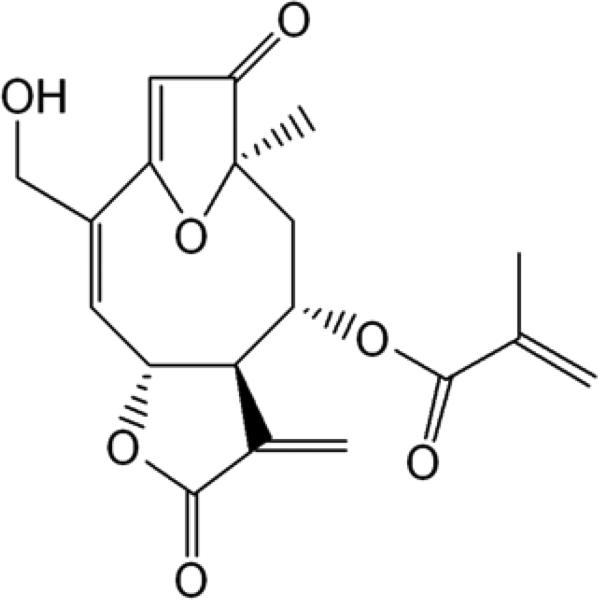
A chromatographically and spectroscopically pure crystalline sample of goyazensolide (GZL) was isolated from the leaves of Piptocoma rufescens in a previous study (Fig. 1) (Ren et al., 2012).
Fig. 1.
Chemical structure of GZL, a sesquiterpene lactone from Piptocoma rufescens.
Cell culture
The colonic cancer HT-29 and cervical HeLa cell lines were purchased from American Type Culture Collection (ATCC), Manassas, VA. The HT-29 cells were grown in RPMI-1640 medium and was purchased from Gibco (Rockville, MD, USA), supplemented with 10% (v/v) fetal bovine calf serum (FBS) and 10% antibiotic-antimycotic. The HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and 10% antibiotic-antimycotic (Gibco). The cells were kept at 37°C and in an atmosphere with 5% CO2.
Western blotting
Immunoblotting experiments were performed following a previously reported protocol with some modifications (Acuna et al., 2012a). To observe the effect of GZL on the NF-κB pathway, HT-29 cells were seeded in 10 cm dishes and treated with GZL at five different concentration levels (0.008, 0.016, 0.4, 2.0 and 50 μM) and with the positive control, rocaglamide from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). Cells were incubated for 8h followed by 30 min treatment with or without human recombinant tumor necrosis factor α (TNFα) (10 ng/ml) from Thermo Scientific (Rockford, IL, USA). Cells were lysed and whole cell protein lysates were prepared using PhosphoSafe Lysis Buffer from Novagen (Madison, WI, USA). The protein concentrations were measured by using a Bradford protein assay kit and albumin standard (Thermo Scientific). To determine the protein concentration present in cell lysates and in albumin standard dilutions, absorbance was measured using a Fluostar Optima plate reader (BMG Labtech Inc, Durham, NC). The protein content in cell-lysates was extrapolated on the standard curve created for albumin standard. Sample volume containing 20 μg of proteins together with lithium dodecyl sulfate sample loading buffer (LDS), were loaded to Nu-PAGE 10% SDS-PAGE Bis-Tris gels, together with SeeBlue® Plus2 Pre-Stained Standard Ladder from Invitrogen (Carlsbad, CA, USA). The proteins were separated by electrophoresis performed with SDS-PAGE running buffer in a Nu-PAGE XCell SureLock Module (Invitrogen), and transferred to a polyvinyldiene fluoride (PVDF) membrane from Thermo Scientific, using TBS-T buffer (TBS-T)( Sigma Aldrich, St. Louis, MO, USA). The blots were blocked overnight at room temperature with non-fat dried milk and subsequently probed by primary antibodies (1:1000) against each target protein using 1% BSA in TBS-T. Primary antibodies (anti-NF-κBp65 and p50, anti-IKKα, anti-IKKβ, anti-intracellular adhesion molecule 1(ICAM-1) and anti-caspase-3) were purchased from Cell Signaling Technologies (Beverly, MA, USA) and detected by western blot analysis with HRP conjugated secondary antibodies (1:2000) from purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Conjugated antibodies were detected using a chemiluminescent substrate, Supersignal Femto LumiGLO kit (Thermo Scientific).
Animals and treatment regimen
Eight- to nine-week old immunodeficient NCr nu/nu mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used in this study. The mice were housed in laminar-flow cages at room temperature and a relative humidity of 50-60% under 12:12 h light-dark cycle with specific pathogen-free conditions and kept under such condition for at least one week before experiment. All animal work was approved by University of Illinois at Chicago Animal Care and Use Committee, and the mice were treated in accordance with the institutional guidelines for animal care. GZL and taxol were dissolved initially in DMSO and subsequently diluted with Cremophor®. The mixture was then diluted with distilled water to its final injection state which consisted of 13% DMSO and 25% Cremophor®. The mice were treated daily with GZL, the positive control (taxol), or the negative control (the vehicle solution) by i.p. injections for four days.
Hollow fiber assay
The antitumor effects of GZL were confirmed in vivo, using the hollow fiber assay that was conducted as described previously (Mi et al., 2002; Mi et al., 2009). Human cancer cell lines designated colon HT-29 and melanoma MDA-MB-435 were propagated in RPMI-1640 medium supplemented with FBS (5% vol/vol) and 2 mM glutamines at 37°C in a 5% CO2 atmosphere. Monolayer cultures in late log-phase growth were released by digestion with trypsin, and suspended in medium. Sterile conditioned polyvinylidene fluoride hollow fibers perforated with 500 kDa molecular weight exclusion pores were filled with the cells (HT 29; 1 × 106 and MDA-MB 435: 2.5 × 106 per fiber) (Hollingshead et al., 1995). The fibers were then heat sealed at two-cm intervals and cut to generate the fibers used for the study. Prior to implantation, the fibers were cultured overnight at 37°C in a 5% CO2 atmosphere. A set of fibers remained in culture to confirm sterility. The remaining fibers were transplanted into immunodeficient female NCr nu/nu mice. For intraperitoneal (i.p.) implants, a small incision was made through the skin and musculature of the dorsal abdominal wall, the fiber samples were inserted into the peritoneal cavity in a craniocaudal direction, and the incision was closed with skin staples. For subcutaneous (s.c.) implants, a small skin incision was made at the nape of the neck and an 11-gauge trocar containing a hollow fiber, was inserted caudally. The incision was closed with skin staples. On day three, the mice were treated with GZL at the 3.125, 6.25 and 12.5 mg/kg in four daily i.p. injections, followed by fiber retrieval on day 7. Taxol was administered at a dose of 3 mg/kg in the same vehicle. Each mouse was weighed daily during the study. On day 7, all remaining mice were sacrificed and the fibers were retrieved and viable cell mass was evaluated by a modified MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Alley et al., 1991). The percent net growth for each cell line in each treatment group was calculated by subtracting the day-zero absorbance from the day 7 absorbance and dividing this difference by the net growth in the day 7 vehicle-treated controls minus the day-zero values. The method of statistical methodology was previously published (Pearce et al., 2012).
ROS assay
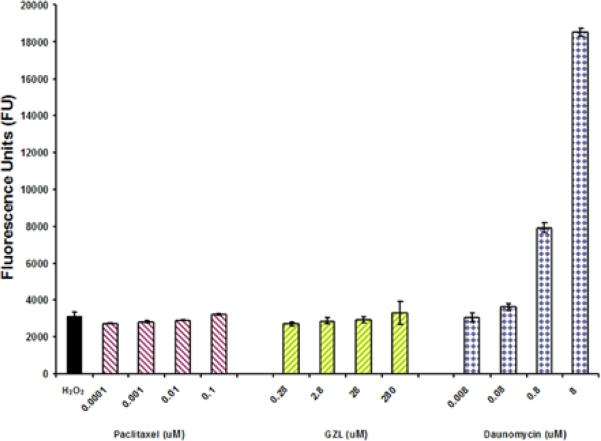
The assay was performed following a previously described procedure (Kimet al., 2010; Acuna et al., 2012b. HT-29 cells were seeded in a 96-well plate then, the cells were treated with GZL (0.028-280 μM), paclitaxel (0.0001-0.1 μM) (Sigma Aldrich), and daunomycin (0.08-8 μM) (Tocris, Bristol, UK) and incubated at 37°C with 5% CO2 for 5h. This was followed by a treatment with H2O2 from Fluka Biochemika (Steinhiem, Switzerland) /FeSO4 (1.25 mM/0.2 mM)( Fischer Scientific Company , Fair Lawn, NJ) (the positive control) and probed with the fluorescent probe DCFH-DA (5 mM) (Sigma Aldrich) for 30 min at 37°C. Vitamin C (Sigma Aldrich) was used as negative control. Fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a FLUOstar Optima fluorescence microplate reader (BMG Labtechnologies Inc., Durham, NC, USA). Each experiment was carried out in triplicate and the data was representative of at least two different experiments.
Cell adhesion assay
Cell suspension was plated in a 48-well plate, grown confluent, and treated in triplicates with different concentrations of GZL (0.028-280 μM) for 5h, following previously reported procedure (Acuna et al., 2012a). The cells were then washed with PBS three times. The attached cells were photographed using the ProgResC10 plus camera from Jenoptik, Carl Zeiss, GmbH, Germany attached to the microscope Axiovert 40 CFL from Zeiss at X20 magnification. After treatment, the cells were then trypsinated and re-suspended in cell media before being counted using hemocytometer and an inverted microsope (Axiovert 40 CFL, Zeiss). Cell adhesion was calculated based on non-treated cells that were used as control.
Cell cycle analysis
Cells (HT-29-colon) were seeded into 10 cm dishes and 24h later treated using two different concentrations of test samples or solvent control. After 24h of incubation, cells were trypsinized, pelleted by centrifugation, washed with PBS and fixed in ice-cold 70% ethanol. DNA was stained with propidium iodine (PI) (10 μg/mL) (Sigma Aldrich) in a reaction solution containing 1 mM EDTA and 100 μg/mL Purelink RNase A, obtained from Invitrogen. Fluorescence emitted from the propidium iodine-DNA complex was quantified using BD FACS Canto II at 488 nm.
Caspase-3/7 assay
Caspase-3/7 activities were tested in HT-29 cells using the caspase- Glo3/7® assay (Promega, Madison, WI, USA). The assay was performed following the manufacturer's instructions and a previously published procedure with some modifications (Acuna et al., 2012b). The HT-29 cells were seeded in a 96-well plate and treated at three concentration levels with GZL (0.08, 0.4 and 2.0 μM), and paclitaxel (0.001, 0.01, 0.1μM). Plate was incubated for 3h and caspase- Glo3/7® reagent was added 30 min before luminescence was measured. Luminescence units (LU) were recorded using Fluostar Optima plate reader (BMG Labtechnologies Inc.) at 37°C.
Statistical analysis
Statistical analysis for obtained results was carried out with the aid of Excel computer software program.
RESULTS
NF-κB inhibiting effect of GZL on HT-29 cells
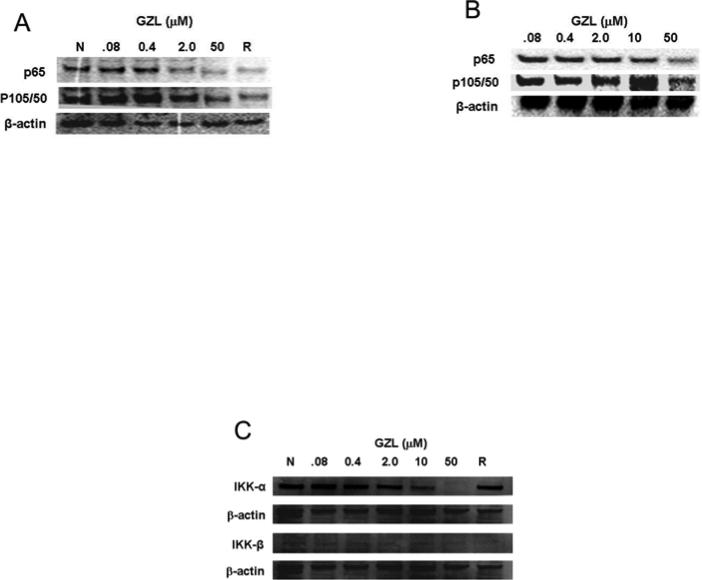
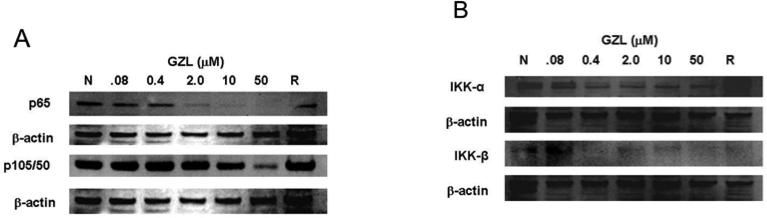
As part of a drug discovery program, GZL was previously identified as a potent NF-κB inhibitor on HeLa cervical cancer cells when using an Elisa assay (Ren et al., 2012). The NF-κB inhibition (IC50 = 3.8 μM) was compared to the effects exhibited by rocaglamide (IC50= 0.075 μM), a potent inhibitor of the NF-κB signaling pathway. A further analysis of the data previously reported, using western blot and HeLa cells, confirmed the NF-κB inhibitory effects of GZL. The expression of NF-κB subunits p65 and p50 decreased in absence (Fig. 2A) and presence of TNFα treatment (Fig. 2B). Inhibition on the activating kinases, upstream in the NF-κB pathway, IKKα and IKKβ was also observed (Fig. 2C). The effects observed on the protein expression, when using western blot and HeLa cells, were more noticeable with increasing concentrations. Thus the underlying molecular effects on the NF-κB pathway by GZL were further evaluated using HT-29 cells and western blot analysis. GZL exhibited dose-dependent inhibition of p65 and p50 both in presence or absence of TNFα (Fig. 3A). The upstream mediators, IKKα and IKKβ, in the NF-κB pathway were also downregulated (Fig. 3B) in a concentration-dependent manner after treatment with TNFα. The suppression was particularly prominent on the IKKβ in both HeLa and HT-29 cells, which shows that the canonical NF-κB pathway was inhibited upstream in the pathway on the treated cells.
Fig. 2.
GZL decreases the expression of NF-κB subunits p65 and p50 in (A) absence and (B) presence of TNFα treatment. The effects were compared to the negative control (N) and to the positive control, rocaglamide (R). Western blot analysis was performed on whole cell HeLa extracts and showed that a decrease in activity was detected upon higher concentrations of GZL. (C) IKKα and IKKβ were down-regulated in treated HeLa cells. β-Actin expression was used as an internal control to establish equivalent protein loading.
Fig. 3.
GZL decreases the expression of NF-κB mediators in HT-29 cells. GZL suppresses NF-κB activation in comparison with the negative control (N) and the positive control rocaglamide (R), in the presence of TNFα treatment. (A) A decrease in NF-κB p65 and p50 subunits was detected at different concentrations of GZL. (B) IKKα and IKKβ were downregulated in treated HT-29 cells. β-Actin expression was used as an internal control to establish equivalent protein loading.
Inhibition of cell growth and suppression of tumor progression in vivo
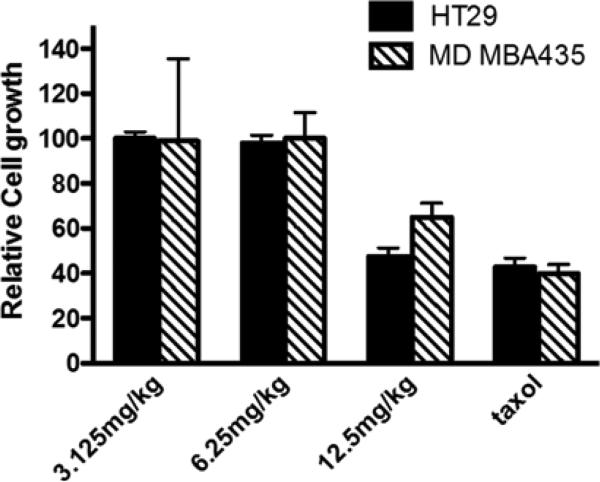
GZL exhibited anti-tumor effects in vivo against HT-29 colon cancer cells, when using the hollow fiber assay (Fig. 4). Taxol was used as a positive control (Fig. 4). The treated animals showed low signs of toxicity at 15 mg/kg and the compound was lethal at 20 mg/kg. At the highest dose (GZL 12.5 mg/kg), the effects on cell growth were comparable to that of the treatment with taxol at the dose of 3 mg/kg. Similar efficacy of GZL and paclitaxel was found in treated HT-29 colon cancer cells.
Fig. 4.
Anti-tumor effects of GZL in vivo. GZL inhibits human colon cancer HT-29 cells and melanoma MDA-MB-435 cells in vivo at 12.5 mg/kg. Taxol was used as a positive control.
Intracellular levels of ROS after treatment with GZL
The effects of GZL on the intracellular levels of ROS were not significant in comparison with the positive control daunomycin (Fig. 5). Daunomycin is known as a potent ROS inducing agent in cancer cells and as a DNA damaging agent (Gervasoni et al., 2004). GZL effects were similar to those of paclitaxel. GZL showed only a low inducing ROS effect after 5h of treatment.
Fig. 5.
GZL inhibits the NF-κB activation through ROS independent pathway. The cells were treated for 5h at four different concentration levels of GZL and taxol. ROS was detected with fluorescent probe DCFH-DA at 485 nm emission wavelength and 530 nm excitation wavelength. Daunomycin was used as a positive control. Each experiment was carried out in triplicate. Values represent the means of fluorescence units (FU) ± SEM of triplicate samples from two separate experiments.
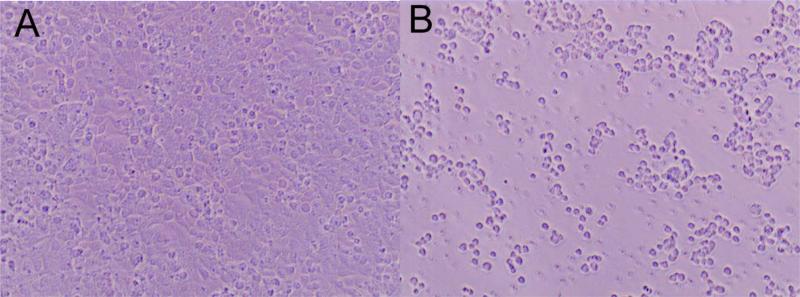
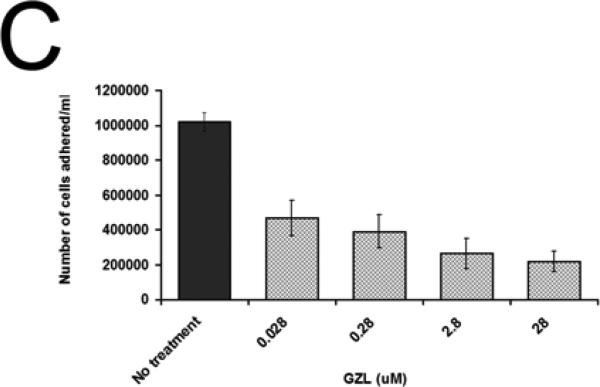
Effects of GZL on cell adhesion of colon HT-29 cancer cells
Reduction of cell adhesion is a downstream effect of NF-κB inhibition. To test if GZL had an inhibitory effect on cell adhesion (Fig. 6A), HT-29 cells were incubated with GZL for 5h over a range of concentrations (0.28-280 μM). Treatment with GZL affected cell adhesion in HT-29 cells (Fig. 6B). After 5h treatment with GZL, 78% of the treated cells were detached from surface when treated at 28 μM. At the lowest concentration 0.028 μM, 54% of cells were detached from surface after 5h of incubation under the same conditions as the untreated cells (Fig. 6C). GZL also dose-dependently downregulated the expression of ICAM1 in HT-29 cells, when using western blot analysis (Fig. 6D). This data strongly suggest that adhesion of cells was negatively affected by GZL.
Fig. 6.

GZL treatment resulted in a decreased number of adhered HT-29 colon cancer cells. (A) Cell adhesion was unaffected in untreated HT-29 cells after 5h incubation. (B)Effect on cell adhesion after 5h of treatment with GZL (28 μM). (C) A dose dependent decrease in cell adhesion of HT-29 was detected at different treatment levels (0.028-28 μM). After treatment at the highest concentration (28 μM) and at the lowest concentration (0.028 μM), 78% and 54% of cells were detached from surface, respectively. Bars on the graph represent the means of the number of cells attached (NCA) ± SEM of triplicate samples from two independent experiments. (D) GZL down-regulated ICAM in a dose-dependent manner in HT-29 cells.
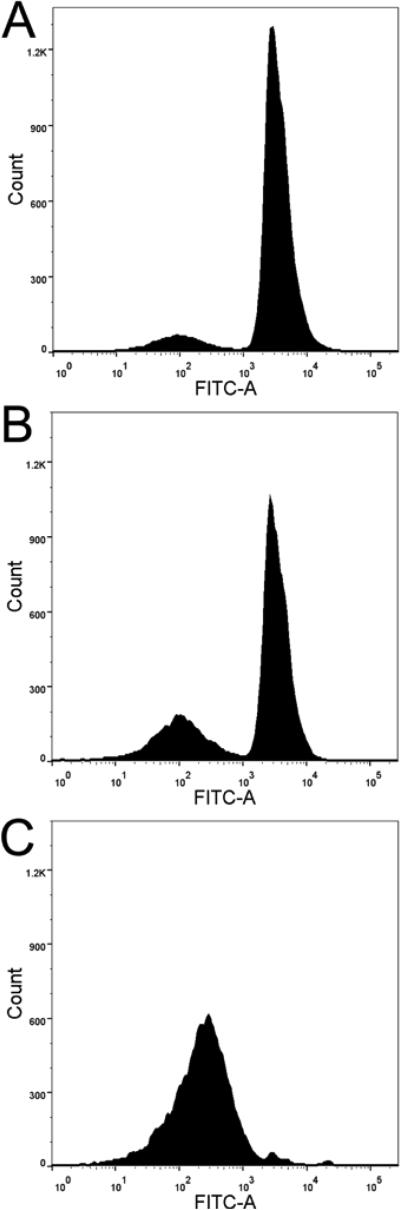
Cell cycle arrest on HT-29 cells
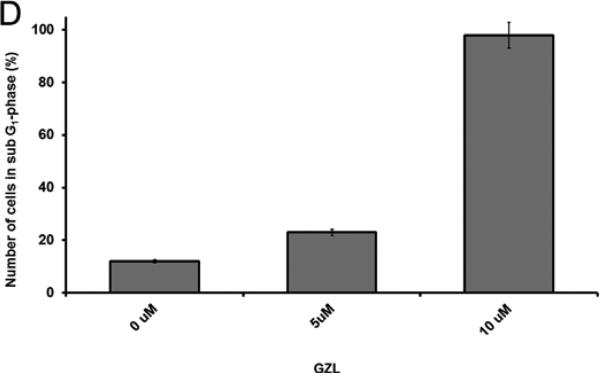
The proapoptotic effect of GZL was confirmed by cell sorting analysis after 24h of treatment, at different levels of concentration (5 and 10 μM). Cell cycle arrest was detected in sub G1-phase by fluorescence-activated cell flow cytometry. Not treated HT-29 cells showed 12% of cells in sub G1-phase and 49% in G1-phase (Fig. 7A). After treatment with GZL at 5 and 10 μM, 23%, and 96% of cells were found in sub G1-phase, respectively (Figs.7B and C). The cells in G1-phase decreased with increasing concentrations of GZL, 48% were in G1-phase at 5 μM and 2 % were in G1-phase at 10 μM, respectively. After treatment with 10 μM of GZL, however; over 90% of HT-29 cells had undergone apoptosis and were in sub G1-phase.
Fig. 7.
The apoptotic effects of GZL were detected in HT-29 cells by cell flow cytometry. Fluorescence emitted from the PI-DNA complex was quantified at 488 nm and presented using a DNA histogram for HT-29 cells. (A) Control showed 12% HT-29 cells in G1- phase. (B) Treatment (5 μM) showed 23% HT-29 cells in sub G1-phase, and 48% in G1-phase. (C) Treatment (10 μM) with GZL induces cell-cycle arrest in sub G1-phase (96%). At the lower concentration of GZL (5 μM), 48% of cells were in G1-phase and at the higher concentration of GZL (10 μM), 2 % of cells were in G1-phase. (D) Effect of GZL on HT-29 colon cancer cells treated at 5 and 10 μM (cells in sub G1-phase) in comparison with untreated cells.
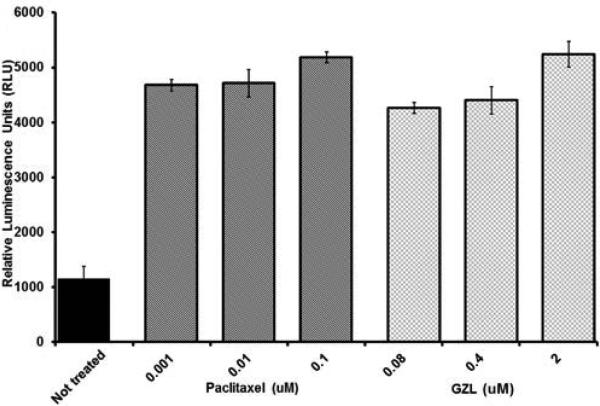
GZL induced caspase-3/7 activity
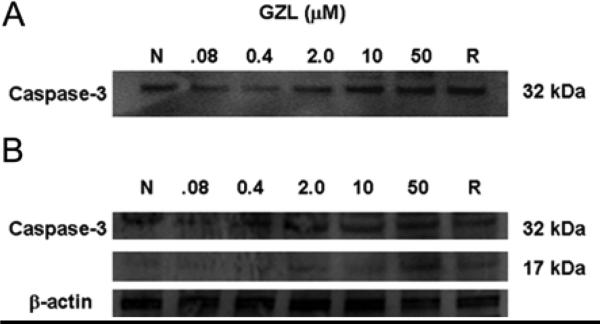
The caspase activity in treated HT-29 cells was tested using the luminescent assay caspase −3/7®GLO. Although the dose of taxol was 20 times lower than GZL, both exhibited caspase enzyme activity in a dose-dependent manner. A 4.6- fold increase of caspase-3 activity was induced after 3h when treated with GZL at a concentration of 2.0 μM (Fig. 8). The immunoblot analysis of caspase-3 showed a dose-dependent (0.08-50 μM) increase of the expression of procaspase-3 (32 kDa) after 3h (Fig. 9A). An increase in the cleavage of procaspase-3 (32 kDa) and a subsequent increase of the subunit of caspase 3 (17 kDa) was detected after 24h. The cleavage of procaspase-3 (32 kDa) and the detection of the fragment of 17 kDa (Fig. 9B) suggested that HT-29 cells undergo caspase-dependent apoptosis in a dose-dependent manner when treated with GZL.
Fig. 8.
GZL increased the activity of caspase 3/7 in treated HT-29 colon cancer cells. Caspase 3/7 activity is presented after 3 h of treatment with GZL (0.08-2.0 μM) and positive control, paclitaxel (0.001-0.1 μM), respectively. Bars on graph represent the means of relative luminescence units (RLU) ± SEM of triplicate samples from two independent experiments.
Fig. 9.
Effects of GZL on caspase-3. (A) GZL increases the levels of caspase-3 in HT-29 cells after 3h. Immunoblot analysis showed an increased level of expression of caspase-3 (32 kDa). The effect was dose dependent, after been treated with increasing concentration of GZL. (B) Cleavage of caspase-3 increased after 24h in a dose dependent manner.
DISCUSSION
The transcription factor NF-κB regulates apoptosis, cell adhesion, and survival of cancer cells (Scaife et al., 2002). Activation of NF-κB through phosphorylation by the IKK complex (IKKα and IKKβ) and subsequent released from IκB in the cytoplasm, occur in the presence of TNFα as an activator, and is known as the canonical NF-κB pathway (Garg and Aggarwal, 2002; Fitzgerald et al., 2007). Inhibition of NF-κB results in apoptosis and suppression of tumor cell growth (Li et al., 2011; Olivera et al., 2012; Sato et al., 2011; Wilken et al., 2011; Wu et al.,2011; Wu and Zhou, 2010; Kishida et al., 2007; Oka et al., 2007; Idris et al., 2009; Moon et al., 2009). Thus, this protein complex has been widely used as a key target for new anticancer drug discovery and development (Garg and Aggarwal, 2002) .
Our previous report on GZL showed that GZL exhibited NF-κB p65 inhibitory activity in an Elisa assay when using HeLa cells (Ren et al., 2012). The present study confirmed this by immunoblot analysis in both HeLa cells and HT-29 cells. Moreover, GZL dose-dependently attenuated the upstream mediators, IKKα and IKKβ in both HeLa and HT-29 cells (Figs. 2 and 3). This is of particular interest since the deletion of IKKβ has resulted in marked decrease of tumor incidence (Greten et al., 2004). These results suggested that GZL mediates its cytotoxicity in HeLa and HT-29 cells through NF-κB inhibition and shows potential as an anti-tumorigenic agent.
As part of our drug discovery program, a panel of cell lines has been standardized for in vivo studies, using the hollow fiber assay. The HT-29 colon cancer cell line is part of this panel of cancer cell lines. After confirming the NF-κB inhibitory effect of GZL in HT-29 cells, an in vivo study was pursued (Fig. 4). This assay was developed to bridge cell-based in vitro assays and a xenograft assay system (Casciari et al., 1994). Several natural products have been found to be active in this assay-system in our discovery program of anticancer agents from diverse organisms (Mi et al., 2009. Inhibition of cell growth in HT-29 cells was further evaluated using this assay system, and it was found that GZL significantly suppressed tumor cell growth at a dose of 12.5 mg/kg by i.p. administration. The in vivo results indicated that GZL warrants for further investigation as a potential anticancer drug candidate and lead compound against colon cancer. Thus further studies on the NF-κB pathway and cell cycle effects were performed using HT-29 cells.
There are several mechanisms of induction of the NF-κB pathway. The NF-κB pathway can be induced by divergent pathways and is affected by cytokinins, ROS and K-Ras. Since GZL did not inhibit K-Ras activity, when induced by EGF (Acuna et al., 2012a) (graphed data is not shown, but GZL exhibited less than 50% inhibition at 100 μM) and previous reports indicate that ROS may regulate NF-κB, acting as an important intracellular second messenger that induces NF-κB activation, we also examined the effect on ROS (Gloire et al., 2006). ROS has been proposed as a second messenger, signaling activation of NF-κB through a delayed ROS-dependent signaling pathway, named the oxidant pathway, which is separate from the TNF-α-mediated NF-κB pathway (Phalitakul et al.. 2011). ROS can also regulate cell adhesion (Vlahopoulos et al., 1999) and increase caspase-3 expression, which initiates apoptosis (Olivera et al., 2012). GZL did not significantly increase ROS generation in the HT-29 cells, when compared to the effects of daunomycin. The ROS-increasing effects in HT-29 cells of both GZL and paclitaxel were comparable to the hydrogen peroxide; however, in comparison with a potent ROS inducer such as daunomycin, GZL did not induce significant levels of ROS. It is possible that the cytotoxic effect is mainly arbitrated by another pathway than the oxidative pathway in treated HT-29 cells. Thus our findings suggests that the ROS generating activity of GZL might be partially responsible for initiating oxidative stress, but it appears that another mechanism is involved in the significant effect exhibited by GZL, leading to cell death of HT-29 colon cancer cells. Hence, our findings confirmed that GZL selectively targets the TNFα induced canonical pathway of NF-κB.
Several independent studies have shown that NF-κB regulates cell adhesion, and that the expression of intracellular adhesion molecule (Bonizzi and Karin, 2004) which is involved in the metastasis of tumors and progression of the disease (Addabbo et al., 2012). It has been suggested that NF-κB inhibition leads to cell adhesion–dependent apoptosis (Scaife et al., 2002), since monolayer cells commit irreversibly to cell cycle progression at a restriction point, late in G1-phase, and both growth factors and cell adhesion are requirements for proliferation of cells (Zhu et al., 1996). Hence, the effect of GZL on adhesion of HT-29 colon cancer cells might be directly correlated to cell death of GZL treated cells, since adhesion is a requirement for the proliferation.
Moreover, it has recently been reported that suppression of NF-kB activation prevents the expression of TNF-α-induced intracellular adhesion molecules, which interferes with monocyte adhesion and angiogenesis (Addabbo et al., 2012, Chen et al., 2011, Thapa et al., 2009). It has also been shown that suppression of NF-kB activation prevents the expression of intracellular adhesion molecules. Furthermore, ICAM1 has shown to mediate the interaction between HT-29 cells and inflammatory cells, which play an important role in cancer progression and chronic colonic conditions (Kim et al., 2012a). In our findings, as downstream effect of NF-κB inhibition, GZL significantly downregulated ICAM1 and interfered with cell adhesion in HT-29 cells, and similar effects on the expression of ICAM1 were recently found as result of treatments with mollugin from Rubia cordifolia L., a Chinese traditional medicinal plant (Kim et al., 2009). Similarly, Mino and co-workers have shown that cancer progression can be suppressed by inhibiting gastric cancer cell adhesion to the peritoneum through NF-κB inhibition (Mino et al., 2011).
The apoptotic effect of GZL in HT-29 was confirmed by cell flow cytometry. The data showed that treatment with GZL increased the number of cells in sub G1-phase while a significant change was also observed in G1-phase, suggesting a block in G1-phase. The restriction point in late G1-phase is crucial to cell cycle progression of adherent cells such as HT-29 colon cancer cells (Zhu et al., 1996). G1-phase is also involved in cellular metabolic changes, which are necessary for cell division. Lack of cell division will then affect cell growth and cell death. This effect might contribute to the cytotoxic effect of GZL on the HT-29 cancer cells. Caspase-3 is an important mediator of apoptosis (Ringer et al.. 2010; Wu and Zhou, 2010; Sa and Das, 2008; Moon et al., 2009), and some natural products have shown to induce apoptosis arbitrated by caspase-3. In this study, GZL exhibited significant dose-dependent caspase 3/7 activity. GZL induced in a dose-dependent manner, the expression of caspase-3 in HT-29 cancer cells. This suggested that the pro-apoptotic effect of GZL in HT-29 cells was mediated through a caspase-dependent pathway, which led to cell cycle arrest in G1-phase.
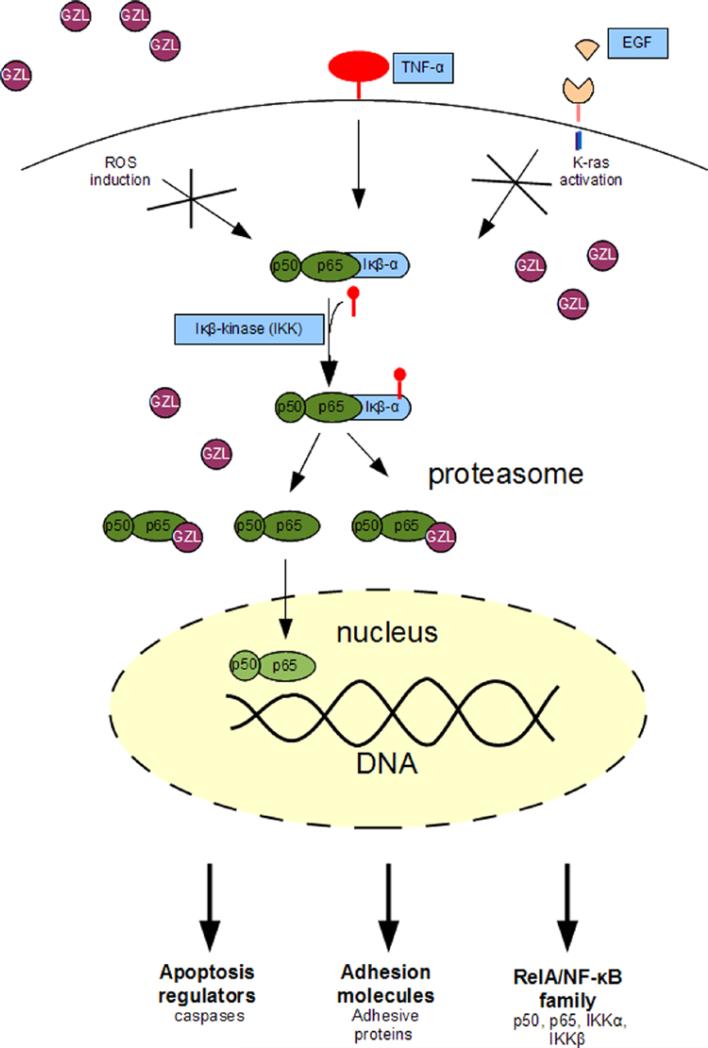
Several goyazensolide-type and related sesquiterpene lactones have shown cytotoxic and NF-κB inhibitory activities (Rungeler et al., 1999; Siedle et al., 2004; Wagner et al., 2006; Ren et al., 2012). The present study shows that the cytotoxicity of GZL toward the HT-29 cells have resulted from the inhibition of TNFα-mediated NF-κB activation which induced procaspase-3 expression in treated cells, leading to apoptosis and cell cycle arrest in G1-phase. These findings are consistent with previous reports showing similar mechanisms of action for parthenolide, an epoxylated sesquiterpene lactone (Shanmugam et al. 2010; Kishida et al., 2007; Oka et al., 2007; Idris et al., 2009; Kim et al., 2012b), and sulforaphane, an organosulfur natural product (Moon et al., 2009). As a result of these effects, target genes are not transcribed, affecting cell adhesion, apoptosis regulators, and also possibly inflammatory cytokines and mediators such as IL-1, IL-2, IL-6, IL-8, TNFα and growth factors. In addition, the present study has shown that GZL inhibits the NF-κB pathway in colon cancer HT-29 cells, in part by downregulating the activating kinase IKKβ, an important mediator in the activation of the canonical NF-κB signaling pathway. A schematic description and overview of the NF-κB effects of GZL on HT-29 cells is presented in Fig. 10.
Fig. 10.
A schematic description of the NF-κB inhibitory effect of GZL on treated HT-29 colon cancer cells.
Previous studies have shown that inhibition of NF-κB activation induce apoptosis and suppress tumor growth (Li et al., 2011; Olivera et al., 2012; Sato et al., 2011; Wilkenet al., 2011.; Wu and Zhou, 2010; Ahmad et al., 2002; Kishida et al., 2007; Oka et al., 2007; Idris et al., 2009; Moon et al., 2009). The animal model confirmed that the NF-κB inhibition resulted in antitumor effects in vivo, by sensitizing malignant cells to undergo apoptosis and decreasing the proliferative activity. In our studies, tumor cell apoptosis was induced through activation of caspase-3 (Ringer et al., 2010; Wu and Zhou, 2010; Moon et al., 2009). Furthermore this treatment could prevent tumor cells from developing resistance to chemotherapeutic agents, and sensitize HT-29 cells to existing cancer treatment (Garg and Aggarwal, 2002; Nakanishi and Toi, 2005). The cytotoxicity of GZL, the inhibition of the NF-κB pathway, and the downstream effects, which consequently arrests the progression of tumor growth in vivo, warrants further investigation of GZL as a potential effective agent to treat colon cancer. The downstream effect of GZL on the NF-κB pathway also affects cell adhesion, which would be preventing metastasis of colon cancer tumors. Therefore, GZL is a promising anticancer lead compound that could be developed to be used alone or in combination with chemotherapeutic agents to improve the efficacy of existing anticancer treatments.
CONCLUSION
GZL inhibits the proliferation of colon cancer cells. GZL had an attenuating effect in a concentration-dependent manner on the upstream mediators IKKα and IKKβ, which resulted in lower levels of expression of NF-κB p65 and p50 in HT-29 colon cancer cells. The NF-κB inhibitory effects decreased the activity of the nuclear p65 and affected cell cycle progression and induced cell death in cancer cells both in vitro and in vivo, preventing tumor growth in mice. Moreover, the effect exhibited by GZL on the NF-κB pathway occurred in the presence and absence of TNFα. Our findings suggest that inhibition of NF-κB expression by GZL sensitizes HT-29 colon cancer cells to apoptosis. GZL is also a promising inhibitor of human cancer cell proliferation in vivo as judged by its activity in the hollow fiber assay. GZL, therefore, represents a potential chemotherapeutic agent with antitumor effects from natural origin with significant NF-κB inhibitory effects. Thus, optimization of the potential of GZL might lead to a more effective cancer treatment, specifically colon cancer.
Acknowledgments
This work was supported by grant P01 CA125066-S2, funded by the National Cancer Institute, NIH, Bethesda, MD, and by a Pelotonia Fellowship from the Ohio State University. Authors would like to thank Dr. Mark E. Drew and Dawn Walker for facilitating the use of a BD FACS Canto II instrument.
Footnotes
Conflict of interest
No conflict of interest was disclosed by the authors.
REFERENCES
- Addabbo F, Nacci C, de Benedictis L, Leo V, Tarquinio M, Quon MJ, Montagnani M. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-kappaB/COX-2 signaling in human aortic endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2012;301:E1143–1154. doi: 10.1152/ajpendo.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Adhami VM, Gupta S, Cheng P, Mukhtar H. Role of the retinoblastoma (pRb)-E2F/DP pathway in cancer chemopreventive effects of green tea polyphenol epigallocatechin-3-gallate. Arch. Biochem. Biophys. 2002;398:125–131. doi: 10.1006/abbi.2001.2704. doi:10.1006/abbi.2001.2704; PMID:11811957 Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11811957. [DOI] [PubMed] [Google Scholar]
- Alley MC, Pacula-Cox CM, Hursey ML, Rubinstein LR, Boyd MR. Morphometric and colorimetric analyses of human tumor cell line growth and drug sensitivity in soft agar culture. Cancer Res. 1991;51:1247–1256. [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The twoNF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Borkosky S, Ponce de Leon S, Juarez G, Sierra MG, Bardon A. Molluscicidal sesquiterpene lactones from species of the tribe Vernonieae (Compositae). Chem. Biodiversity. 2009;6:513–519. doi: 10.1002/cbdv.200800156. [DOI] [PubMed] [Google Scholar]
- Casciari JJ, Hollingshead MG, Alley MC, Mayo JG, Malspeis L, et al. Growth and chemotherapeutic response of cells in a hollow-fiber in vitro solid tumor model. J. Natl. Cancer Inst. 1994;86:1846–1852. doi: 10.1093/jnci/86.24.1846. doi: 10.1093/jnci/86.24.1846; PMID:7990159 Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7990159. [DOI] [PubMed] [Google Scholar]
- Chen CY, Yi L, Jin X, Zhang T, Fu YJ, et al. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-kappaB pathway. Cell Biochem. Biophys. 2011;61:337–348. doi: 10.1007/s12013-011-9216-2. doi: 10.1007/s12013-011-9216-2; PMID:21695376 Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21695376. [DOI] [PubMed] [Google Scholar]
- Dos Santos PA, Amarante MFC, Pereira AMS, Bertoni B, Franca SC, et al. Production of an antiproliferative furanoheliangolide by Lychnophora ericoides cell culture. Chem. Pharm. Bull. 2004;52:1433–1435. doi: 10.1248/cpb.52.1433. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Meade KG, McEvoy AN, Lillis L, Murphy EP, MacHugh DE, Baird AW. Tumour necrosis factor-alpha (TNF-α) increases Nuclear Factor κB (NF-kappaB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2007;116:59–68. doi: 10.1016/j.vetimm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Garg A, Aggarwal BB. Nuclear transcription Factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. doi:10.1038/sj/leu/2402482; PMID:12040437. [DOI] [PubMed] [Google Scholar]
- Gervasoni JE, Jr., Hindenburg AA, Vezeridis MP, Schulze S, Wanebo HJ, Mehta S. An effective in vitro antitumor response against human pancreatic carcinoma with paclitaxel and daunorubicin by induction of both necrosis and apoptosis. Anticancer Res. 2004;24:2617–2626. PMID:15517865. [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. http://dx.doi.org/10.1016/j.bcp.2006.04.011, PMID: 16723122 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16723122. [DOI] [PubMed] [Google Scholar]
- Grael CF, Albuquerque S, Lopes JL. Chemical constituents of Lychnophora pohlii and trypanocidal activity of crude plant extracts and of isolated compounds. Fitoterapia. 2005a;76:73–82. doi: 10.1016/j.fitote.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Grael CF, Vichnewski W, Souza GE, Lopes JL, Albuquerque S, Cunha WR. A study of the trypanocidal and analgesic properties from Lychnophora granmongolense (Duarte) semir and feitao filho. Phytother. Res. 2005b;14:203–206. doi: 10.1002/(sici)1099-1573(200005)14:3<203::aid-ptr565>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, et al. Ikkbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. http://dx.doi.org/10.1016/j.cell.2004.07.013 PMID:15294155. [DOI] [PubMed] [Google Scholar]
- Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L, Grever MR. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995;57:131–141. doi: 10.1016/0024-3205(95)00254-4. [DOI] [PubMed] [Google Scholar]
- Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH. Pharmacologic inhibitors of Ikappab kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasis in vivo. Mol. Cancer Ther. 2009;8:2339–2347. doi: 10.1158/1535-7163.MCT-09-0133. doi: 10.1158/1535. [DOI] [PubMed] [Google Scholar]
- Kim H, Jung J, Jung H, Kim JY, Chung SK, Chung DK. Lactobacillus plantarum lipoteichoic acid alleviates TNF-alpha in the HT-19 intestinal epithelial cell line. Mol. Cell. 2012a;33:479–486. doi: 10.1007/s10059-012-2266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Lau EK, Pan L, de Blanco EJ. NF-kappaB inhibitors from Brucea javanica exhibiting intracellular effects on reactive oxygen species. Anticancer Res. 2010;30:3295–3300. PMID:20944100. [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Lee JS, Kwak MK, Choi HG, Yong CS, et al. Anti-inflammatory ction of mollugin and its synthetic derivatives in HT-29 human colonic epithelial cells is mediated through inhibition of NF-kappaB activation. Eur. J. Pharmacol. 2009;622:52–57. doi: 10.1016/j.ejphar.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Kim SL, Trang KT, Kim SH, Kim IH, Lee SO, et al. Parthenolide suppresses tumor growth in a xenograph model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int. J. Oncol. 2012b doi: 10.3892/ijo.2012.1587. [DOI] [PubMed] [Google Scholar]
- Kinghorn AD, Carcache de Blanco EJ, Chai HB, Orjala J, Farnsworth NR, et al. Discovery of anticancer agents of diverse natural origin. Pure Appl. Chem. 2009;81:1051–1063. doi: 10.1351/PAC-CON-08-10-16. doi: 10.1351/PAC-CON-08-10-16; PMID: 20046887 Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20046887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of Nuclear Factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin. Cancer Res. 2007;13:59–67. doi: 10.1158/1078-0432.CCR-06-1559. doi: 10.1158/1078-0432.CCR-06-1559 PMID:17200339. [DOI] [PubMed] [Google Scholar]
- Li Y, Yu Y, Zhang Y, Zhou Y, Li C, et al. Mafip is a tumor suppressor in cervical cancer that inhibits activation of the Nuclear Factor-kappaB pathway. Cancer Sci. 2011;102:2043–2050. doi: 10.1111/j.1349-7006.2011.02061.x. doi: 10.1111/j.1349-7006.2011.02061; PMID:21834855 Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21834855. [DOI] [PubMed] [Google Scholar]
- Lunardello MA, Tomaz JC, Vichnewski W, Lopes JLC. Sesquiterpene lactones and flavonoids from Eremanthus mattogrossensis and Eremanthus eriopus. J. Braz. Chem. Soc. 1995;6:307–311. [Google Scholar]
- Mantovani MS, Takahashi CS, Vichnewski W. Evaluation of the genotoxic activity of the sesquiterpene lactone goyazensolide in mammalian systems in vitro and in vivo. Rev. Bras. Genet. 1993;16:967–975. [Google Scholar]
- Mi Q, Lantvit D, Reyes-Lim E, Chai H, Zhao W, et al. Evaluation of the potential cancer chemotherapeutic efficacy of natural product isolates employing in vivo hollow fiber tests. J. Nat. Prod. 2002;65:842–850. doi: 10.1021/np010322w. [DOI] [PubMed] [Google Scholar]
- Mi Q, Pezzuto JM, Farnsworth NR, Wani MC, Kinghorn AD, Swanson SM. Use of the in vivo hollow fiber assay in natural products anticancer drug discovery. J. Nat. Prod. 2009;72:573–580. doi: 10.1021/np800767a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino K, Ozaki M, Nakanishi K, Haga S, Sato M, et al. Inhibition of nuclear factor-kappaB suppresses peritoneal dissemination of gastric cancer by blocking cancer cell adhesion. Cancer Sci. 2011;102:1052–1058. doi: 10.1111/j.1349-7006.2011.01901.x. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Kang SH, Choi YH, Kim GY. Sulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009;274:132–142. doi: 10.1016/j.canlet.2008.09.013. doi: 10.1016/j.canlet.2008.09.013; PMID:18952368. [DOI] [PubMed] [Google Scholar]
- Acuna UM, Wittwer J, Ayers S, Pearce CJ, Oberlies NH, De Blanco EJC. Effects of (5Z)-7-Oxozeaenol on the oxidative pathway of cancer cells. Anticancer Res. 2012a;32:2665–2671. [PMC free article] [PubMed] [Google Scholar]
- Acuna UM, Wittwer J, Ayers S, Pearce CJ, Oberlies NH, De Blanco EJC. Effects of (5Z)-7-oxozeaenol on MDA-MB-231 breast cancer cells. Anticancer Res. 2012b;32:2415–2421. [PMC free article] [PubMed] [Google Scholar]
- Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. doi: 10.1038/nrc1588; PMID: 15803156. [DOI] [PubMed] [Google Scholar]
- Oka D, Nishimura K, Shiba M, Nakai Y, Arai Y, et al. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-κB. Int. J. Cancer. 2007;120:2576–2581. doi: 10.1002/ijc.22570. doi: 10.1002/ijc.22570. [DOI] [PubMed] [Google Scholar]
- Olivera A, Moore TW, Hu F, Brown AP, Sun A, et al. Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012;12:368–377. doi: 10.1016/j.intimp.2011.12.009. doi: 10.1016/j.intimp.2011.12.009; PMID:22197802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce CJ, Lantvit DD, Shen Q, Jarjoura X, Zhang NH, et al. Use of the Hollow Fiber Assay for Discovery of Novel Anticancer Agents from Fungi. In: Keller NP, Turner G, editors. Fungal Secondary Metabolism: Methods and Protocols. Humana Press/Springer. Totawa, New Jersey: 2012. pp. 267–277. [DOI] [PubMed] [Google Scholar]
- Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol. Res. 2011;64:493–500. doi: 10.1016/j.phrs.2011.06.001. doi: 10.1016/j.phrs.2011.06.001; PMID:21683791. [DOI] [PubMed] [Google Scholar]
- Ren Y, Acuna UM, Jimenez F, Garcia R, Mejia M, et al. Cytotoxic and NF-κB inhibitory sesquiterpene lactones from Piptocoma rufescens. Tetrahedron. 2012;68:2671–2678. doi: 10.1016/j.tet.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer L, Sirajuddin P, Yenugonda VM, Ghosh A, Divito K, et al. VMY-1-103, a dansylated analog of purvalanol B, induces caspase-3-dependent apoptosis in LNCaP prostate cancer cells. Cancer Biol. Ther. 2010;10:320–325. doi: 10.4161/cbt.10.4.12208. doi: 10.4161/cbt.10.4.12208; PMID:20574155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungeler P, Castro V, Mora G, Goren N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: A proposed molecular mechanism of action. Bioorg. Med. Chem. 1999;7:2343–2352. doi: 10.1016/s0968-0896(99)00195-9. [DOI] [PubMed] [Google Scholar]
- Sa G, Das T. Anti cancer effects of curcumin: Cycle of life and death. Cell Div. 2008;3:14. doi: 10.1186/1747-1028-3-14. doi: 10.1186/1747-1028-3-14; PMID:18834508 Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18834508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kudo C, Yamakoshi H, Uehara Y, Ohori H, Ishioka C, Iwabuchi Y, Shibata H. Curcumin analog GO-Y030 is a novel inhibitor of IKKβ that suppresses NF-κB signaling and induces apoptosis. Cancer Sci. 2011;102:1045–1051. doi: 10.1111/j.1349-7006.2011.01886.x. doi: 10.1111/j.1349-7006.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- Scaife CL, Kuang J, Wills JC, Trowbridge DB, Gray P, et al. NF-kappaB inhibitors induce adhesion-dependent colon cancer apoptosis: Implications for metastasis. Cancer Res. 2002;62:6870–6878. PMID:12460901. [PubMed] [Google Scholar]
- Shanmugam R, Kusumanchi P, Cheng L, Crooks P, Neelakantan S, et al. A water-soluble parthenolide analogue suppresses in vivo prostate cancer growth by targeting NF-kappaB and generating reactive oxygen species. Prostate. 2010;70:1074–1086. doi: 10.1002/pros.21141. doi: 10.1002/pros.21141; PMID:20209491. [DOI] [PubMed] [Google Scholar]
- Siedle B, Alfonso JGP, Murillo R, Schulte-Monting J, Castro V, et al. Quantisttative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. J. Med. Chem. 2004;47:6042–6054. doi: 10.1021/jm049937r. [DOI] [PubMed] [Google Scholar]
- Thapa D, Lee JS, Park MA, Cho MY, Park YJ, et al. Inhibitory effects of clotrimazole on tnf-alpah-induced adhesion molecule expression and angiogenesis. Arch. Pharm. Res. 2009;32:593–603. doi: 10.1007/s12272-009-1416-6. [DOI] [PubMed] [Google Scholar]
- Valdes DA, Bardon A, Catalan CA, Gedris TE, Herz W. Goyazensolides and isogoyazensolides from argentine Centratherum punctatum ssp. punctatum. Biochem. Syst. Ecol. 1998;26:805–808. [Google Scholar]
- Vichnewski W, Sarti SJ, Gilbert B, Herz W. Goyazensolide, a schistosomicidal heliangolide from Eremanthus goyazensis. Phytochemistry. 1976;15:191–193. [Google Scholar]
- Vlahopoulos S, Boldogh I, Casola A, Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: Evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood. 1999;94:1878–1889. PMID: 10477716. [PubMed] [Google Scholar]
- Vongvanich N, Kittakoop P, Charoenchai P, Intamas S, Sriklung K, Thebtaranonth Y. Antiplasmodial, antimycobacterial and cytotoxic principles from Camchaya calcarea. Planta Med. 2006;72:1427–1430. doi: 10.1055/s-2006-951711. doi: 10.1055/s-2006-951711; PMID: 17089326. [DOI] [PubMed] [Google Scholar]
- Wagner S, Hofmann A, Siedle B, Terfloth L, Merfort I, Gasteiger J. Development of a structural model for NF-kappaB inhibition of sesquiterpene lactones using self-organizing neural networks. J. Med. Chem. 2006;49:2241–2252. doi: 10.1021/jm051125n. [DOI] [PubMed] [Google Scholar]
- Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;1012 doi: 10.1186/1476-4598-10-12. doi: 10.1186/1476-4598-10-12; PMID: 21299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Huang AC, Yang JS, Liao CL, Lu HF, et al. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 os cells. J. Orthop. Res. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhou BP. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. doi: 10.1038/sj.bjc.6605530; PMID:20087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2 and phosphorylation of the retinoblastoma protein. J. Cell Biol. 1996;133:391–403. doi: 10.1083/jcb.133.2.391. PMID: 8609171. [DOI] [PMC free article] [PubMed] [Google Scholar]