Abstract
Inflammation of the retina is a contributing factor in ocular diseases such as uveitis, diabetic retinopathy, and age-related macular degeneration (AMD). The M013 immunomodulatory protein from myxoma virus has been shown to interfere with the proinflammatory signaling pathways involving both the NLRP3 inflammasome and NF-κB. We have developed and characterized an adeno-associated viral (AAV) vector that delivers a secretable and cell-penetrating form of the M013 protein (TatM013). The expressed TatM013 protein was secreted and blocked the endotoxin-induced secretion of interleukin (IL)-1β in monocyte-derived cells and the reactive aldehyde-induced secretion of IL-1β in retinal pigment epithelium cells. The local anti-inflammatory effects of AAV-delivered TatM013 were evaluated in an endotoxin-induced uveitis (EIU) mouse model after intravitreal injection of mice with an AAV2-based vector carrying either TatM013 fused to a secreted green fluorescent protein (GFP) tag (sGFP-TatM013) or GFP. Expression of the sGFP-TatM013 transgene was demonstrated by fluorescence funduscopy in living mice. In EIU, the number of infiltrating cells and the concentration of IL-1β in the vitreous body were significantly lower in the eyes injected with AAV-sGFP-TatM013 compared with the eyes injected with control AAV-GFP. These results suggest that a virus-derived inhibitor of the innate immune response, when delivered via AAV, could be a generalized therapy for various inflammatory diseases of the eye.
Introduction
Viruses have evolved to infect host cells and disperse to target tissues from the point of initial infection. Viral dissemination and host-to-host spread create strong selection pressure to evade the early stages of the host immune and inflammatory responses. Viral genes that express inhibitors capable of blocking inflammatory signaling pathways have been discovered in many viruses, but poxviruses are especially adept at acquiring immune-inhibitory functions.1–3 These functions are generally not required for the replication of the virus in cultured cells, but are essential for successful infection of immunocompetent host animals. Myxoma virus is a member of the poxvirus family that infects and causes disease only in European rabbits, but many of its specific immunomodulatory proteins can still target pathways in nonrabbit host cells.4,5 The M013 gene from the myxoma virus was previously shown to express a small cytoplasmic pyrin domain (PYD)-containing immunomodulatory protein with at least two distinct mechanisms of action. First, it binds to the ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) component of inflammasomes and inhibits the proteolytic processing of caspase-1 and thus activation/secretion of interleukin (IL)-1β and IL-18.6,7 Second, the M013 protein also directly binds to the precursor NF-κB (p105), preventing its cleavage and activation. Thus, M013 blocks this aspect of the NF-κB signaling pathway and prevents the induction of other proinflammatory cytokines under its control, such as IL-6 and tumor necrosis factor (TNF)8 (see Fig. 1A). These dual inflammasome/NF-κB inhibitory properties of M013 are fully active in rabbit, mouse, and human cells.
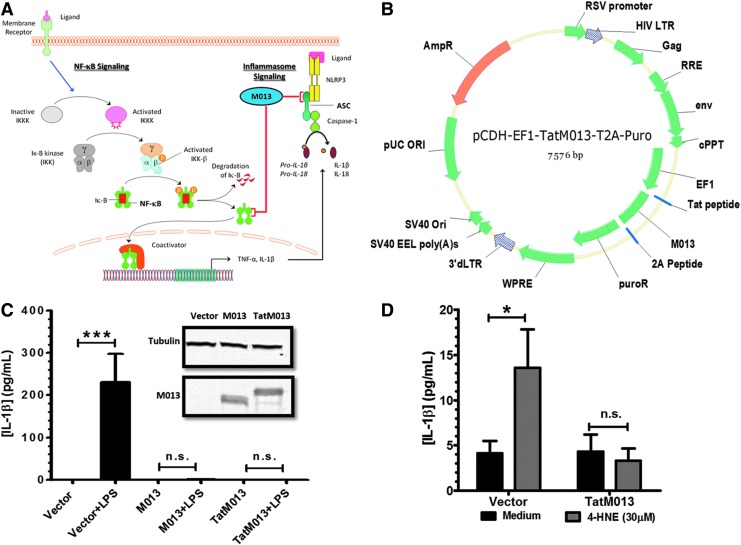
FIG. 1.
The myxoma virus M013 fusion protein inhibits interleukin (IL)-1β secretion. (A) The native M013 protein from myxoma virus has been demonstrated to inhibit both nuclear factor (NF)-κB and NLRP3 inflammasome signaling pathways by interacting with key effector components. (B) Map of lentiviral vector delivering the TatM013 fusion gene. The M013 and TatM013 fusion genes were cloned in-frame with the 2A peptide and puromycin resistance (puroR) sequence. (C) Monocyte-derived THP-1 cells were transduced with a lentiviral vector delivering the M013-puroR fusion gene, the TatM013-puroR fusion, or the puroR gene alone as a control. Stably transduced cells were incubated with IFN-γ (4 hr) and lipopolysaccharide (LPS) (18 hr). Secreted IL-1β was quantified by ELISA. M013 protein was measured in cell extracts by Western blotting, and compared with tubulin control (inset) (n=3, average±SEM). (D) ARPE-19 cells were transduced with either the TatM013-puroR or puroR lentiviral vector. Stably transduced cells were incubated with 30 μM 4-hydroxynonenal (4-HNE, 18 hr). Secreted IL-1β was quantified by ELISA (n=3, average±SEM). ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; AmpR, ampicillin resistance gene; cPPT, central polypurine tract; EF1, elongation factor-1; HIV LTR, human immunodeficiency virus long terminal repeat; Iκ-B, inhibitor of NF-κB; IKKK, Iκ-B kinase kinase; n.s., not significant; ORI/Ori, origin of replication; RRE, Rev response element; RSV, Rous sarcoma virus; SV40, simian virus 40; TNF-α, tumor necrosis factor-α; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. *p≤0.05; ***p≤0.0001. Color images available online at www.liebertpub.com/hum
Although the eye is an immune-privileged organ, inflammatory processes can adversely affect the ocular tissue, either acutely, after injury or infection, or chronically, as a consequence of conditions such as age-related macular degeneration.9,10 Uveitis is characterized by a massive inflammatory reaction within the anterior and posterior chambers of the eye. The trigger for this inflammation is often unknown, but recurrent uveitis is thought to have an autoimmune component.11 Although the disease may be treated with corticosteroids, their prolonged use leads to elevated intraocular pressure and risk of glaucoma.12 Proinflammatory cytokines such as IL-1β and TNF-α have been associated with the ocular damage ascribed to this disease.13,14
In this paper, we describe the development of an adeno-associated viral (AAV) vector that delivers a secretable and cell-penetrating form of the M013 dual inflammasome/NF-κB inhibitor gene derived from myxoma virus. We have characterized the function of this AAV-expressed antiinflammatory protein, using in vitro models of inflammation. We have also tested the efficacy of this novel anti-inflammatory treatment in mice, using the endotoxin-induced uveitis model. Our results suggest that this AAV-mediated expression of the modified M013 immunomodulator is a potential treatment for inflammatory eye disease, and possibly other tissue-specific chronic inflammatory diseases that are amenable to AAV therapy.
Materials and Methods
Study design
The goal of these studies was to develop and characterize an adeno-associated viral vector that could deliver the anti-inflammatory gene M013 from the myxoma virus. To validate previously published results demonstrating the inhibitory activity of M013 on inflammasome signaling, we used lentiviral vectors to generate stable cell lines in which we induced the activation of this signaling pathway via various agents. In these in vitro experiments the conditioned media of three independent cultures were analyzed. Animal studies were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Florida (Gainesville, FL). These studies were conducted with the minimal number of mice needed to investigate end points that could not be addressed with in vitro experiments. Animal were properly anesthetized with ketamine and xylazine for in-life assessment by funduscopy and humanely killed, using carbon dioxide inhalation, for the analysis of tissue.
Cell culture
Human HEK293T and mouse RAW 264.7 cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (Pen-Strep) solution. The human ARPE-19 cell line was grown in DMEM/F12 (50/50) medium supplemented with 10% FBS and 1% Pen-Strep. The human THP-1 cell line was grown in RPMI 1640 medium supplemented with 10% FBS and 1% Pen-Strep. All the cell cultures were maintained in an incubator at 37°C with 5% CO2. All stable cell lines generated by lentiviral vector transduction were grown in the corresponding medium supplemented with puromycin at a dose of 1 μg/ml.
Concentration of conditioned medium
Conditioned M199 low-protein medium (Invitrogen Life Technologies, Grand Island, NY) was harvested and placed in a 15-ml conical tube and centrifuged at 2500×g for 5 min to remove any cellular debris. The conditioned medium was then passed through a 50-kDa molecular weight cut off (MWCO) Amicon-Ultra centrifugal filter (EMD Millipore, Billerica, MA) by centrifugation at 14,000×g for 15 min in a tabletop microcentrifuge. The flow-through was then harvested and passed through a 3-kDa Amicon-Ultra centrifugal filter (EMD Millipore) by centrifugation at 14,000×g for 30 min in a tabletop microcentrifuge. The concentrated medium containing molecules with molecular weights between 50 and 3 kDa was collected by inversion and centrifugation of the filter at 1000×g for 1 min.
Viral vectors
All the lentiviral vectors were created with the pCDH-EF1-MCS-T2A-Puro plasmid (Systems Biosciences, Mountain View, CA). The transgenes were cloned, using the EcoRI and the NotI restriction sites in the multiple cloning sites. Plasmids were propagated in DH5α cells and sequenced by the dideoxy chain termination method. To generate viral particles, the plasmids were cotransfected with the pPACKH1 lentiviral vector packaging kit (Systems Biosciences) in HEK293T cells. The lentiviral vector-containing media were harvested 48 hr after the cotransfection and were centrifuged at 2500×g for 5 min at 4°C. The vector-containing media were passed through a 0.22-μm (pore size) syringe filter.
The sGFP-TatM013 vector plasmid was created by subcloning the TatM013 fusion construct downstream of Igκ-GFP (IgG kappa light chain leader sequence fused to the GFP sequence) while maintaining the reading frame of these elements. This gene construct was then excised, using the XbaI (5′) and XhoI (3′) sites, and cloned in the pTR-smCBA AAV plasmid. The final pTR-smCBA-Igκ-GFP- TatM013 plasmid was grown in SURE 2 cells (Agilent, Santa Clara, CA) and purified by passage through a CsCl gradient. The DNA was packaged, purified, and titered by the Center for Vision Research Vector Core at the University of Florida according to previously published methods.15,16
Isolation of vitreous
Mouse vitreous was isolated by the protocol developed by Skeie and colleagues.17 Briefly, mice were humanely killed, using CO2 as approved by the University of Florida IACUC. A transverse incision was made in the cornea with a surgical scalpel to allow the aqueous humor to flow. Excess aqueous humor was absorbed with a paper towel. The retina, vitreous, and lens were eviscerated by squeezing the eye from the back. Tissues were collected in a sterile 1.5-ml tube and later transferred into an Amicon Ultra centrifugal filter (cutoff, 50 kDa; EMD Millipore). An additional 100 μl of phosphate-buffered saline (PBS) supplemented with protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL) was added to the tube. Samples were centrifuged at 14,000×g for 15 min and vitreous flow-through was collected. Protein concentration of the vitreous was determined by DC protein assay (Bio-Rad, Hercules, CA) according to the manufacturer's protocol and stored at –20°C until assayed.
ELISA
The ELISA kit for mouse IL-1β was purchased from Peprotech (Peprotech, Rocky Hill, NJ). The concentration of IL-1β was determined according to the manufacturer's protocol. The concentration of human IL-1β was determined with a RayBiotech human IL-1β ELISA kit (RayBiotech, Norcross, GA) according to the manufacturer's protocol. In both cases, a total of 100 μl of either tissue homogenate or cell culture medium was analyzed.
Western blot
The cells corresponding to the conditioned media were lysed in NP-40 lysis buffer supplemented with protease inhibitor cocktail (Thermo Fisher Scientific) and 2 mM EDTA. The protein concentration of the samples was measured with the DC protein assay (Bio-Rad) according to the manufacturer's protocol. Protein lysates were diluted in Laemmli buffer containing 100 μM dithiothreitol (DTT) and boiled for 5 min.18 Equal amounts of protein were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride (PVDF) membrane, using an iBlot system (Invitrogen Life Technologies) as recommended by the manufacturer. This membrane was blocked with a proprietary blocking buffer from Li-Cor Biosciences (Lincoln, NE) for 1 hr at room temperature and incubated overnight with the designated primary antibody at 4°C. The membrane was then washed four times with PBS–0.1% Tween 20 and incubated with the corresponding IRDye secondary antibody (1:5000 dilution in blocking buffer; Li-Cor Biosciences). Finally, the membrane was washed as done previously and scanned with an Odyssey infrared imaging system (Li-Cor Biosciences).
Endotoxin-induced uveitis mouse model
Mice of the C57BL/6J strain were injected intravitreally with 3×109 vector genomes in each eye. Two weeks after injection the mice were inspected by spectral domain optical coherence tomography, using a Bioptigen high-resolution instrument, to exclude inflammatory infiltrates resulting from injection of vector. One month after injection, green fluorescent protein (GFP) expression was observed by fluorescence funduscopy. The next day mice were injected intravitreally in each eye with 25 ng of lipopolysaccharide (LPS). After 24 hr, these mice were killed, and their eyes were enucleated and placed in 4% paraformaldehyde at 4°C overnight. The eyes were placed in PBS after fixation and stored at 4°C until embedding. Eyes were dehydrated and embedded in paraffin in preparation for sectioning. Embedded eyes were sectioned through the cornea–optic nerve axis, at a thickness of 12 μm. Eight step sections were collected in independent slides, with sections on the same slide having a difference of 80 μm. Slides were stained with hematoxylin and eosin to visualize infiltrating cells. These cells were quantified in images of the sections by an individual who was ignorant of the treatment group.
Funduscopy
A Micron III digital fundus retinal imaging microscope (Phoenix Research Laboratories, Pleasanton, CA) was used to monitor GFP expression in life. Conscious mice had their eyes dilated with 1% atropine and 2.5% phenylephrine. Mice were then anesthetized with a mixture of ketamine and xylazine in normal saline (0.9% sodium chloride solution USP, pH 5.0; Baxter, Deerfield, IL). To avoid loss of moisture from the ocular surface during the procedure, mice received a drop of 2.5% hypromellose ophthalmic demulcent solution (Gonak; AKORN, Lake Forest, IL). Bright-field fundus images were acquired using the same exposure times. GFP fluorescence was measured with the fluorescence filter, using the same exposure times for all the eyes.
Statistical analysis
Data from in vitro experiments are reported as averages±standard deviation. Values were compared by analysis of variance followed by a Student Newman–Keuls t test to determine significant differences between groups. For animal studies, values are reported as averages±standard error of the mean and statistical comparison was done by Mann–Whitney U test to avoid potential biases introduced by outliers. p<0.05 was considered significant (*p≤0.05, **p≤0.01, ***p≤0.001).
Results
AAV-mediated expression of myxoma virus gene M013 can inhibit the secretion of IL-1β in vitro
On inflammasome activation by appropriate stimulatory ligands, IL-1β precursor is cleaved and secreted, thus providing a cytokine readout for inflammasome signaling. To validate the inhibitory effect of fusion constructs of M013 on the secretion of IL-1β, we first used a lentiviral vector to generate human myeloid THP-1 cells that stably express the following: (1) M013 fused at its C terminus to the puromycin resistance gene (puroR), (2) M013 with an N-terminal cell-penetrating peptide (TatM013) fused to puroR, or (3) the puroR gene alone (Fig. 1B). Activation of the inflammasome was induced by incubating the transduced THP-1 cell lines with interferon (IFN)-γ in the presence or absence of lipopolysaccharide (LPS). The concentration of secreted IL-1β in the conditioned media was increased in the presence of LPS only in cells expressing the control puromycin resistance gene alone (Fig. 1C). In the THP-1 cells expressing either the unmodified M013 protein (∼19 kDa) or the TatM013 protein (∼20 kDa) fused to puroR, the secretion of IL-1β was significantly inhibited. Interestingly, the level of detectable expression of the TatM013 protein was considerably less than that of the unmodified M013 (Fig. 1C, inset) in total cellular extracts prepared from these THP-1 cell clones. Nevertheless, both proteins prevented the IFN/LPS-induced secretion of IL-1β.
In the retina, induced IL-1β can be secreted from microglia, endothelial cells,19 and cells of the retinal pigmented epithelium (RPE).20 The RPE-derived cell line ARPE-19 was stably transfected with the same lentiviral vectors as described previously to study the effect of TatM013 on IL-1β secretion by a biologically relevant ocular cell type. The modified ARPE-19 cells expressed the TatM013-puroR fusion or the control puromycin resistance gene alone (data not shown). Inflammasome activation was induced by incubation of these cells with 4-hydroxy-2-nonenal (4-HNE), a reactive aldehyde generated by oxidative stress in the retina and reported to activate the inflammasome in these cells.20 In ARPE-19 cells, 4-HNE led to a 3-fold induction of IL-1β secretion, but this induction was blocked by expression of the TatM013 gene compared with cells expressing GFP only (Fig. 1D). Together, these results demonstrate that ectopic expression of M013 or TatM013 protein inhibited inflammasome-activated IL-1β secretion in vitro. Furthermore, this fusion of the M013 gene with the cell-penetrating peptide Tat did not seem to compromise the efficiency of this inhibitory function of M013.
Development of an AAV vector that expresses secretable and cell-penetrating M013
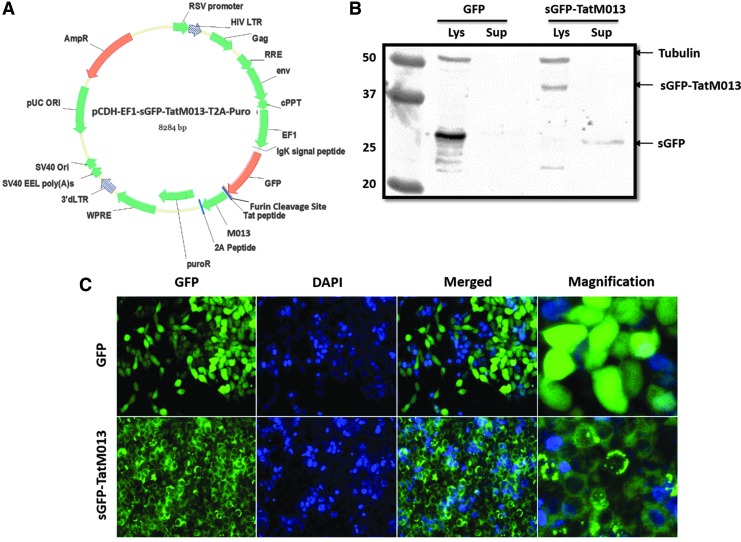
The various cellular sources of IL-1β in the eye make it difficult to prevent its secretion by a gene therapy strategy that blocks IL-1β secretion only from cells transduced with the AAV vector. To permit the visualization of TatM013 protein, which has the potential to impact surrounding cells that may not be directly transduced by the gene therapy vector, the TatM013 coding sequence was fused to a sequence encoding a secretable version of GFP. This fusion construct was expressed as a single recombinant protein containing the N-terminal secretory signal from the Igκ gene fused to GFP and linked at the C terminus to the TatM013 gene and separated by a furin cleavage site sequence (Fig. 2A). This secretion system has been demonstrated to deliver and secrete a small peptide derived from angiotensin.21 To study the expression of the novel sGFP-TatM013 fusion construct, we cloned it in a lentiviral plasmid under the control of the elongation factor (EF)-1α promoter and fused it to the puromycin resistance (puroR) gene through a self-cleaving 2A peptide (Fig. 2A). Expression of the fused sGFP-TatM013 protein was demonstrated by Western blot, which revealed a band of the predicted size using either an antibody against the 2A peptide (not shown) or against GFP itself (Fig. 2B). By using fluorescence microscopy, we observed that control GFP had a cytoplasmic distribution, whereas sGFP-TatM013 had a punctate pattern characteristic of proteins within the secretory pathway22–24 (Fig. 2C). This result suggested that the sGFP-TatM013 gene product can be expressed and targeted for secretion.
FIG. 2.
Development of a secretable and cell-penetrating TatM013 fusion construct. (A) Map of pCDH-EF1-sGFP-TatM013-T2A-Puro plasmid. The TatM013 gene was fused to the secretable green fluorescent protein (sGFP) cDNA, separated by a furin cleavage site sequence. The sGFP-TatM013 fusion gene was cloned in the lentiviral vector plasmid upstream and fused to the 2A signal peptide and puroR sequence. All these sequences were cloned in-frame, which on translation generated the fused protein sGFP-TatM013 targeted to the secretory pathway. (B) Expression of sGFP-TatM013 in lentivirus-transduced cells. HEK293T cells stably expressing GFP, a control peptide fused to GFP or sGFP-TatM013, were lysed in NP-40 lysis buffer (Lys). Proteins in the culture supernatant (Sup) were concentrated as described in Materials and Methods. A total of 30 μg of protein from each sample was separated by SDS-PAGE, using a 12% gel. Expression of M013 was determined by Western blot with an anti-GFP antibody. (C) sGFP-TatM013 protein exhibited a discrete perinuclear pattern of distribution in cells. Stably transduced HEK293T cells were generated by transfection with either unmodified GFP or sGFP-TatM013 lentiviral vector particles and further selection with puromycin. The expression of GFP was visualized by fluorescence microscopy. DAPI, 4′,6-diamidino-2-phenylindole; Lys, lysate; Sup, supernatant. Color images available online at www.liebertpub.com/hum
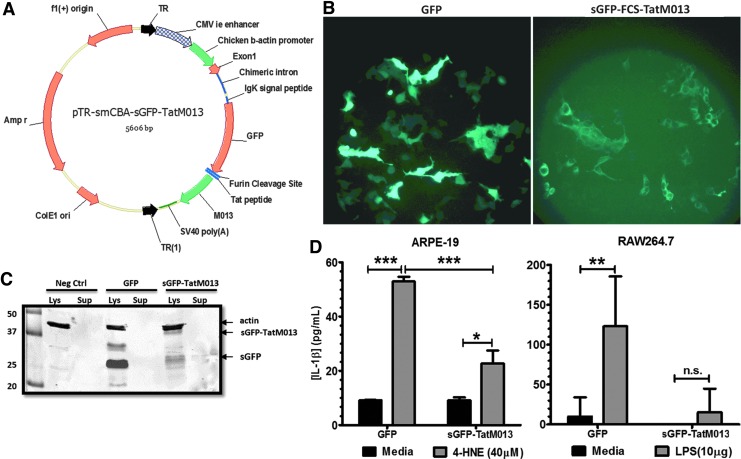
For stable delivery to the eye, we next designed an AAV vector to express sGFP-TatM013. AAV has proven to be safe and effective in multiple clinical trials intended to treat retinal disease.25–29 This construct was subcloned in a plasmid containing the inverted terminal repeats (TRs) of AAV2 and a truncated chimeric cytomegalovirus (CMV) β-actin (smCBA) promoter, which is known to be ubiquitously active in the retina30 (Fig. 3A). HEK293T cells were transfected with the AAV plasmid delivering either control GFP or sGFP-TatM013 to determine the effect of the secretion signal on the distribution of the fused protein. By using fluorescence microscopy, we observed a similar punctate pattern of distribution for GFP only among cells transfected with sGFP-TatM013 (Fig. 3B). To demonstrate that the sGFP-TatM013 protein was proteolyzed by furin and secreted, we harvested conditioned media from cells transfected with either control GFP or sGFP-TatM013. Medium was concentrated and a portion of it was fractionated on an SDS-polyacrylamide gel. In a Western blot (Fig. 3C), GFP was detected in the conditioned medium of sGFP-TatM013-expressing cells and was absent in the conditioned medium of GFP-expressing cells, suggesting that secretion and proteolysis at the furin cleavage site of the fusion protein did occur. The biological activity of the extracellular proteolyzed TatM013 was demonstrated by its ability to inhibit inflammasome signaling in cells incubated with this conditioned medium. Incubation of cells with TatM013-conditioned medium caused a significant decrease in the amount of IL-1β produced by cells treated with an inflammatory agent (LPS or 4-HNE) when compared with cells that were incubated with control medium (Fig. 3D). Although IL-1β release was repressed by the TatM013 conditioned medium, no cell death was observed after exposure to any of the conditioned media. These results demonstrate that secretable and cell-penetrating TatM013 can be expressed by AAV and that this protein retains its anti-inflammasome activity when exported into the conditioned medium.
FIG. 3.
In vitro confirmation of the sGFP-TatM013-expressing AAV vector. (A) Map of the pTR-smCBA-sGFP-TatM013 plasmid. The sGFP-TatM013 sequence was cloned in an AAV plasmid between AAV2 inverted terminal repeats (TRs). (B) Cellular distribution of sGFP-TatM013. HEK293T cells were transfected with the pTR-smCBA-sGFP-TatM013 plasmid. GFP expression was validated at 48 hr, by fluorescence microscopy. As a control, HEK293T cells were transfected with pTR-smCBA-GFP plasmid, which delivers an unmodified GFP gene. (C) Secretion of the sGFP-TatM013 fusion protein into the medium of transfected cells. Conditioned media from the cells imaged in (B) were concentrated. Protein samples from lysed cells (Lys) and conditioned media (Sup) were separated by SDS-PAGE, and GFP was detected with an anti-GFP antibody. (D) The biological activity of secreted TatM013 was measured in human ARPE-19 cells (left). A total of 500 μl of medium from cells transfected as in (B) (conditioned for 48 hr) was overlaid on ARPE-19 cells for 1 hr. Cells were incubated with or without 4-HNE (40 μM) for 18 hr by adding this reagent to the wells without removing the conditioned medium, and secreted IL-1β was quantified by ELISA. The biological activity of secreted TatM013 was measured in murine RAW 264.7 cells (right). Conditioned medium containing either GFP- or sGFP-TatM013-transfected cells was overlaid on RAW 264.7 cells. Cells were then incubated with or without LPS (18 hr), and secreted IL-1β was quantified by ELISA (n=3, average±SEM). *p≤0.05; **p≤0.001; ***p≤0.0001. Color images available online at www.liebertpub.com/hum
AAV vector-mediated expression of sGFP-TatM013 protein is biologically active
With the goal of treating inflammatory retinal disease, delivery of therapeutic vector to the vitreous compartment of the eye may be clinically advantageous, because intravitreal injections are routinely performed in outpatient office visits. We therefore packaged the sGFP-TatM013 plasmid in an AAV-based vector containing four tyrosine–phenylalanine (Y–F) and one threonine–valine (T–V) mutation on its capsid surface: AAV2(quadY-F+T-V). Y–F mutations were at positions 272, 444, 500, and 730 and the T–V mutation was at position 491. This variant is capable of infecting multiple cell types in the retina after injection into the vitreous compartment.31 AAV vector was injected into the vitreous fluid of right eyes (3×109 vector genomes delivered) of C57B/6J mice. As a control, the left eyes were injected with the same capsid mutant driving only GFP. This vector was chosen to control for the impact of ocular injection on the release of neurotrophic factors such as basic fibroblast growth factor (bFGF) and ciliary neurotrophic factor (CNTF) as reported in the literature.32,33 Two weeks after injection all mice were inspected by spectral domain optical coherence tomography (SD-OCT) to rule out retinal detachments and inflammatory infiltrates in the vitreous. Importantly, neither the GFP-expressing virus nor the sGFP-TatM013-expressing virus led to ocular inflammation. Mice were evaluated by fluorescence funduscopy 4 weeks after vector injection. Fluorescence imaging revealed that AAV2(quadY-F+T-V)-smCBA-mediated GFP expression was robust and panretinal, with the most intense labeling surrounding retinal blood vessels. In contrast, AAV2(quadY-F+T-V)-mediated sGFP-TatM013 exhibited a diffuse pattern of fluorescence suggesting secretion of the fused protein. This diffuse fluorescence, however, was not observed in noninjected control animals, indicating that the signal was caused by the expression of sGFP (Fig. 4A).
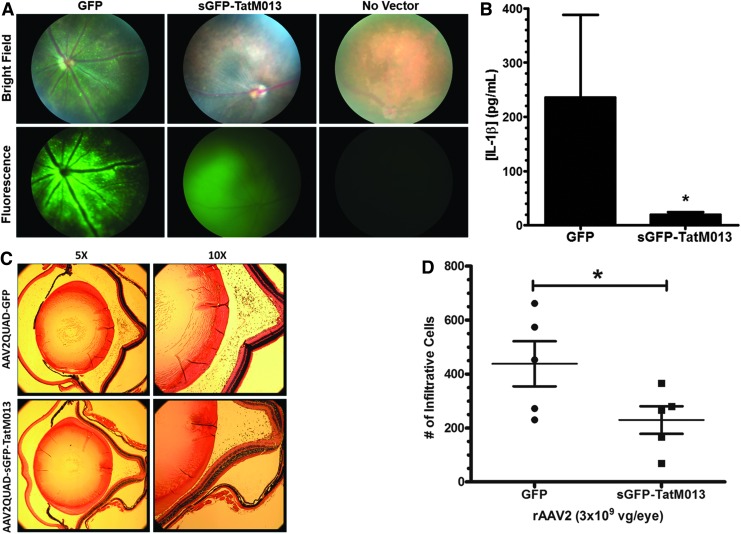
FIG. 4.
Expression of the AAV-vectored TatM013 gene product inhibits the inflammatory response observed in the endotoxin-induced uveitis (EIU) model. (A) Fundus of mice injected intravitreally with either AAV-GFP (left eye) or AAV-sGFP-TatM013 (right eye). Bright-field and fluorescence images of a noninjected eye (No Vector) are shown for comparison. (B) One week later, three mice were injected intravitreally with 175 ng of LPS (both eyes), and their vitreous humor was harvested 24 hr later. The secreted IL-1β concentration was quantified by ELISA (average±SEM). (C) Representative micrographs from eyes injected with either GFP or sGFP-TatM013 AAV vector after induction of uveitis (with 25 ng of LPS). Paraffin-embedded eyes were sectioned and stained (hematoxylin–eosin, H&E). Bright-field images were taken at original magnifications of ×5 and ×10 to observe infiltrating cells. (D) Expression of the TatM013 gene product from AAV decreased inflammation in the EIU mouse model. Five mice were injected as described in (A). Twenty-four hours after the injection of 25 ng of LPS mouse eyes were harvested and processed for paraffin sections. The eye sections were stained with H&E and the infiltrating cells were counted by a masked observer (average±SEM). vg, vector genomes. *p≤0.05. Color images available online at www.liebertpub.com/hum
To test the biological activity of our AAV vector in a model of acute ocular inflammation, we used the well-characterized endotoxin-induced uveitis (EIU) mouse model first described by Rosenbaum and colleagues.34 Increases in IL-1β and other cytokines have been reported in this model,35 making it a suitable in vivo system for testing the efficacy of our viral vector. The biological activity of AAV- mediated sGFP-TatM013 was next evaluated by inducing an inflammatory response in the treated eyes by an injection of LPS 5 weeks after AAV injection. Levels of IL-1β in the vitreous body were quantified by subjecting the harvested vitreous (as described in Materials and Methods) to an ELISA. The concentration of IL-1β was significantly lower in the samples from eyes treated with sGFP-TatM013 than in the GFP-treated control eyes (Fig. 4B). To quantify the effect of our vector on the recruitment of infiltrating cells, we used the minimal dose of LPS (25 ng) that would induce uveitis, allowing us to count these cells by light microscopy. To quantify the amount of infiltrating leukocytes in response to LPS challenge, eyes were harvested 24 hr after LPS injection, when inflammatory cell infiltration response peaks in the C57BL/6J strain,35 and fixed and embedded in paraffin for sectioning and staining with hematoxylin and eosin (Fig. 4C). The average number of immune cells infiltrating the vitreous chamber in AAV-sGFP-TatM013-treated eyes was reduced by nearly 50% relative to eyes treated with control AAV-GFP (Fig. 4D). These results indicate that expression of the fusion gene sGFP-TatM013 significantly reduced the infiltrating inflammatory cell response in this widely used model of ocular inflammation. Furthermore, these results indicated that the TatM013 fusion gene had anti-inflammatory activities that could be measured both in cultured cells and in the eyes of test animals.
Discussion
Inflammation contributes to the pathology of chronic diseases affecting many organ systems and tissues, including the CNS. In the brain, activation of glial cells is thought to play a role in the etiology of Alzheimer disease36 and amyotrophic lateral sclerosis (ALS).37,38 In the retina, chronic inflammation has been implicated in age-related macular degeneration (AMD), but the sources of that inflammation are a matter of conjecture and include such disparate molecules as oxidized lipids,39 bis-retinoids,40 double-stranded RNA,41 and amyloid peptides.42,43 To suppress inflammation that may arise from more than one stimulus affecting a variety of cell types within complex tissues such as the eye, we exploited a generalized anti-inflammatory strategy perfected by poxviruses. The myxoma virus M013 gene is expressed as a small (∼19 kDa) cytoplasmic PYD-containing protein which interacts with two important regulatory components of innate immunity: NF-κB/p105 and the ASC of the inflammasomes. Expression of M013 protein thus blocks two important innate immune signaling cascades and reduces the secretion of inflammatory cytokines under their control: for example, IL-1β and IL-18 under inflammasome control and TNF and IL-6 under NF-κB control.6–8 In infections of susceptible hosts (rabbits of the genus Oryctolagus), suppression of the immune response by myxoma is so successful that a single infectious virion is sufficient to kill an adult rabbit.4 Viruses deleted for the M013 gene, however, are severely attenuated because of the inability of the mutant virus to inhibit innate immune and inflammatory responses of the host.6,7 Some of the immunomodulators expressed by this virus act only within this rabbit species, but others, such as M013, inhibit host cell targets in a wide variety of species.1,44 Thus, the M013 protein inhibits both NF-κB and inflammasome signaling in human and murine cells, as well as in rabbits.7
Although the retinal pigment epithelium (RPE) plays a major role in the secretion of inflammatory cytokines in diseases such as uveitis and AMD, other important sources of proinflammatory molecules include Müller glia, the major resident macroglial cells of the retina, retinal microglia, and vascular endothelial cells. Because there are multiple sources of induced inflammatory pathways, we constructed an AAV2-based capsid mutant that expresses a secretable and cell-penetrating fused version of M013. The N-terminal signal sequence from immunoglobulin κ led to efficient section of an sGFP-TatM013 fusion protein, with the GFP cleaved from TatM013 by furin during the secretion phase. The cell penetration signal derived from HIV tat permitted entry of the TatM013 fusion protein into intact nontransduced cells in the surrounding tissue. Cell culture experiments indicated that this fused TatM013 gene retained the immune suppressive properties of the parental M013 protein. The sGFP-TatM013 fusion protein inhibited the secretion of ligand-induced IL-1β in relevant cell types, the mouse monocyte line RAW 246.7, human myeloid THP-1 cells, and the RPE-like human ARPE-19 line, even after incubation in media from various cells that had secreted M013. When injected in mouse vitreous this capsid mutant of AAV coexpressed recombinant M013 protein and GFP with a pattern of fluorescence consistent with a secreted protein. Furthermore, transduction with this AAV vector also led to a significant decrease in the recruitment of inflammatory cells in the vitreous, when eyes were challenged with LPS 1 month after vector delivery in the EIU mouse model.
The eye presents a particularly useful tissue to test immunomodulatory therapy against chronic inflammatory syndromes, using AAV gene therapy vectors armed with this secreted tat-fusion version of M013. Inflammation in the eye is localized and, because the TatM013 protein is secreted from cells that take up and express the AAV-TatM013 vector, a low dose of vector delivered to the vitreous compartment should protect all layers of the retina from inflammatory signals that arise locally. Indeed, monoclonal antibodies that bind vascular endothelial growth factor (VEGF)-A are delivered directly to the vitreous in order to block neovascularization from the choroid, on the other side of the neural retina.45 Tseng and colleagues demonstrated that activation of the inflammasome could be associated with the low-grade inflammation associated with dry AMD.46 Activation of the NLRP3 inflammasome has also been reported in donor samples and in mouse models of AMD.41,47 In addition to uveitis, there are other diseases of the eye that may be amenable to therapy with this novel AAV-TatM013 vector. For example, we developed a mouse model of geographic atrophy, based on oxidative stress in the RPE.48 We will next evaluate the secreted, cell-penetrating M013 for its ability to protect photoreceptor structure and function in the face of increased oxidative stress.
This is the first report of a virus-derived inhibitor of innate immunity expressed from a gene therapy vector that demonstrates usefulness for protecting neighboring, nontransduced cells against inflammatory insults that emanate from the target tissue. As a regimen of immunosuppressive gene therapy, systemic treatment with TatM013 might be more applicable when production of the fusion protein can be regulated with exogenous inducers. But even without specific regulation of the TatM013 gene, localized AAV-mediated expression of the secreted M013 may be of value for chronic autoimmune disorders or other organ-specific inflammatory diseases.
Acknowledgments
The authors acknowledge the technical help of Mr. James Thomas, Jr. for the preparation of plasmids for viral packaging. The authors also thank Dr. Zhaoyang Wang and Brian Rossmiller for help with animal injections. The authors thank Mr. Vince Chiodo (UF Vector Core Laboratory) for production of the vectors used in these experiments. Finally, the authors appreciate the thoughtful review of this manuscript by Dr. Chulbul Ahmed. This research was funded by grants from the National Eye Institute (R01 EY02025) and the Florida Biomedical Research Foundation (e-10KG-s), and by the Macula Vision Research Foundation. Core facilities were supported by NEI grant P30 EY02172. The G.M. laboratory is supported by NIAID R01 AI100987. Author contributions: C.J.I. and A.S.L. designed experiments. C.J.I. and H.J. conducted the experiments and analyzed the data. C.J.I., H.J., M.M.R., Q.L., S.E.B., W.W.H., A.R.L., G.M., and A.S.L. contributed to manuscript preparation and review.
Author Disclosure Statement
Patent protection for the AAV-sGFP-TatM013 technology has been filed by the University of Florida.
References
- 1.Liu J, Wennier S, and McFadden G. The immunoregulatory properties of oncolytic myxoma virus and their implications in therapeutics. Microbes Infect 2010;12:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratke KA, McLysaght A, and Rothenburg S. A survey of host range genes in poxvirus genomes. Infect Genet Evol 2013;14:406–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith GL, Benfield CTO, Maluquer de Motes C, et al. . Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol 2013;94:2367–2392 [DOI] [PubMed] [Google Scholar]
- 4.Shope RE. A filtrable virus causing a tumor-like condition in rabbits and its relationship to virus myxomatosum. J Exp Med 1932;56:803–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerr PJ. Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Res 2012;93:387–415 [DOI] [PubMed] [Google Scholar]
- 6.Johnston JB, Barrett JW, Nazarian SH, et al. . A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 2005;23:587–598 [DOI] [PubMed] [Google Scholar]
- 7.Rahman MM, and McFadden G. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways. J Virol 2011;85:12505–12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman MM, Mohamed MR, Kim M, et al. . Co-regulation of NF-κB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog 2009;5:e1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hori J. Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation. J Ocul Biol Dis Infor 2008;1:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mochizuki M, Sugita S, and Kamoi K. Immunological homeostasis of the eye. Prog Retin Eye Res 2013;33:10–27 [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum JT, and Kim HW. Innate immune signals in autoimmune and autoinflammatory uveitis. Int Rev Immunol 2013;32:68–75 [DOI] [PubMed] [Google Scholar]
- 12.Foster CS, and Vitale AT, eds. Diagnosis and Treatment of Uveitis (W.B. Saunders, Philadelphia, PA: ). 2002; pp. 17–23 [Google Scholar]
- 13.Valentincic NV, de Groot-Mijnes JDF, Kraut A, et al. . Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol Vis 2011;17:2003–2010 [PMC free article] [PubMed] [Google Scholar]
- 14.Cordero-Coma M, Calleja S, Llorente M, et al. . Serum cytokine profile in adalimumab-treated refractory uveitis patients: decreased IL-22 correlates with clinical responses. Ocul Immunol Inflamm 2013;21:212–219 [DOI] [PubMed] [Google Scholar]
- 15.Zolotukhin S, Potter M, Zolotukhin I, et al. . Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 2002;28:158–167 [DOI] [PubMed] [Google Scholar]
- 16.Jacobson SG, Acland GM, Aguirre GD, et al. . Safety of recombinant adeno-associated virus type 2–RPE65 vector delivered by ocular subretinal injection. Mol Ther 2006;13:1074–1084 [DOI] [PubMed] [Google Scholar]
- 17.Skeie JM, Tsang SH, and Mahajan VB. Evisceration of mouse vitreous and retina for proteomic analyses. J Vis Exp 2011;50:2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685 [DOI] [PubMed] [Google Scholar]
- 19.Kohno H, Chen Y, Kevany BM, et al. . Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem 2013;288:15326–15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kauppinen A, Niskanen H, Suuronen T, et al. . Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells—implications for age-related macular degeneration (AMD). Immunol Lett 2012;147:29–33 [DOI] [PubMed] [Google Scholar]
- 21.Verma A, Shan Z, Lei B, et al. . ACE2 and Ang-(1–7) confer protection against development of diabetic retinopathy. Mol Ther J Am Soc Gene Ther 2012;20:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakihana T, Araki K, Vavassori S, et al. . Dynamic regulation of Ero1α and peroxiredoxin 4 localization in the secretory pathway. J Biol Chem 2013;288:29586–29594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stow JL, and Murray RZ. Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev 2013;24:227–239 [DOI] [PubMed] [Google Scholar]
- 24.Reinhardt TA, Lippolis JD, and Sacco RE. The Ca2+/H+ antiporter TMEM165 expression, localization in the developing, lactating and involuting mammary gland parallels the secretory pathway Ca2+ ATPase (SPCA1). Biochem Biophys Res Commun 2014;445:417–421 [DOI] [PubMed] [Google Scholar]
- 25.Hauswirth WW, Aleman TS, Kaushal S, et al. . Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire AM, Simonelli F, Pierce EA, et al. . Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonelli F, Maguire AM, Testa F, et al. . Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Testa F, Maguire AM, Rossi S, et al. . Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 2013;120:1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLaren RE, Groppe M, Barnard AR, et al. . Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 2014;383:1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beltran WA, Boye SL, Boye SE, et al. . rAAV2/5 gene-targeting to rods: dose-dependent efficiency and complications associated with different promoters. Gene Ther 2010;17:1162–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kay CN, Ryals RC, Aslanidi GV, et al. . Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 2013;8:e62097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen R, Song Y, Cheng T, et al. . Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J Neurosci 1995;15:7377–7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin S, and Rodrigues GA. Differential roles of AMPKα1 and AMPKα2 in regulating 4-HNE-induced RPE cell death and permeability. Exp Eye Res 2010;91:818–824 [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum JT, McDevitt HO, Guss RB, et al. . Endotoxin-induced uveitis in rats as a model for human disease. Nature 1980;286:611–613 [DOI] [PubMed] [Google Scholar]
- 35.Shen DF, Buggage RR, Eng HC, et al. . Cytokine gene expression in different strains of mice with endotoxin-induced uveitis (EIU). Ocul Immunol Inflamm 2000;8:221–225 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, and Chan C. The role of inflammasome in Alzheimer's disease. Ageing Res Rev 2014;15:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall ED, Oostveen JA, and Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia 1998;23:249–256 [DOI] [PubMed] [Google Scholar]
- 38.Chiu IM, Phatnani H, Kuligowski M, et al. . Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci U S A 2009;106:20960–20965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollyfield JG, Perez VL, and Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol 2010;41:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson OA, Finkelstein A, and Shima DT. A2E induces IL-1β production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS One 2013;8:e67263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarallo V, Hirano Y, Gelfand BD, et al. . DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 2012;149:847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao L, Wang H, Wang F, et al. . Aβ-induced senescent retinal pigment epithelial cells create a proinflammatory microenvironment in AMD. Invest. Ophthalmol Vis Sci 2013;54:3738–3750 [DOI] [PubMed] [Google Scholar]
- 43.Liu RT, Gao J, Cao S, et al. . Inflammatory mediators induced by amyloid-β in the retina and RPE in vivo: implications for inflammasome activation in age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:2225–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas A, and McFadden G. Secreted immunomodulatory viral proteins as novel biotherapeutics. J Immunol 2004;173:4765–4774 [DOI] [PubMed] [Google Scholar]
- 45.Abedi F, Wickremasinghe S, Islam AFM, et al. . Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 2014;34:1531–1538 [DOI] [PubMed] [Google Scholar]
- 46.Tseng WA, Thein T, Kinnunen K, et al. . NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerur N, Hirano Y, Tarallo V, et al. . TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci 2013;54:7395–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo S, Krebs MP, Mao H, et al. . Pathological consequences of long-term mitochondrial oxidative stress in the mouse retinal pigment epithelium. Exp Eye Res 2012;101:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]